SUMMARY
The generation of induced pluripotent stem (iPS) cells and induced neuronal (iN) cells from somatic cells provides new avenues for basic research and potential transplantation therapies for neurological diseases. However, clinical applications must consider the risk of tumor formation by iPS cells and the inability of iN cells to self-renew in culture. Here we report the generation of induced neural stem cells (iNSCs) from mouse and human fibroblasts by direct reprogramming with a single factor, Sox2. iNSCs express NSC markers and resemble wild-type NSCs in their morphology, self-renewal, ability to form neurospheres, and gene expression profiles. Cloned iNSCs differentiate into several types of mature neurons, as well as astrocytes and oligodendrocytes, indicating multipotency. Implanted iNSCs can survive and integrate in mouse brains and, unlike iPS cell-derived NSCs, do not generate tumors. Thus, self-renewable and multipotent iNSCs without tumorigenic potential can be generated directly from fibroblasts by reprogramming.
INTRODUCTION
After seminal studies that succeeded in reprogramming mouse and human somatic cells to iPS cells (Takahashi et al., 2007; Takahashi and Yamanaka, 2006; Yu et al., 2007), researchers have taken great strides to improve reprogramming methods and to apply the technology to the understanding and potential future treatment of human diseases (Hanna et al., 2008; Hockemeyer et al., 2008; Kaji et al., 2009; Kim et al., 2009; Okita et al., 2008; Park et al., 2008a; Park et al., 2008b; Shi et al., 2008; Stadtfeld et al., 2008; Wernig et al., 2008; Woltjen et al., 2009; Yu et al., 2009). A number of disease- and patient-specific iPS cell lines have been established, including those from patients with amyotrophic lateral sclerosis (Dimos et al., 2008), spinal muscular atrophy (Ebert et al., 2009), Parkinson’s disease (Park et al., 2008a; Soldner et al., 2011), schizophrenia (Brennand et al., 2011), Huntington’s disease (Park et al., 2008a), and Alzheimer’s disease (Israel et al., 2012). Furthermore, correction of genetic mutations in disease-specific iPS cells can rescue phenotypes in cultured cells (Soldner et al., 2011; Yusa et al., 2011) or in mouse models of human diseases, such as sickle cell anemia (Hanna et al., 2007). However, for successful therapeutic application, iPS cells need to be efficiently differentiated into the desired cell type. Moreover, pluripotent stem cells, including embryonic stem (ES) cells and iPS cells, can form teratomas in vivo, whereas multipotent, lineage-restricted stem cells, such as hematopoietic stem cells and NSCs, do not (Fong et al., 2010; Miura et al., 2009; Yamanaka, 2009). Thus, direct reprogramming of somatic cells into multipotent, lineage-restricted stem cells should complement iPS cell technology and sidestep the difficulty of differentiating iPS cells. It would also lower the risk of immature teratoma formation after the transplantation of iPS cell–derived multipotent stem cells or their progeny as a result of potential iPS cell contamination or incomplete reprogramming.
Recently, transcription factors have been used to induce cell fate change from one type of somatic cell to another in cell cultures and in mice (Huang et al., 2011; Ieda et al., 2010; Sekiya and Suzuki, 2011; Zhou et al., 2008). Mouse and human fibroblasts and other types of cells have been transdifferentiated directly into postmitotic neurons with combinations of transcription factors (Ambasudhan et al., 2011; Caiazzo et al., 2011; Kim et al., 2011b; Marro et al., 2011; Pang et al., 2011; Qiang et al., 2011; Son et al., 2011; Vierbuchen et al., 2010; Yoo et al., 2011). iN cells have typical neuronal cell properties and exhibit proper electrical function in culture. Although iN cells can be generated with relatively high efficiency (5–20%), current protocols generate a mixture of neuronal cells and other unknown types of cells, limiting the direct use of iN cells in transplantation therapy. The addition of neuronal fate–specifying factors to the reprogramming cocktail can influence the efficiency with which a specific neuronal subtype can be generated (Caiazzo et al., 2011; Son et al., 2011). However, this technology is limited by the fact that iN cells are terminally differentiated and cannot self-renew. Most recently, it has been reported that the combination of three or more factors can reprogram mouse fibroblasts into induced neural stem cells (iNSCs) with self-renewing ability (Han et al., 2012; Kim et al., 2011a; Lujan et al., 2012; Sheng et al., 2011; Thier et al., 2012). Here, we present a novel method for generating self-renewable, multipotent, neural lineage–restricted, and non-tumorigenic iNSCs from mouse and human fibroblasts by direct reprogramming with one factor.
RESULTS
Generation and characterization of iNSCs from mouse fibroblasts
The protocol for generating iNSCs from mouse embryonic fibroblasts (MEFs) is shown in Figure S1A. In choosing the reprogramming factors, we considered five key transcription factors that are important in NSC production, maintenance, and self-renewal—Sox2, Bmi-1, TLX, Hes1, and Oct1 (Graham et al., 2003; Jin et al., 2009; Kageyama et al., 2008; Molofsky et al., 2003; Shi et al., 2004; Williams et al., 2004). These factors were expressed individually or in different combinations in MEFs by retroviral-mediated gene transduction. In pilot studies, the morphology of MEFs cultured on gelatin-coated plastic in NSC medium supplemented with growth factors (Epidermal Growth Factor (EGF) and Fibroblast Growth Factor (FGF2)) was unchanged for up to 4 weeks after transduction (Figures S1A-Step 1 and S1D). However, when MEFs were cultured on glass coverslips coated with gelatin, their morphology was drastically altered by retroviral Sox2 alone (Figures S1A-Step 2, 1A, 1B and S1B) or by Sox2 plus additional transcription factors. Since the combination of different transcription factors with Sox2 did not enhance the reprogramming efficiency, and in some cases yielded less encouraging results, we focused on using Sox2 alone.
By 2–10 days after transduction with Sox2, transformed cells had formed networks with colonies at the intersections of these networks (Figures S1A-Step 2, 1B, and S1B), many of which stained positive for Sox2 and Nestin (Figures 1C and S1C). The efficiency of generating Sox2 and Nestin double-positive colonies on gelatin-coated coverslips was 0.13% at day 8 post infection and 0.52% at day 12, and was further enhanced to 0.96% at day 8 by culturing infected cells on coverslips coated with NSC-permissive substrates poly-L-ornithine and laminin (Table S1) (Lee et al., 2007). Furthermore, immunostaining of Sox2-infected cells 14 days post infection in NSC-media with growth factors revealed the lack of MAP2-positive neurons, GFAP-positive astrocytes, and O4-positive oligodendrocytes, indicating that Sox2 transduction does not generate differentiated neural cells directly (Table S2). Importantly, no morphological changes and no Nestin- or Sox2-positive cells were observed in MEFs not transduced with Sox2 (Figure 1D) or in Sox2-transduced MEFs cultured on gelatin-coated plastic for up to 4 weeks (Figure S1D). Furthermore, untransfected MEFs did not stain positive for the differentiated cell markers MAP2, GFAP, and O4 (Table S2).
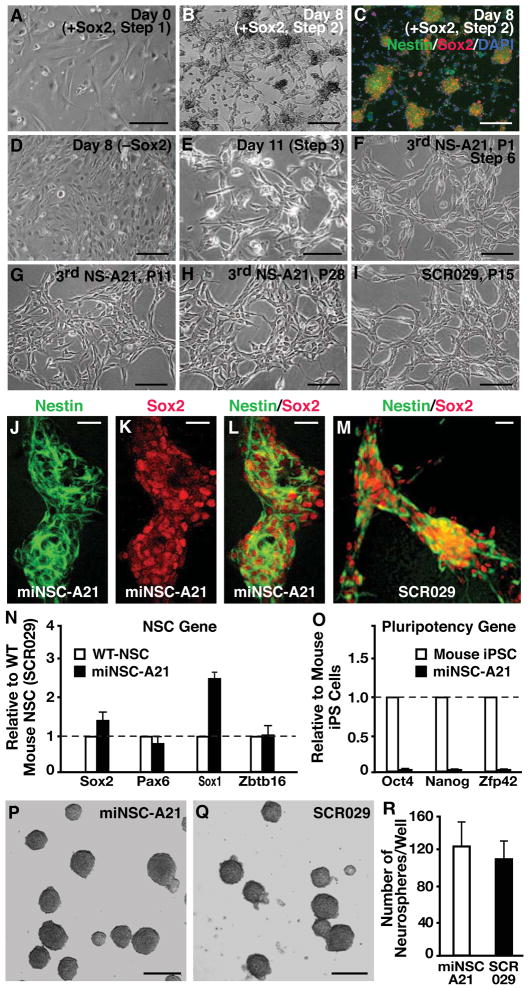
Figure 1. Generation and characterization of iNSCs from mouse fibroblasts.
(A) Phase-contrast image of MEFs after overnight treatment with Sox2 retrovirus in fibroblast medium.
(B) Sox2-infected cells in NSC medium with growth factors generate networks and colonies on gelatin-coated glass coverslips by 8 days after infection.
(C) Sox2-transformed colonies are positive for the NSC markers Nestin and Sox2.
(D) Fibroblasts cultured in NSC medium with growth factors but without Sox2 retroviral transduction do not generate colonies or networks.
(E) Sox2-transduced cells after 11 days have drastically different morphology from their fibroblast counterparts.
(F) After three rounds of neurosphere generation, reprogrammed cells take on the characteristic bipolar NSC morphology.
(G) After multiple passages as a monolayer, NSC-like cells are a morphologically homogenous population.
(H) Morphology of NSC-like cells stays the same over prolonged passaging, and reprogrammed cells can proliferate over 28 passages.
(I) The morphology of NSC-like cells is similar to that of wild-type cortical-derived NSCs such as the commercial cell line SCR029 (Millipore).
(J–M) For the miNSC-A21 cell line, expression of Nestin and Sox2 is similar to that of brain-derived wild-type NSCs as revealed by immunostaining.
(N and O) qRT-PCR reveals that miNSC-A21 express typical NSC markers (N), but do not express pluripotency related genes (O). Error bars denote standard deviation of triplicate reactions.
(P–R) In suspension culture, miNSC-A21 generates neurospheres similar to wild-type NSCs and with similar efficiency (n=3).
Scale bars = 50 μm in A and D–I; scale bars = 100 μm in B and C; scale bars = 50 μm in J–M; scale bars = 100 μm in P and Q.
See also Figures S1 and S2 and Tables S1, S2, and S4.
Six to ten days after retroviral Sox2 transduction, cell mixtures containing multiple colonies were collected and re-cultured to promote cell proliferation and expansion (Figures S1A-Step 3 and 1E). Five days later, Sox2-infected cells were released for primary neurosphere culture in suspension to select for NSC-like cells (Figures S1A-Step 4 and S1E). The primary neurospheres were seeded, and cells with NSC-like morphology grew gradually from adhered neurospheres (Figures S1A-Step 5, S1F, and S1G). To further enrich reprogrammed NSC-like cells, we repeated the neurosphere culture procedures twice (Figure S1A-Steps 4 and 5). NSC-like cells were then grown in a monolayer for many generations to generate a homogenous population of NSC-like cells (Figures S1A-Step 6 and 1F). Reprogrammed NSC-like cells at passages 8 (Figure S1H), 11 (Figure 1G), and 28 (Figure 1H) had morphologies very similar to those of wild-type mouse NSCs (Figures 1I and S1I). Similar results were obtained from independent reprogramming experiments using the same protocol (Figures S1J–S1L), demonstrating the repeatability of this reprogramming method.
The reprogrammed mouse NSC-like cells expressed NSC markers, including Sox2 and Nestin, similarly to the wild-type mouse NSC line SCR029 (Figures 1J–1M), as well as Pax6 and BLBP (Figures S2A–S2D). Quantitative real-time RT-PCR (qRT-PCR) confirmed that NSC-like cells expressed Sox2, Nestin, Sox1, and Zbtb16 (Figure 1N); however, they did not express pluripotency-related genes, such as Oct4, Nanog, and Zfp42 (Figure 1O). In contrast, MEFs cultured in fibroblast or NSC medium for up to 4 weeks did not show significant expression of Sox2, Nestin, Pax6, Zbtb16, or Msi1 (Figures S2E–S2H). We next determined whether reprogrammed NSC-like cells had silenced exogenous Sox2 expression and turned on endogenous Sox2 expression by qRT-PCR (Figure S2I). Interestingly, endogenous Sox2-specific qRT-PCR detected low levels of endogenous Sox2 expression in reprogrammed NSC-like cells at passage 7 and significant levels of exogenous Sox2 expression compared to wild-type NSCs. In contrast, reprogrammed NSC-like cells at later passage had more comparable levels of endogenous Sox2 expression to wild-type NSCs (Figure S2I), suggesting that the endogenous Sox2 gene was gradually turned on in reprogrammed NSC-like cells over continuous passaging. Exogenous Sox2-specific qRT-PCR did not detect a significant signal in either reprogrammed NSC-like cells at passage 16 or in wild-type NSCs, consistent with silencing of the retroviral Sox2 transgene in reprogrammed NSC-like cells during continuous passaging (Figure S2I). Thus, the reprogrammed NSC-like cells do not require the expression of exogenous Sox2 to maintain their NSC identity at later passages.
Methylation patterns of NSC (Sox2 and Nestin) and embryonic stem (ES) cell (Oct3/4) gene promoters were next analyzed in reprogrammed NSC-like cells, wild-type NSCs, and MEFs at passages 12, 17, and 2, respectively (Han et al., 2009; Imamura et al., 2006, Western et al., 2010). Methylation analysis of bisulfite-treated DNA revealed that the Oct3/4 promoter was hypermethylated in all three cell lines, indicating the transcriptional silencing of that gene (Figures S2J–S2L). In contrast, both the Sox2 and Nestin promoters were hypomethylated in reprogrammed NSC-like cells similarly to wild-type NSCs, indicating that these genes are transcriptionally activated (Figures S2M–S2P).
Microarray studies demonstrated that the global gene expression pattern of the reprogrammed NSC-like cells was similar to that of wild-type mouse NSCs but different fromthat of MEFs (Figures S2Q and S2R). Furthermore, like wild-type mouse NSCs, the reprogrammed NSC-like cells formed neurospheres in suspension cultures and did so with similar efficiency (Figures 1P–1R). Taken together, these data strongly suggest that a single factor plus NSC-permissive culture conditions can reprogram MEFs into self-renewing NSCs that appear similar to wild-type NSCs at the transcriptional level and in forming neurospheres. We therefore refer to them as mouse induced NSCs (miNSCs).
Multipotency of miNSCs in culture
Under neuronal differentiation conditions involving removal of growth factors from the NSC medium, miNSCs differentiated into immature neurons (Tuj1-positive) at 1 week in culture similarly to wild-type NSCs (Figures 2A and 2B) and mature neurons (MAP2- and Tau-positive) at 2 weeks (Figures 2E–2H). At 4 weeks, miNSCs developed into MAP2-positive neurons with extensive and complex neurites similar to those of mouse primary neurons in culture (Figure 2I). At 14–28 days, miNSCs differentiated into vGluT1-positive excitatory neurons (Figures 2J and S3E) and GABA-positive inhibitory neurons (Figure 2K). Importantly, MAP2-positive mature neurons could be generated from miNSCs at passages P11–P28 (Figures S3A–S3D) and beyond. Thus, miNSCs have stable neurogenesis capability during continuous passaging.
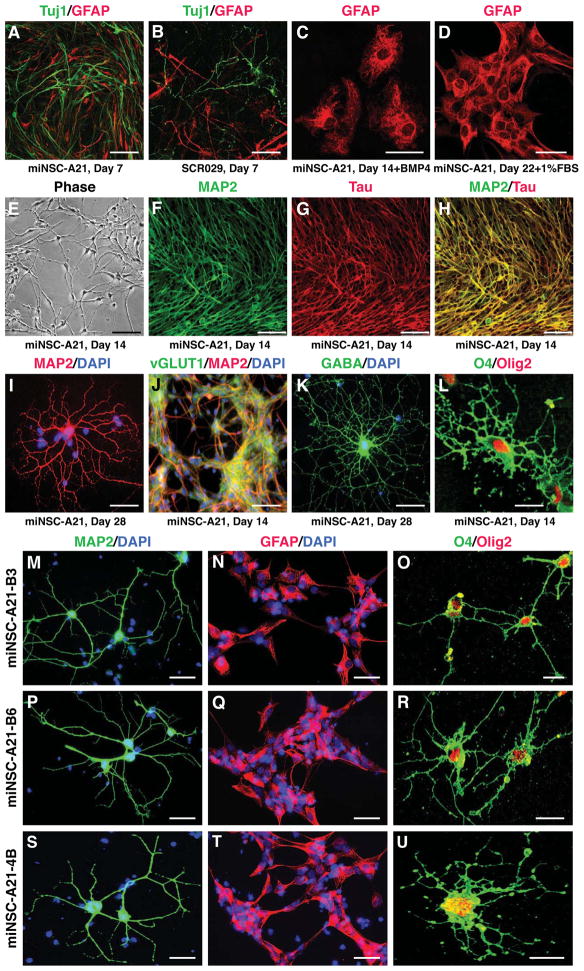
Figure 2. Multipotency of miNSCs in vitro.
(A and B) Like wild-type NSCs, miNSC-A21 can differentiate into Tuj1+ neurons and GFAP+ astrocytes by 7 days in culture after growth factor withdrawal.
(C and D) miNSC-A21 can robustly generate GFAP+ astrocytes by 14 days in vitro in the presence of BMP4 or FBS.
(E) miNSC-A21 can generate mature looking neurons and neuronal networks by 14 days in culture without growth factors.
(F–H) Neurons derived from miNSC-A21 stain positive for the mature neuronal markers MAP2 and Tau.
(I) miNSC-A21 can differentiate into mature arborized neurons by 28 days in vitro.
(J and K) miNSC-A21 can differentiate into subtypes of neurons, including excitatory vGluT1+ neurons (J) and inhibitory GABA+ neurons (K).
(L) miNSC-A21 can generate O4+ and Olig2+ oligodendrocytes by 14 days in culture.
(M–U) After 14 days in culture, subcloned lines B3 (M–O), B6 (P–R), and 4B (S–U) of miNSC-A21 can differentiate into MAP2+ mature neurons (M, P, S), GFAP+ astrocytes (N, Q, T), and O4+/Olig2+ oligodendrocytes (O, R, U).
Scale bars = 50 μm in A, B, E, and J; scale bars = 25 μm in C and D; scale bars = 75 μm in F–H; scale bars = 10 μm in I, K, L, and M–U.
See also Figure S3.
Immunostaining revealed robust GFAP-positive astrocytes derived from both miNSCs and wild-type NSCs cultured for 7–22 days under various differentiation conditions (Figures 2C, 2D, and S3F). Importantly, the ability of miNSCs to generate neurons and astrocytes was confirmed in different batches of miNSCs (Figures S3G–S3L). Furthermore, miNSCs also developed into O4- and Olig2-positive oligodendrocytes (Figure 2L). Thus, miNSCs are multipotent, being able to differentiate into neurons, astrocytes, and oligodendrocytes.
To further confirm the multipotency of miNSCs, we subcloned miNSC-A21 line at passage 13 when we observed stable NSC gene expression and neuronal differentiation, and tested the multipotency of each clone. All five clones tested could differentiate into MAP2-positive neurons, GFAP-positive astrocytes, and O4/Olig2 double-positive oligodendrocytes (Figures 2M–2U for three clones). Thus, miNSCs are a population of truly multipotent NSCs and are not a heterogenous population of different neural progenitor cells.
Lastly, we determined the efficiency of neuronal and glial differentiation in vitro at 14 days for two miNSC clones (A21-B8 and A21-C1). We found a similar yield in neurons (MAP2-positive cells normalized to DAPI-positive nuclei) between the two miNSC-A21 clones (Clone B8: 67 ± 5%; Clone C1: 59 ± 6%) and wild-type brain-derived NSCs (76 ± 6%). However, we saw a higher percentage in astrocytes (GFAP-positive cells normalized to DAPI-positive nuclei) generated from miNSC-A21 (Clone B8: 25 ± 2%; Clone C1: 18 ± 2%) compared to wild-type brain-derived NSCs (6 ± 4%).
Functional neurons derived from miNSCs
Neurons derived from miNSCs, under a condition conducive to primary neuron culture, expressed Synapsin with punctate distribution, suggesting synaptic formation in vitro (Figures 3A and 3B). Whole-cell patch-clamp recordings (Figure 3C) revealed that miNSC-derived neurons had hyperpolarized resting membrane potentials (−40 to −80 mV) (Figure 3D) and membrane resistance properties (Figure 3E). Action potentials could be elicited by depolarizing the membrane in current-clamp mode (Figure 3F). Furthermore, in voltage-clamp mode, both fast inactivating inward and outward currents, which correspond to opening of voltage-dependent Na+ and K+ channels, respectively, were recorded from miNSC-derived neurons (Figure 3G). Thus, miNSC-derived neurons appear to exhibit the functional membrane properties and activities of normal neurons.
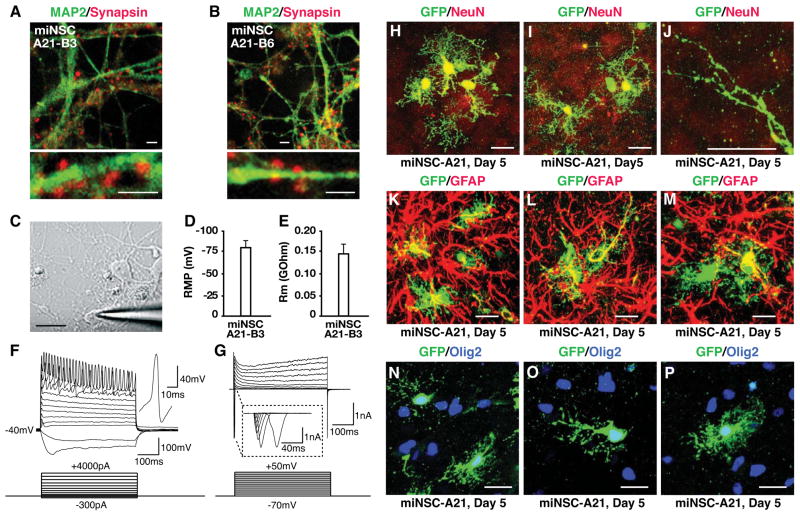
Figure 3. miNSC-derived functional neurons in vitro and multipotency of miNSCs in vivo.
(A and B) Neurons derived from subclones miNSC-A21-B3 or miNSC-A21-B6 at 14 days in culture express MAP2 (green) and Synapsin (red), a presynaptic marker of mature neurons.
(C) A patched neuron derived from miNSC-A21-B3 at 17 days in culture.
(D and E) Whole-cell capacitance and membrane resistance of neurons derived from miNSC-A21 were determined from a transient 5-mV hyperpolarizing step from a holding potential of −70 mV.
(F) Current-clamp recordings of neurons derived from miNSC-A21 at −40mV reveal action potentials with stepwise current injection.
(G) Voltage-clamp recordings of neurons derived from miNSC-A21 reveal both fast inactivating inward and outward currents indicating functional voltage-dependent Na+ and K+ channels.
(H–P) GFP-labeled miNSC-A21 were grown in suspension cultures for one day to generate small neurospheres and then microinjected into the cortex of P2–3 wild-type pups. Five days after transplantation, mouse brains were collected, fixed, sectioned, and immunostained.
(H–J) Immunostainings reveal that miNSC-A21 can differentiate into NeuN+ neurons (H and I) with mature looking dendritic spines (J) in vivo.
(K–M) miNSC-A21 can also differentiate into GFAP+ astrocytes in vivo.
(N–P) miNSC-A21 can also differentiate into Olig2+ oligodendrocytes in vivo.
Scale bars = 2 μm in A and B; scale bar = 10 μm in C; scale bars = 10 μm in H–P.
See also Table S3.
miNSCs can survive, integrate, and differentiate in vivo and do not generate tumors
We microinjected GFP-labeled miNSC neurospheres into the cortex of P2–3 wild-type pups. Immunostaining revealed that miNSCs survived and differentiated into NeuN-positive neurons with mature-looking dendritic spines (Figures 3H–3J), GFAP-positive astrocytes (Figures 3K–3M), and Olig2-positive oligodendrocytes (Figures 3N–3P) 5 days post transplantation. Thus, miNSCs are capable of differentiating into neurons, astrocytes, and oligodendrocytes in vivo.
Since transplantation of iPS cell-derived neurospheres into mouse brains often results in teratoma formation, we also assessed the ability of miNSCs to generate tumors or teratomas in vivo (Yamanaka, 2009). Transplantation of miNSCs or wild-type brain-derived NSCs into mouse brains did not generate tumors; however, teratomas formed in over 60% of mice transplanted with mouse iPS cell-derived NSCs (Table S3). The observation that miNSCs did not form tumors in vivo in 28 separate hippocampal injections involving three different miNSC-A21 subclones suggests that miNSCs have little or no tumorigenic potential.
Generation and characterization of iNSCs from human fetal fibroblasts
We generated human iNSCs (hiNSCs) from human fetal foreskin fibroblasts (HFFs) using a similar protocol (Figure S1A), in which mouse Sox2 was replaced with human SOX2 and reprogrammed cells were cultured in human NSC (hNSC) culture medium supplemented with human EGF and FGF2. During reprogramming, the morphological changes in HFFs were similar to those in mouse fibroblasts (compare Figures S4A–S4G to Figures 1 and S1). Immunostaining revealed that, within 5 days after SOX2 retroviral transduction, hiNSC colonies were positive for SOX2 and NESTIN (Figure S4E). After three or four rounds of neurosphere culture, hiNSCs had morphology different from the original HFFs (Figures S4F and S4A) and further passaging resulted in morphology similar to that of wild-type NSCs derived from human iPS cells (Figures S4G and S4H). hiNSCs did not express pluripotency-related genes as determined by qRT-PCR (Figure S4I) and had neurosphere-forming ability similar to that of NSCs derived from human iPS cells (Figure S4J).
Multipotency of hiNSCs in culture
At 2–4 weeks in culture under conditions that favor neuronal differentiation (hNSC medium without growth factors in the presence of WNT5A (100 ng/ml) or retinoic acid (1 μM) plus forskolin (5 μM)), hiNSCs differentiated into immature neurons (TUJ1-positive) and mature neurons (MAP2-positive) (Figures 4A–4D). At 4 weeks, hiNSCs developed into MAP2-positive neurons with extensive and complex neurites (Figure 4E). Importantly, MAP2-positive neurons could be generated from hiNSCs at various passages from P8 to P22, suggesting stable neurogenic capacity of hiNSCs. Immunostaining revealed GFAP-positive astrocytes derived from two separate hiNSC lines cultured for 14 days in the presence of 50 ng/ml BMP4 (Figures 4F and 4G). Furthermore, hiNSCs also developed into O4- and OLIG2-positive oligodendrocytes (Figure 4H). Importantly, hiNSCs did not generate tumors upon transplantation into mouse brains (Table S3). Thus, hiNSCs are multipotent, being able to differentiate into neurons, astrocytes, and oligodendrocytes, and may not harbor any tumorigenic potential in vivo.
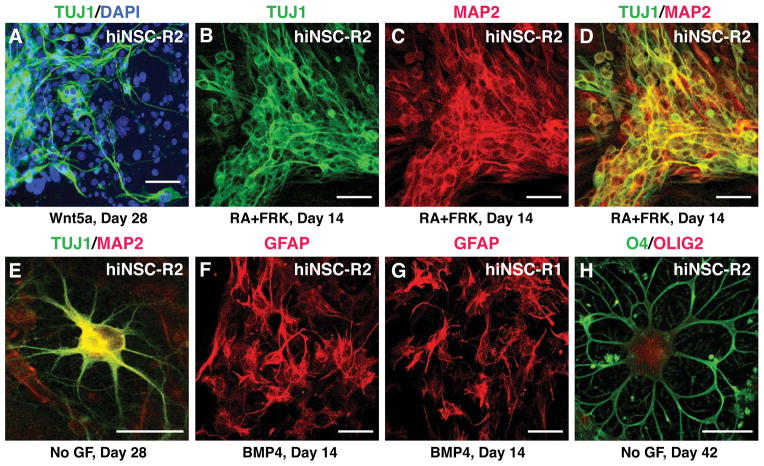
Figure 4. Multipotency of hiNSCs in vitro.
(A) hiNSCs can differentiate into TUJ1+ immature neurons in the presence of the signaling protein WNT5A by 28 days in culture.
(B) The addition of retinoic acid (RA) and forskolin (FRK) to neuronal differentiation conditions pushed hiNSCs to differentiate into TUJ1+ neurons by 14 days in vitro.
(C and D) hiNSCs can generate TUJ1+/MAP2+ neurons by 14 days in the presence of RA and FRK.
(E) hiNSC can generate mature looking neurons that are MAP2+ by 28 days in vitro in hNSC medium without growth factors.
(F) hiNSCs can also generate GFAP+ astrocytes in the presence of BMP4 by 14 days.
(G) A separate hiNSC line can also robustly generate GFAP+ astrocytes at 14 days in vitro.
(H) hiNSCs can generate O4+/OLIG2+ oligodendrocytes by 40 days in culture in hNSC medium lacking growth factors.
Scale bars = 20 μm in A–D; scale bars = 10 μm in E–H.
See also Figure S4.
DISCUSSION
The ability to reprogram somatic cells into self-renewable iNSCs has major implications for regenerative medicine. iNSCs can serve as a model system for unveiling disease pathogenesis, for drug screening and toxicity tests, and ultimately for cell transplantation therapies. Many studies have focused on generating NSCs from pluripotent sources such as ES cells or iPS cells (Hochedlinger and Plath, 2009; Yamanaka, 2009). However, these methods are plagued by ethical and practical issues, such as the origin of ES cells and the tendency for teratoma formation of cells derived from iPS cells (Fong et al., 2010; Miura et al., 2009; Yamanaka, 2009). Interestingly, transplantation of Sox2-reprogrammed iNSCs into mouse brains does not generate tumors, making iNSCs more attractive than NSCs derived from iPS cells. iN cells can be generated from fibroblasts and other somatic cell sources (Ambasudhan et al., 2011; Caiazzo et al., 2011; Kim et al., 2011b; Marro et al., 2011; Pang et al., 2011; Qiang et al., 2011; Son et al., 2011; Vierbuchen et al., 2010; Yoo et al., 2011), but iN cells are terminally differentiated and restricted to the subtypes of neurons they can generate. Having a patient-derived population of multipotent iNSCs would bypass some of the disadvantages of pluripotent and terminally differentiated cell populations. Thus, direct reprogramming of somatic cells into self-renewable and multipotent iNSCs should not only complement the iPS cell and iN technologies but also sidestep their shortcomings.
It has recently been reported that the four Yamanaka reprogramming factors in combination with NSC-permissive culture conditions can reprogram fibroblasts to induced neural progenitors (iNPCs) that can generate multiple neuronal cell types as well as astrocytes (Kim et al., 2011a). However, these iNPCs can only self-renew for 3–5 passages in culture and have not been shown to differentiate into oligodendrocytes. In a separate study, a combination of nine factors reprogramed Sertoli cells into iNSCs (Sheng et al., 2011). However, exogenous expression of eight out of the nine factors was not silenced even after multiple passages, raising the question of whether these iNSCs would revert back to their original state without constant overexpression of those factors. It also has been reported that three factors, Brn2, Sox2, and FoxG1, can reprogram mouse fibroblasts to tripotent, self-renewing iNPCs (Lujan et al., 2012). However, when the authors attempted to generate iNPCs with only two factors, they found that Sox2 and FoxG1 generated only bipotent iNPCs and that the combination of FoxG1 and Brn2 generated tripotent iNPCs that were unable to form mature and functional neurons in vitro. Interestingly, the three-factor-reprogrammed iNPCs could generate oligodendrocytes in vivo although it was not tested for generation of neurons or astrocytes. Most recently, two studies have shown that the combinations of Sox2, Klf4 and c-Myc or Brn4, Sox2, Klf4, c-Myc, and E47/Tcf3 can reprogram mouse fibroblasts into iNSCs (Thier et al., 2012; Han et al., 2012). While these studies do show that iNSCs can self-renew, generate functional neurons in vitro, and integrate in vivo, both reprogramming methods require overexpression of the potent c-Myc oncogene, which has been reported to be a cause of brain tumorigenesis from transplanted iPS cell-derived NSCs (Okita et al., 2007).
The studies mentioned above are in line with our findings that mouse fibroblasts can be directly reprogrammed into iNSCs that exhibit typical NSC properties and differentiation abilities in vitro and in vivo. However, our iNSC reprogramming protocol is advantageous because it requires a single factor to generate tripotent iNSCs from both mouse and human fibroblasts. The miNSCs described here can be passaged more than 40 times, can generate functional neurons with synaptic connections in vitro, and can survive, integrate, and are multipotent in vivo without tumor formation. Furthermore, our results uniquely show that our miNSCs are a homogeneous tripotent population, rather than a heterogenous population of different neural progenitor cells. Additionally, retroviral expression of Sox2 in miNSCs is silenced at later passage, suggesting that Sox2-reprogrammed miNSCs have turned on endogenous expression of NSC genes and can maintain a stable cell fate. Finally, our SOX2 reprogramming protocol can reprogram human fibroblasts into hiNSCs that express the typical NSC markers, can self-renew over 20 passages, generate neurospheres comparable to NSCs derived from human iPS cells, and are tripotent in vitro.
Sox2 functions as a master regulator gene for NSC identity and maintenance, as knocking down Sox2 expression leads to immediate cell cycle exit and terminal differentiation of NSCs (Bylund et al., 2003; Graham et al., 2003). Thus, it is conceivable that under conditions conducive to NSC expansion, including the presence of growth factors and proper surface and substrates, overexpression of Sox2 can reprogram fibroblasts to multipotent NSCs. If one factor can generate a multipotent population of NSCs from somatic cells, then certain combinations of more lineage-defined factors may generate subtype-specific NSCs, such as motor neuron, dopaminergic neuron, oligodendrocyte, or astrocyte progenitors. Overexpression of specific transcription factors such as Lmx1a in combination with extrinsic factors can bias NSCs toward differentiation into dopaminergic neurons that constitute 75–90% of the total neuronal cell population (Panman et al., 2011). Thus, Sox2 might be used in combination with such factors to create neural progenitors that can develop into subtype-specific neurons, which would be invaluable for mechanistic studies, drug screening, and potential cell therapies for different neurodegenerative diseases.
EXPERIMENTAL PROCEDURES
Generation of iNSCs from mouse and human fibroblasts
MEFs were isolated from wild-type E18 embryos, and 7.5×103 to 4×105 cells were transduced at passages 1–3 with pMX-Sox2 retrovirus to induce miNSC reprogramming. Sox2 transformed MEFs were cultured in NSC medium supplemented with EGF and FGF2, and NSC-like cells were selected during three rounds of neurosphere suspensions. NSC-like cells were further enriched by monolayer passaging and then characterized for their in vitro and in vivo properties. 7.5×103 HFFs at passages 5–8 were transduced with human SOX2 retrovirus to generate hiNSCs using a similar reprogramming procedure. Detailed methods, including iNSC reprogramming protocol, in vitro characterization and differentiation, immunostaining, qRT-PCR, microarray analysis, bisulfite sequencing, electrophysiology, transplantation, and tumorigenesis studies, can be found in Supplemental Information.
Supplementary Material
HIGHLIGHTS.
iNSCs are generated from mouse and human fibroblasts with a single factor.
iNSCs express NSC markers and are able to self-renew in culture.
iNSCs differentiate into astrocytes, oligodendrocytes, and functional neurons.
Implanted iNSCs can survive and integrate and do not form tumors in mouse brains.
Acknowledgments
This work was supported in part by the J. David Gladstone Institutes, grant RN2-00952 from the California Institute for Regenerative Medicine, grant P01 AG022074 from the National Institutes of Health, a graduate research fellowship from National Science Foundation, and a gift from the S.D. Bechtel, Jr. Foundation. We thank Drs. Li Gan, Eric Huang, Deepak Srivastava, and Sheng Ding for critical discussions. We also thank Stephen Ordway and Gary Howard for editorial assistance and Linda Turney for manuscript preparation.
Footnotes
ACCESSION NUMBER
The accession number for the microarray data reported here is GSE37859.
Supplemental Information includes Supplemental Experimental Procedures, four figures, and four tables and can be found with this article online at __.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong CY, Gauthaman K, Bongso A. Teratomas from pluripotent stem cells: A clinical hurdle. J Cell Biochem. 2010;111:769–781. doi: 10.1002/jcb.22775. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Han DW, Do JT, Araúzo-Bravo MJ, Lee SH, Meissner A, Lee HT, Jaenisch R, Schöler HR. Epigenetic hierarchy governing Nestin expression. Stem Cells. 2009;27:1088–1097. doi: 10.1002/stem.43. [DOI] [PubMed] [Google Scholar]
- Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Soldner F, Cook EG, Gao Q, Mitalipova M, Jaenisch R. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 2008;3:346–353. doi: 10.1016/j.stem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura M, Miura K, Iwabuchi K, Ichisaka T, Nakagawa M, Lee J, Kanatsu-Shinohara M, Shinohara T, Yamanaka S. Transcriptional repression and DNA hypermethylation of a small set of ES cell marker genes in male germline stem cells. BMC Dev Biol. 2006;6:34. doi: 10.1186/1471-213X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Liu L, Bian W, Chen Y, Xu G, Cheng L, Jing N. Different transcription factors regulate nestin gene expression during P19 cell neural differentiation and central nervous system development. J Biol Chem. 2009;284:8160–8173. doi: 10.1074/jbc.M805632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Kobayashi T. Roles of Hes genes in neural development. Dev Growth Differ. 2008;50:S97–S103. doi: 10.1111/j.1440-169X.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci USA. 2011a;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Su SC, Wang H, Cheng AW, Cassady JP, Lodato MA, Lengner CJ, Chung CY, Dawlaty MM, Tsai LH, et al. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011b;9:413–419. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Lee G, Kim H, Elkabetz Y, Shamy AIG, Panagiotakos G, Barberi T, Tabar V, Studor L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nature Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Südhof TC, Wernig M. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9:374–382. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panman L, Andersson E, Alekseenko Z, Hedlund E, Kee N, Mong J, Uhde CW, Deng Q, Sandberg R, Stanton LW, et al. Transcription factor-induced lineage selection of stem-cell-derived neural progenitor cells. Cell Stem Cell. 2011;8:663–675. doi: 10.1016/j.stem.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008a;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008b;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, Vanti WB, et al. Directed conversion of Alzheimer’s disease patients skin fibroblasts into functional neurons. Cell. 2011;146:359–371. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- Sheng C, Zheng Q, Wu J, Xu Z, Wang L, Li W, Zhang H, Zhao XY, Liu L, Wang Z, et al. Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res. 2012;22:208–218. doi: 10.1038/cr.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Desponts C, Do JT, Hahm HS, Schöler HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lie DC, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- Soldner F, Laganière J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe LI, Myers RH, Lindquist S, et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146:318–331. doi: 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thier M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nöthen MM, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western PS, van den Bergen JA, Miles DC, Sinclair AH. Male fetal germ cell differentiation involves complex repression of the regulatory network controlling pluripotency. FASEB J. 2010;24:3026–3035. doi: 10.1096/fj.09-151555. [DOI] [PubMed] [Google Scholar]
- Williams DC, Jr, Cai M, Clore GM. Molecular basis for synergistic transcriptional activation by Oct1 and Sox2 revealed from the solution structure of the 42-kDa Oct1.Sox2.Hoxb1-DNA ternary transcription factor complex. J Biol Chem. 2004;279:1449–1457. doi: 10.1074/jbc.M309790200. [DOI] [PubMed] [Google Scholar]
- Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, Cowling R, Wang W, Liu P, Gertsenstein M, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yusa K, Rashid ST, Strick-Marchand H, Varela I, Liu PQ, Paschon DE, Miranda E, Ordóñez A, Hannan NRF, Rouhani FJ, et al. Targeted gene correction of α1-antitrypsin deficiency in induced pluripotent stem cells. Nature. 2011;478:391–394. doi: 10.1038/nature10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.