Abstract
Purpose
Although the cause of prune belly syndrome is unknown, familial evidence suggests a genetic component. Recently 2 nonfamilial cases of prune belly syndrome with chromosome 17q12 deletions encompassing the HNF1β gene have made this a candidate gene for prune belly syndrome. To date, there has been no large-scale screening of patients with prune belly syndrome for HNF1β mutations. We assessed the role of HNF1β in prune belly syndrome by screening for genomic mutations with functional characterization of any detected mutations.
Materials and Methods
We studied patients with prune belly syndrome who were prospectively enrolled in our Pediatric Genitourinary DNA Repository since 2001. DNA from patient samples was amplified by polymerase chain reaction, sequenced for coding and splice regions of the HNF1β gene, and compared to control databases. We performed functional assay testing of the ability of mutant HNF1β to activate a luciferase construct with an HNF1β DNA binding site.
Results
From 32 prune belly syndrome probands (30 males, 2 females) HNF1β sequencing detected a missense mutation (V61G) in 1 child with prune belly syndrome. Absent in control databases, V61G was previously reported in 2 patients without prune belly syndrome who had congenital genitourinary anomalies. Functional testing showed similar luciferase activity compared to wild-type HNF1β, suggesting the V61G substitution does not disturb HNF1β function.
Conclusions
One genomic HNF1β mutation was detected in 3% of patients with prune belly syndrome but found to be functionally normal. Thus, functionally significant HNF1β mutations are uncommon in prune belly syndrome, despite case reports of HNF1β deletions. Further genetic study is necessary, as identification of the genetic basis of prune belly syndrome may ultimately lead to prevention and improved treatments for this rare but severe syndrome.
Keywords: genetics, medical, HNF1B protein, human, prune belly syndrome
Prune belly syndrome (Online Mendelian Inheritance in Man code 100100) is a severe multisystem congenital anomaly complex affecting 3.8 per 100,000 live male births.1 By definition the syndrome includes 1) hypoplastic or absent abdominal wall musculature, 2) moderate to severe urinary tract dilatation and 3) bilateral undescended testes in males.2 Prune belly syndrome is an extremely morbid condition, as 20% of patients are stillborn, 30% die during the initial hospitalization and the remaining 50% have degrees of urinary pathology during their lifetime, including renal failure in 67%. In addition, 43% of patients are born premature, 48% require respiratory intubation and mechanical ventilation, and 25% have congenital cardiovascular anomalies. Despite advances in perinatal care, the overall initial mortality rate does not seem to have improved since the middle of the last century. Abdominal wall weakness increases susceptibility to pulmonary infection, decreases physical mobility and causes psychological concerns due to poor cosmesis. Fertility is decreased and extensive surgery is required for reconstruction.
While the cause of PBS is unknown, several aspects of the syndrome, including a high concordance rate in twins (12.2 per 100,000 live births), monozygotic male twin case reports, familial case reports and a higher incidence in males, suggest that it is influenced by a sex-linked genetic factor, although some have suggested an autosomal recessive mode of inheritance.3–6 A recent 9-year study from a national database found a 5:3:1:1 white-to-black-to-Hispanic-to-other racial distribution, with a twofold higher proportion of PBS in blacks than in the general population.1 Thus, evidence suggests that PBS might be a genetic disorder. In a genetic disease the spectrum of DNA alterations ranges from point mutations and small deletions/insertions (detectable by direct DNA sequencing) to DNA rearrangements (detectable by karyotyping and fluorescence in situ hybridization) to single and multi-exon deletions and duplications (detectable by comparative genomic hybridization).7 A panel that tests for multiple types of genomic alterations may be required to define a genetic disease.
Recently 2 case reports of PBS with interstitial deletions in chromosome 17q12 encompassing HNF1β (also known as TCF2) have made HNF1β a candidate PBS gene.8,9 HNF1β is a transcription factor that regulates gene expression necessary for mesodermal and endodermal development and is expressed in numerous tissues, including mesonephric duct derivatives. Since HNF1β mutations and deletions have been postulated to contribute to urinary tract abnormalities,10,11 we performed a large-scale screening of HNF1β mutations in patients with PBS.
MATERIALS AND METHODS
Sequencing
With institutional review board approval, we identified patients prospectively enrolled since 2001 in the University of Texas Southwestern Pediatric Urology DNA and Tissue Repository with a diagnosis of PBS. Genomic DNA was extracted from whole blood of PBS probands using standard procedures and screened for mutations by sequencing coding regions and intron-exon boundaries of HNF1β (www.polymorphicdna.com). Detected HNF1β mutations were cross-referenced to 2 large multiethnic databases, the NCBI dbSNP (www.ncbi.nih.gov/projects/SNP/) and 1000 Genomes Project (www.1000genomes.org/).
In Silico Functional Characterization
Interspecies comparison of HNF1β amino acid conservation was performed by using the NCBI protein-protein Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/BLAST.cgi). To predict the structural and functional impact of detected HNF1β amino acid substitutions, the detected substitutions were analyzed using PolyPhen-2 (http://genetics.bwh.harvard.edu/) and SIFT (http://sift.jcvi.org/).
Functional Testing
Plasmid construction
The pcDNA 3.1 plasmid encoding the truncated form of mouse HNF1β lacking 236 amino acids at the C-terminal end (pcDNA3-HNF1βΔC) has been described previously,12 as well as a luciferase reporter plasmid containing approximately 1.9 kb of the autosomal recessive polycystic kidney and hepatic disease 1 (Pkhd1) promoter linked to the coding region of firefly luciferase (pGL3-Pkhd1).13 A plasmid (pRSVcat-N809) containing the human wild-type HNF1β cDNA was obtained from the CNRS Institut Pasteur, Paris. The HNF1β coding region was amplified from the pRSVcat-N809 with forward primer 5′-ATGGTGTCCAAGCTCACGTC and reverse primer 5′-CCAGGCTTGTAGAGGACACTG, and the 1.6kb band was cloned in the TA site of the pcDNA3.1/V5-HIS-TOPO vector to produce the pcDNA3.1-WT HNF1β vector. The V61G mutation was introduced in the pcDNA3.1-WT HNF1β plasmid using the forward primer 5′-GGGGCCGAGCCCGACACCAAGCCGGGCTTCCATACTCTCACCAACGGCCAC and the reverse primer 5′-GTGGCCGTTGGTGAGAGTATGGAAGCCCGGCTTGGTGTCGGGCTCGGCCCC to produce the pcDNA3.1-V61G-HNF1β plas-mid. Site directed mutagenesis was performed using the QuikChange® kit, as described previously,12 and the presence of the desired mutation was verified by DNA sequencing.
Luciferase assay
mIMCD3 and HeLa cells were grown as described previously.12 Cells were plated in 6-well dishes (1.2 × 105 cells per well) and transfected with 0.6 μg luciferase reporter plasmids using FuGENE® 6. Cells were cotransfected with 20 ng pRL plasmid encoding Renilla luciferase to control for differences in transfection efficiency. After growth for 48 hours the cells were lysed in 250 μl passive lysis buffer (Promega Corp., Madison, Wisconsin), freeze-thawed once and centrifuged. Supernatants (20 μl) were added to 96-well plates, and firefly and Renilla luciferase activities were measured using the Dual-Luciferase® Reporter Assay System according to the manufacturer directions. Luciferase assay reagent II (100 μl) was added, and light output was measured for 10 seconds using a VICTOR™ V multilabel counter. Firefly luciferase activity was normalized to Renilla luciferase activity, which was measured by adding 100 μl Stop & Glo® reagent and measuring light output for 5 seconds. ANOVA using Dunnett’s test (GraphPad Prism®, version 5) was used to test for significance of differences in the means, expressed as false discovery rate q values.
RESULTS
HNF1β Gene Sequencing
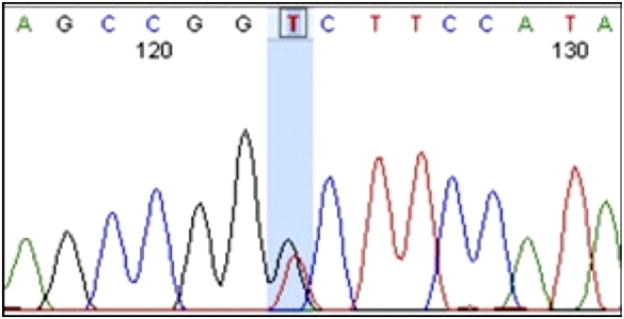
DNA samples from 32 probands affected with PBS were identified in the repository, including 30 males (23 white non-Hispanics, 3 black non-Hispanics, 4 white Hispanics) and 2 females (both white non-Hispanics). One heterozygous mutation was discovered in exon 1 of the HNF1β gene, yielding a missense mutation encoding glycine instead of valine at amino acid position 61 (V61G, fig. 1). Parental samples of this individual with PBS were unavailable to assess whether the mutation was inherited or de novo. This nsSNP was not reported in the NCBI dbSNP and is not contained in the 1000 Genome Project. However, the V61G mutation was previously reported in 2 patients without PBS, 1 with VACTERL association,14 and 1 with müllerian anomaly and a solitary kidney.15 Neither of these publications described functional testing of the V61G missense mutation to prove causality.
Figure 1.
Electropherogram of V61G mutation (blue shaded area) in patient with PBS, occurring at position 36, 104, 694 (hg19).
The V61G mutation is found in exon 1 of the HNF1β gene between the dimerization domain and the DNA binding domain,16 and is conserved across multiple species (see Appendix). However, both in silico analyses predict that the V61G substitution is not functionally significant. PolyPhen-2 analysis predicts V61G to be benign (PolyPhen-2 score = 0.000 [scale of 0 to 1.0]) and SIFT analysis predicts V61G is tolerated (SIFT score = 0.08 [scale of 0 to 1.0, with score less than 0.05 considered damaging]).
Functional Assays
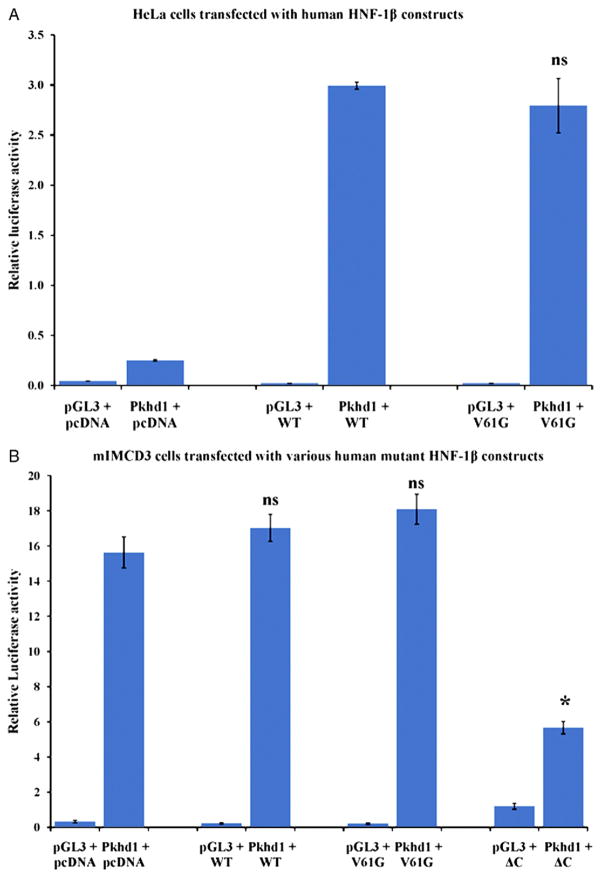
HNF1β consists of an N-terminal dimerization domain, a Pit-1/Oct-1/Unc-86 domain and homeodomain that mediate DNA binding, and a C-terminal transcriptional activation domain. HNF1β is known to regulate the expression of Pkhd1 by directly binding to the Pkhd1 promoter.13 To test whether V61G HNF1β can activate the Pkhd1 promoter, reporter gene assays were performed in HeLa cells that do not endogenously express HNF1β. Plasmids encoding either the WT HNF1β, V61G HNF1β or empty parent vector were cotransfected with the luciferase reporter plasmid containing the Pkhd1 promoter into HeLa cells, and luciferase activity was measured 48 hours later (fig. 2, A). To correct for differences in transfection efficiency, cells were transfected with a constant amount of pRL encoding Renilla luciferase, and transcription activity was calculated from the ratio of firefly and Renilla luciferase activities. Each transfection was completed in triplicate and each experiment was repeated 3 times. Calculated average ± SE relative luciferase activity for WT HNF1β and V61G HNF1β was 2.99 ± 0.06 and 2.79 ± 0.47, respectively, suggesting that the V61G mutation does not affect the ability of HNF1β to bind to the Pkhd1 promoter and drive expression of luciferase.
Figure 2.
Transactivation of Pkhd1 promoter by WT and mutant V61G HNF1β proteins. A, HeLa cells were cotransfected with 0.6 μg pGL3-Pkhd1 and 0.3 μg pcDNA3.1 or pcDNA3.1-WT HNF1β or pcDNA3.1-V61G HNF1β. Cells were cotransfected with 2 ng pRL, and luciferase activity was normalized to Renilla luciferase. Data are means ± SE for 3 independent transfections. B, mIMCD3 cells were cotransfected with 0.6 μg pGL3-Pkhd1 and 0.3 μg pcDNA3.1, pcDNA3.1-WT HNF1β, pcDNA3.1-V61G HNF1β or pcDNA3-HNF1βΔC. Cells were cotransfected with 2 ng pRL, and luciferase activity was normalized to Renilla luciferase. Data are means ± SE for 3 independent transfections. Asterisk indicates p <0.001 compared to cells transfected with pcDNA3.1. ns, nonsignificant.
Activation of the Pkhd1 promoter by HNF1β is stimulated by cyclic adenosine monophosphate responsive element binding protein and p300/CBP associated factor, which directly interact with the transcriptional activation domain at the C-terminal of HNF1β. These findings suggest that HNF1β activates Pkhd1 transcription by recruiting coactivators that promote histone acetylation and chromatin remodeling at the promoter. Deletion mutants lacking the C-terminal domain (ΔC HNF1β) function as dominant negative mutants, possibly by preventing the recruitment of coactivators.17 To determine if the V61G mutation encodes for a dominant negative mutant form of HNF1β, reporter assays were performed in mouse inner medullary collecting duct cells (mIMCD3), which endogenously express HNF1β. Plasmids encoding either the WT HNF1β, V61G HNF1β, ΔC HNF1β or an empty vector were cotransfected with the luciferase reporter plasmid containing the Pkhd1 promoter into mIMCD3 cells, and luciferase activity was measured 48 hours later (fig. 2, B).
To correct for differences in transfection efficiency, cells were transfected with a constant amount of pRL encoding Renilla luciferase, and transcription activity was calculated from the ratio of firefly and Renilla luciferase activities. Each transfection was completed in triplicate and each experiment was repeated 3 times. Calculated average ± SE relative luciferase activity for WT HNF1β and V61G HNF1β was 17.0 ± 1.3 and 18.1 ± 1.5, respectively, and for the dominant negative mutant ΔC HNF1β was 5.661 ± 0.601. Statistical analysis of these averages suggests that the V61G mutation does not encode for a dominant negative form of HNF1β (q = 2.14).
DISCUSSION
PBS, also known as Eagle-Barrett syndrome, is lethal in approximately 50% of affected individuals and is associated with high morbidity in the survivors. In addition to anomalies of the abdominal wall and urinary tract and cryptorchidism, accompanying birth defects can include cardiovascular, respiratory, orthopedic and gastrointestinal anomalies. Although the majority of surviving patients with PBS have normal cognitive functioning, many surviving children have lifelong disabilities, including renal insufficiency and renal failure requiring renal replacement therapy. Multiple surgical interventions are necessary in surviving children, and despite surgical advances, the disorder remains challenging. PBS is associated with long-term diminished self-esteem from poor cosmesis, constipation, pulmonary and urinary dysfunction, impaired physical mobility and sexual health issues. For PBS survivors and their families the lifelong disabilities can be devastating psychologically, financially, socially and medically.
Two mechanistic theories have been proposed to explain embryonic maldevelopment, which begins at approximately 6 to 10 weeks of gestation. In the first theory, known as the theory of mesodermal arrest, an unknown primary defect in the development of the lateral plate mesoderm between 6 and 10 weeks of gestation produces primary maldevelopment of the abdominal wall and urinary tract musculature. In the second theory, involving in utero bladder obstruction, a hypoplastic/dysplastic prostate or abnormal urethra prevents urine passage. This obstruction of urine flow causes bladder, ureteral and renal distention with secondary abdominal wall and urinary muscle maldevelopment. Despite these anatomical and mechanistic theories, the molecular basis is unknown.
Originally a possible genetic basis for PBS was largely discounted due to reports of monozygotic twins discordant for PBS. Nevertheless, 2 publications have described concordant PBS occurring in monozygotic twins.3,4 Additionally 12 published case reports of familial PBS primarily affecting brothers have suggested a possible autosomal or X-linked recessive mode of inheritance (see table).3–6,18–25 Thus, a genetic basis for PBS is highly suggested.
Currently HNF1β is the only candidate PBS gene based on 2 published PBS cases with chromosome 17q12 microdeletions encompassing the HNF1β gene.8,9 In 2008 an adult with PBS, type 2 diabetes, early onset gout and pancreatic atrophy was tested for mutations in HNF1β, which identified no variants.8 Further testing identified a de novo heterozygous chromosome 17q12 microdeletion of 3.2Mb deleting the entire HNF1β gene. In 2010 a severely affected infant with PBS, who died at birth, was described to have a de novo 1.3Mb chromosome 17q12 interstitial microdeletion including the HNF1β gene.9
HNF1β is a transcription factor that regulates gene expression necessary for normal mesodermal and endodermal development, and is expressed in numerous tissues, including kidney, prostate, mesonephric duct derivatives, pancreas, gut and liver. The renal cysts and diabetes syndrome (Online Mendelian Inheritance in Man code 137920, also known as maturity onset diabetes of the young, type 5) represents the most common HNF1β phenotype.11,26–28 Other clinical features described in association with the HNF1β phenotype include müllerian duct anomalies, increased liver enzymes and hyperuricemia.15,29,30 Given the temporal and spatial expression pattern of HNF1β, it is conceivable that HNF1β haploinsufficiency or HNF1β loss of function mutations could disturb normal intermediate mesoderm differentiation, yielding the urinary tract maldevelopment seen in PBS.10,11
Given the rarity of PBS, nearly all publications on PBS are single case studies describing large genomic rearrangements. Currently there are no publications investigating the genetic basis of PBS in a large cohort. Thus, our cohort of 32 patients with PBS may represent the largest series published to date. Despite our large-scale screening, only 1 mutation of HNF1β, V61G, was detected in 1 patient with PBS. This missense mutation has previously been reported in 2 individuals without PBS, 1 with VACTERL association,14 and 1 with müllerian duct anomaly and solitary kidney.15 However, no testing was performed by either group to confirm or refute whether the V61G substitution disturbed HNF1β function. This valine is highly conserved between species but its location between important protein motifs decreases the impact of amino acid substitution in this position.
Since the V61G substitution had not been detected in normal controls but had been detected in 2 patients with congenital genitourinary anomalies, we performed functional characterization of the V61G variant. The V61G mutant HNF1β protein revealed normal transcription factor binding without dominant negative effect via luciferase assay, suggesting that V61G is not a functionally significant mutation.
Nevertheless, this study does not completely discount the role of HNF1β in PBS. Given that all of our patients are living survivors with PBS, we could have enrollment bias in our study. HNF1β may have a strong genetic role in PBS nonsurvivors, a cohort that is not included in our series.
Also our patients may have HNF1β mutations positioned outside the regions analyzed in our study, such as within regulatory regions or introns. In addition, mutations in upstream or downstream signaling events in the HNF1β pathway may affect HNF1β expression and function. However, these pathways are only currently being elucidated and may comprise hundreds of genes. Our functional assay does not comprehensively assess these unknown pathways.
Finally, polymerase chain reaction sequencing does not test for copy number variations or gene rearrangements. Since polymerase chain reaction does not test for the previously reported HNF1β heterozygous deletions found in the 2 PBS cases, an HNF1β copy number variation assay is necessary to discount the role of HNF1β in PBS. Having screened the only known candidate gene for PBS, mutations of genes other than HNF1β might cause PBS. Until additional candidate genes are identified, the genetic basis of PBS remains unknown.
CONCLUSIONS
Despite PBS case reports of multi-exonic HNF1β deletions, functionally significant mutations detectable by coding and splice site sequencing of HNF1β are uncommon in PBS. The V61G HNF1β mutation was detected in 1 of 34 patients (3%) with PBS in this large-scale screening but was observed to be functionally normal. Further genetic study is warranted in PBS to lead ultimately to prevention and improved treatments for this rare but severe disease.
Table 1.
Reported cases of familial prune belly syndrome
| References | PBS Cases | Suggested Mode of Inheritance |
|---|---|---|
| Grenet et al18 | 2 Brothers | Autosomal/X-linked recessive |
| Harley et al19 | 2 Brothers | Autosomal/X-linked recessive |
| Afifi et al20 | 2 Brothers of first cousin parents | Autosomal/X-linked recessive |
| Garlinger and Ott4 | 2 Brothers | Autosomal/X-linked recessive |
| Riccardi and Grum21 | 2 Brothers | Autosomal/X-linked recessive |
| Lockhart et al5 | 2 Brothers + 1 sister | Autosomal recessive |
| Gaboardi et al22 | 2 Brothers + 1 sister | Autosomal recessive |
| Adeyokunnu and Familusi23 | 1 Brother + 1 sister + 1 cousin | Autosomal recessive |
| Feige et al24 | 1 Brother + 1 sister | Autosomal recessive |
| Balaji et al3 | 2 Monozygotic male twins | Autosomal recessive |
| Chan and Bird25 | Family (2 brothers + mother + maternal grandmother) | Autosomal dominant/mitochondrial |
| Ramasamy et al6 | 2 Brothers | Autosomal/X-linked recessive |
Acknowledgments
Supported by the Prune Belly Syndrome Network, Inc. (http://www.prunebelly.org/), NIH Grant R01DK042921 and the UT Southwestern O’Brien Kidney Research Core Center (P30DK079328).
Dr. Moshe Yaniv, CNRS Institut Pasteur, Paris, supplied the plasmids. Emma Sanchez, research coordinator, provided integral help with recruitment and consenting PBS probands and families. Wen-Xiu Zhang and Patricia Cobo-Stark assisted with research.
Abbreviations and Acronyms
- HNF1β
hepatocyte nuclear factor-1beta
- mIMCD3
mouse inner medullary collecting duct cells
- NCBI
National Center for Biotechnology Information
- PBS
prune belly syndrome
- Pkhd1
autosomal recessive polycystic kidney and hepatic disease 1
- SIFT
sorting intolerant from tolerant
- SNP
single nucleotide polymorphism
- WT
wild-type
APPENDIX
Conserved region of V61G mutation (red) within HNF1β across species

|
References
- 1.Routh JC, Huang L, Retik AB, et al. Contemporary epidemiology and characterization of newborn males with prune belly syndrome. Urology. 2010;76:44. doi: 10.1016/j.urology.2009.12.072. [DOI] [PubMed] [Google Scholar]
- 2.Smith E, Woodard JR. Prune-belly syndrome. In: Gearhart JP, Garrett RA, Mouriquand PDE, et al., editors. Pediatric Urology. Philadelphia: WB Saunders; 2001. pp. 577–592. [Google Scholar]
- 3.Balaji KC, Patil A, Townes PL, et al. Concordant prune belly syndrome in monozygotic twins. Urology. 2000;55:949. doi: 10.1016/s0090-4295(00)00452-0. [DOI] [PubMed] [Google Scholar]
- 4.Garlinger P, Ott J. Prune belly syndrome: possible genetic implications. Birth Defects Orig Artic Ser. 1974;10:173. [PubMed] [Google Scholar]
- 5.Lockhart JL, Reeve HR, Bredael JJ, et al. Siblings with prune belly syndrome and associated pulmonic stensosis, mental retardation, and deafness. Urology. 1979;14:140. doi: 10.1016/0090-4295(79)90145-6. [DOI] [PubMed] [Google Scholar]
- 6.Ramasamy R, Haviland M, Woodard JR, et al. Patterns of inheritance in familial prune belly syndrome. Urology. 2005;65:1227. doi: 10.1016/j.urology.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 7.Tayeh MK, Chin EL, Miller VR, et al. Targeted comparative genomic hybridization array for the detection of single- and multiexon gene deletions and duplications. Genet Med. 2009;11:232. doi: 10.1097/GIM.0b013e318195e191. [DOI] [PubMed] [Google Scholar]
- 8.Murray PJ, Thomas K, Mulgrew CJ, et al. Whole gene deletion of the hepatocyte nuclear factor-1b gene in a patient with the prune-belly syndrome. Nephrol Dial Transplant. 2008;23:2412. doi: 10.1093/ndt/gfn169. [DOI] [PubMed] [Google Scholar]
- 9.Haeri S, Devers PL, Kaiser-Rogers KA, et al. Deletion of hepatocyte nuclear factor-1-beta in an infant with prune belly syndrome. Am J Perinatol. 2010;27:559. doi: 10.1055/s-0030-1248943. [DOI] [PubMed] [Google Scholar]
- 10.Mefford HC, Clauin S, Sharp AJ, et al. Recurrent reciprocal genomic rearrangements of 17q12 are associated with renal disease, diabetes, and epilepsy. Am J Hum Genet. 2007;81:1057. doi: 10.1086/522591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bingham C, Hattersley AT. Renal cysts and diabetes syndrome resulting from mutations in hepatocyte nuclear factor-1beta. Nephrol Dial Transplant. 2004;19:2703. doi: 10.1093/ndt/gfh348. [DOI] [PubMed] [Google Scholar]
- 12.Bai Y, Pontoglio M, Hiesberger T, et al. Regulation of kindey-specific Ksp-cadherin gene promoter by hepatocyte nuclear factor-1B. Am J Physiol. 2002;283:F389. doi: 10.1152/ajprenal.00128.2002. [DOI] [PubMed] [Google Scholar]
- 13.Hiesberger T, Bai Y, Shao X, et al. Mutations of hepatocyte nuclear factor-1B inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest. 2004;113:814. doi: 10.1172/JCI20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoskins BE, Cramer CH, Tasic V, et al. Missense mutations in EYA1 and TCF2 are a rare cause of urinary tract malformations. Nephrol Dial Transplant. 2008;23:777. doi: 10.1093/ndt/gfm685. [DOI] [PubMed] [Google Scholar]
- 15.Oram RA, Edghill EL, Blackman J, et al. Mutations in the hepatocyte nuclear factor-1B (HNF1B) gene are common with combined uterine and renal malformations but are not found with isolated uterine malformations. Am J Obstet Gynecol. 2010;203:364. doi: 10.1016/j.ajog.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Edghill EL, Bingham C, Ellard S, et al. Mutations in hepatocyte nuclear factor-1B and their related phenotypes. J Med Genet. 2006;43:84. doi: 10.1136/jmg.2005.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi P, Shao X, McNally BT, et al. Roles of HNF-1beta in kidney development and congenital cystic diseases. Kidney Int. 2005;68:1944. doi: 10.1111/j.1523-1755.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 18.Grenet P, Le Calvé G, Badoual J, et al. Congenital aplasia of the abdominal wall: a familial case. Ann Pediatr (Paris) 1972;19:523. [PubMed] [Google Scholar]
- 19.Harley LM, Chen Y, Rattner WH. Prune belly syndrome. J Urol. 1972;108:174. doi: 10.1016/s0022-5347(17)60675-x. [DOI] [PubMed] [Google Scholar]
- 20.Afifi AK, Rebeiz J, Mire J, et al. The mycopathology of the prune belly syndrome. J Neurol Sci. 1972;15:153. doi: 10.1016/0022-510x(72)90003-2. [DOI] [PubMed] [Google Scholar]
- 21.Riccardi VM, Grum CM. The prune belly anomaly: heterogeneity and superficial X-linkage mimicry. J Med Genet. 1977;14:266. doi: 10.1136/jmg.14.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaboardi F, Sterpa A, Thiebat E, et al. Prune-belly syndrome: report of three siblings. Helv Paediatr Acta. 1982;37:283. [PubMed] [Google Scholar]
- 23.Adeyokunnu AA, Familusi JB. Prune belly syndrome in two siblings and a first cousin. Possible genetic implications. Am J Dis Child. 1982;136:23. doi: 10.1001/archpedi.1982.03970370025005. [DOI] [PubMed] [Google Scholar]
- 24.Feige AF, Rempen A, Osterhage HR. Prenatal diagnosis of prune belly syndrome occurring in siblings in 2 consecutive pregnancies. Z Geburt-shilfe Perinatol. 1984;188:239. [PubMed] [Google Scholar]
- 25.Chan Y, Bird LM. Vertically transmitted hyp-oplasia of the abdominal wall musculature. Clin Dysmorphol. 2004;13:7. doi: 10.1097/00019605-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Bingham C, Bulman MP, Ellard S, et al. Mutations in the hepatocyte nuclear factor-1B gene are associated with familial hypoplastic glomerulo-cystic kidney disease. Am J Hum Genet. 2001;68:219. doi: 10.1086/316945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woolf AS, Feather SA, Bingham C. Recent insights into kidney disease associated with glomerular cysts: roles of fetal urinary tract obstruction and mutations of HNF-1B and OFD1. Pediatr Nephrol. 2002;17:229. doi: 10.1007/s00467-001-0819-5. [DOI] [PubMed] [Google Scholar]
- 28.Fajans SS, Bell GI, Polonsky KS, et al. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345:971. doi: 10.1056/NEJMra002168. [DOI] [PubMed] [Google Scholar]
- 29.Montoli A, Colussi G, Massa O, et al. Renal cysts and diabetes syndrome linked to mutations of the hepatocyte nuclear factor-1β gene: description of a new family with associated liver involvement. Am J Kidney Dis. 2002;40:397. doi: 10.1053/ajkd.2002.34538. [DOI] [PubMed] [Google Scholar]
- 30.Bingham C, Ellard S, van’t Hoff WG, et al. Atypical familial juvenile hyperuricemic nephropathy associated with a hepatocyte nuclear factor-1β gene mutation. Kidney Int. 2003;63:1645. doi: 10.1046/j.1523-1755.2003.00903.x. [DOI] [PubMed] [Google Scholar]