Sudden cardiac death (SCD) generally refers to an unexpected death from a cardiovascular cause in a person with or without preexisting heart disease. The specificity of this definition varies depending on whether the event was witnessed; however, most studies include cases that are associated with a witnessed collapse, death occurring within one hour of an acute change in clinical status or an unexpected death that occurred within the previous 24 hours.1-3 Further, sudden cardiac arrest (SCA) describes SCD cases with resuscitation records or aborted SCD cases in which the individual survived the cardiac arrest.
The incidence of SCD in the United States ranges between 180,000 to 450,000 cases annually.4 These estimates vary due to differences in SCD definitions and surveillance methods for case ascertainment.4, 5 In recent prospective studies utilizing multiple sources in the United States,6, 7 Netherlands,8 Ireland,9 and China,10 SCD rates range from 50-100 per 100,000 in the general population.3 Despite the need for multiple sources of surveillance to provide a more accurate estimate of SCD incidence, it is clear that the overall burden in the population remains high. Although improvements in primary and secondary prevention have resulted in substantial declines in overall coronary heart disease (CHD) mortality over the past 30 years,11, 12 SCD rates specifically have declined to a lesser extent.13-16 SCD still accounts for over 50% of all CHD deaths and 15-20% of all deaths.17, 18 For some segments of the population, rates are not decreasing19 and may actually be increasing.14, 19 As a result, SCD prevention represents a major opportunity to further reduce mortality from CHD.
Despite major advances in cardiopulmonary resuscitation20 and post-resuscitation care, survival to hospital discharge after cardiac arrest in major metropolitan centers remains poor.21 Survival to hospital discharge was recently estimated to be only 7.9% among out of hospital cardiac arrests that were treated by emergency medical services (EMS) personnel.6 In addition, the majority of SCDs occur at home, often where the event is unwitnessed.8, 22 As a result, automated external defibrillators, which improve resuscitation rates for witnessed arrests,21 may have limited effectiveness on reducing overall mortality from SCD. Therefore, substantial reductions in SCD incidence will require effective primary preventive interventions. Since the majority of SCDs occur in the general population, an in depth understanding of the epidemiology of SCD may lead to possible low risk interventions that could be applied broadly to populations. Also, recent data emerging related to the genetics of SCD may eventually aid in the identification of high risk subsets within the general population or provide new molecular targets for intervention.
Demographics: Age, Sex, and Race
The incidence of SCD increases markedly with age regardless of sex or race (Figure 1). For example, the annual incidence for 50 year-old men is about 100 per 100,000 population compared to 800 per 100,000 for 75 year-old men.23 Although SCD increases with age, the proportion of deaths that are sudden is larger in the younger age groups2, 24, 25 where the socioeconomic impact of SCD is greater. At any age,26 women have a lower incidence of SCD than men, even after adjustment for CHD risk factors.27 This discrepancy may be decreasing over time.7, 16 The decline in SCD rates among women has been less than that observed for men, particularly in the younger age groups.14 This may be due, in part, to a lower overall burden of CHD in women with SCD. Approximately two-thirds of women who present with SCD have no known history of heart disease, as compared to 50% of men.8, 24, 28 In addition, among cardiac arrest survivors29 and SCD victims,30 women appear to have a higher prevalence of structurally normal hearts (Figure 2).
Figure 1.
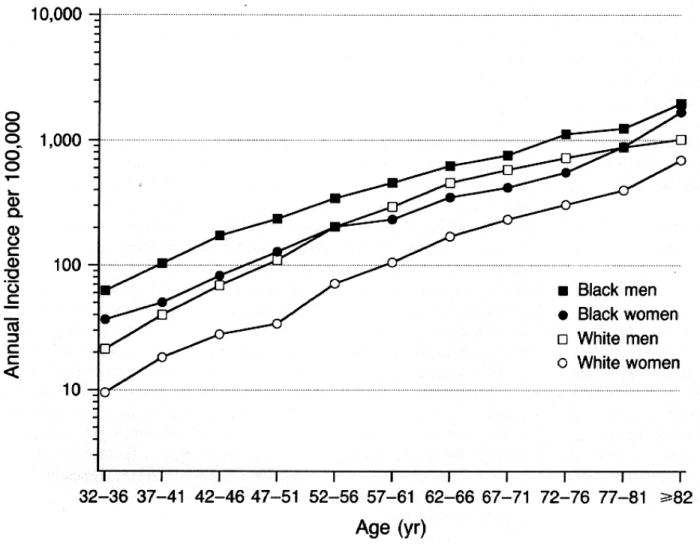
Incidence of sudden cardiac arrest according to age, sex, and race in the Chicago CPR project. The study population was comprised of 6,451 patients including 3,207 whites and 2,910 blacks.23
Figure 2.
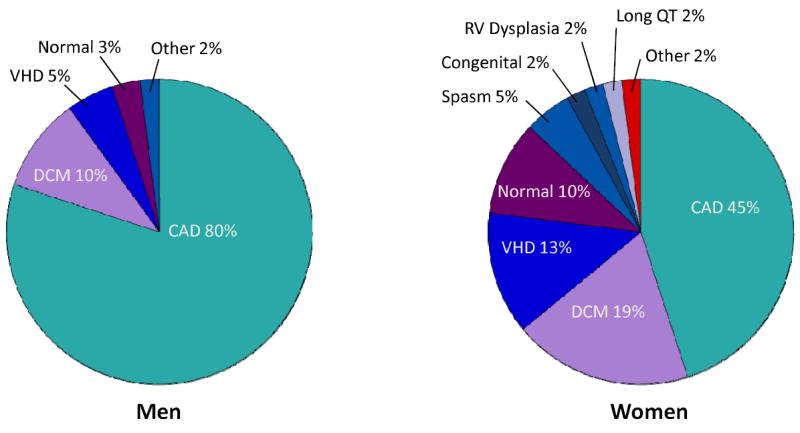
Structural Heart Disease in Cardiac Arrest Survivors. These pie charts depict the proportions of underlying cardiac disease among men and women who survive out-of-hospital cardiac arrests. The mean age was 58±12 years for men and 55±17 years for women. Coronary artery disease comprised the principal diagnosis in the majority of men. In contrast, women had more nonischemic heart disease compared to men including dilated cardiomyopathy (19%) and valvular heart disease (13%).29 CAD indicates coronary artery disease; DCM, dilated cardiomyopathy; VHD, valvular heart disease; SPASM, coronary vasospasm; and RV, right ventricular.
There are also racial differences in the incidence of SCD that are not well understood. African American men and women appear to experience out-of-hospital cardiac arrest several years earlier than Caucasians. In two American cities, blacks had higher rates (relative risk = 1.3 to 2.8) of cardiac arrest than whites (Figure 3).23, 31 Data from death certificates also suggest that SCD is more common among black Americans than other ethnicities, and Hispanic Americans may have lower SCD rates than non-Hispanic populations.14, 32 In addition, survival rates after cardiac arrest are lower for African Americans.23, 33 In Chicago, the overall survival rate after an out-of-hospital cardiac arrest among blacks was only 31 percent of that among whites.23 African Americans are more likely to have an unwitnessed arrest with an unfavorable rhythm such as PEA documented at the time of the arrest.23, 34 However, the disparity in survival does not appear to be entirely due to the initial rhythm at time of arrest. Even when limited to cardiac arrests due to VF or pulseless VT, rates of survival to hospital discharge are 27% lower among black patients.35 In the National Registry of Cardiopulmonary Resuscitation, much, but not all, of this disparity appeared to be explained by black patients receiving treatment at hospitals with worse outcomes.35 As in all studies of racial differences, it is difficult to separate socioeconomic influences from a true genetic predisposition.
Figure 3.
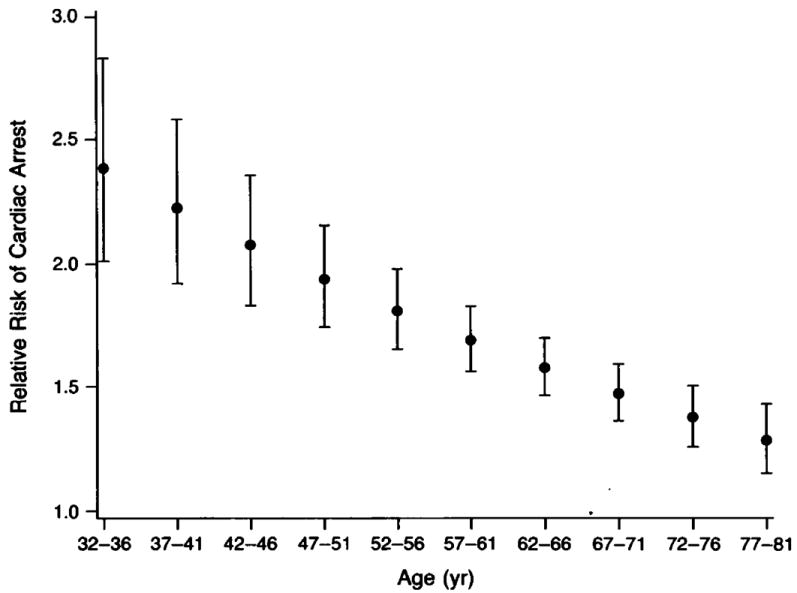
Relative risk of cardiac arrest in blacks compared to whites by age group. The bars represent 95% confidence intervals.23
Underlying Pathophysiology
The pathophysiology of SCD is complex and is believed to require the interaction between a transient event and underlying substrate. This process induces electrical instability and lethal ventricular arrhythmias followed by hemodynamic collapse. Although the challenge remains to predict when such interactions prove harmful, a variety of risk factors have been proposed (Figure 4).
Figure 4.
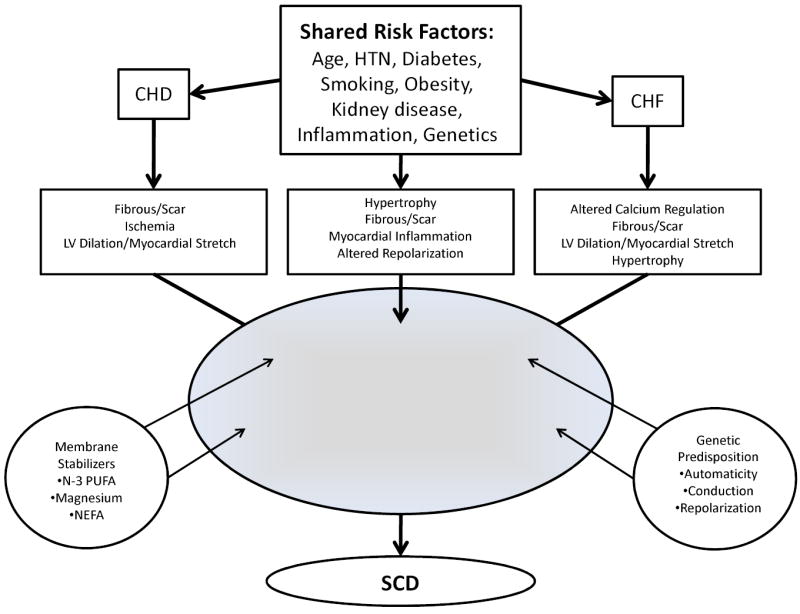
Critical Pathways Leading to Electrical Instability and Sudden Cardiac Death. HTN indicates hypertension; CHD, coronary heart disease; CHF, congestive heart failure; LV, left ventricular; PUFA, polyunsaturated fatty acids; NEFA, non-esterified fatty acids; and SCD, sudden cardiac death.
CHD is the most common substrate underlying SCD in the western world, being responsible for approximately 75% of SCDs.8, 18, 36, 37 Cardiomyopathies (dilated, hypertrophic, and arrhythmogenic right ventricular cardiomyopathy) and primary electrical disorders related to channelopathies account for most of the remainder.18 In approximately 5% of SCDs or cardiac arrests, a significant cardiac abnormality is not found after extensive evaluation or at autopsy.29, 38, 39 CHD predisposes to SCD in three general settings: (1) acute myocardial infarction, (2) ischemia without infarction and (3) structural alterations such as scar formation or ventricular dilatation secondary to prior infarction or chronic ischemia. In those who die suddenly from CHD, 19-27%40, 41 have pathologic evidence for myocardial necrosis, and only 38% of cardiac arrest survivors will develop enzymatic evidence of myocardial infarction.42 In autopsy studies, stable plaques and chronic changes alone are found in approximately 50% of SCD victims with CHD41, 43, 44 suggesting that plaque rupture and acute MI is present in some, but not the majority, of SCD cases.
Presumably, the mechanism of SCD in cases without acute MI is an electrical event due to a ventricular arrhythmia triggered by ischemia or other arrhythmogenic stimuli in the setting of a chronically diseased heart.45 This hypothesis is difficult to prove as most deaths are not monitored, and those that are comprise a highly selected population. Ventricular fibrillation degenerates to asystole over the course of several minutes; as a result, the majority of SCD victims demonstrate asystole or pulseless electrical activity (PEA) when first examined by rescue teams.34 In cases where there has been a relatively short delay between collapse and the initial determination of rhythm, the proportion with documented ventricular tachyarrhythmias increases to 75-80% (Figure 5). 42, 46-49 Studies in epidemiologic cohorts of men50 and women24 from the 1970s to 1990s suggest that 88 to 91 percent of deaths that occur within one hour of symptom onset are arrhythmic in nature. However, the proportion of SCD deaths due to VF may be decreasing over time. VF is less often encountered as the initial rhythm in recent EMS series,19 and the decline does not appear to be entirely accounted for by changing resuscitation patterns or patient characteristics.51
Figure 5.
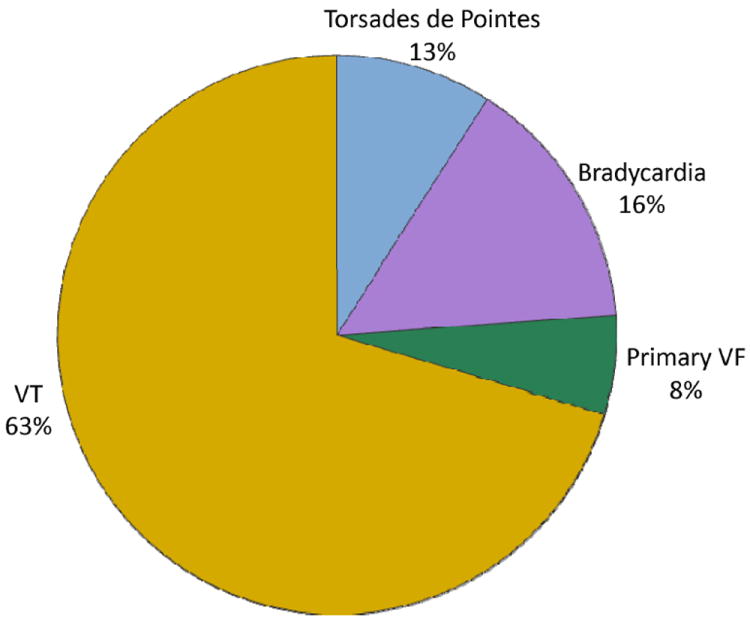
Underlying arrhythmias of sudden cardiac arrest.46 VT indicates ventricular tachycardia; and VF, ventricular fibrillation.
Risk Factors
Structural Heart Disease
Coronary heart disease or congestive heart failure markedly increases SCD risk in the population.52 In the Framingham Study, pre-existing CHD was associated with a 2.8 to 5.3 fold increase in risk of SCD, and CHF was associated with a 2.6 to 6.2 fold increased risk.27 After experiencing an MI, women and men have a 4 to10-fold higher risk of SCD respectively.24, 28 The absolute rate is highest in the first 30 days after MI and decreases gradually with time.53, 54 The incidence of SCD after MI has declined in parallel with CHD mortality over time,54 and rates as low as 1% per year in patients receiving optimal medical therapy and revascularization have been documented.55, 56 However, rates are still high in certain subsets of post-MI patients with SCD.53 Both left ventricular dysfunction and NYHA class are powerful risk factors for SCD in patients with either ischemic or non-ischemic cardiomyopathy,57 and implantable cardioverter defibrillators (ICDs) prolong life in these high-risk patients.58, 59 Other markers of structural heart disease associated with elevated SCD risk include left ventricular hypertrophy,60, 61 QTc prolongation,62 and abnormal heart rate profile during exercise.63 At the present time, none of these markers have been incorporated into risk stratification algorithms.
Although overt structural heart disease markedly increases SCD risk, most patients who suffer a cardiac arrest will not have an LVEF less than 35% documented prior to SCD.2, 18,30, 64 This finding presents a major challenge when designing SCD preventive strategies since those most at risk by current criteria comprise a small percentage of the total number of SCDs in the population. One recent study among post-menopausal women with overt CHD and relatively preserved systolic function raised the possibility that a combination of easily accessible clinical and epidemiologic risk factors might be able to better reclassify SCD risk into clinically meaningful risk categories as compared to LVEF alone.65 However, as is the case for LVEF and most other clinical predictors, high risk patients identified by this approach were also at a similarly high risk for competing forms of cardiovascular death.53, 66 The high risk for competing causes of death limits the effectiveness of therapies such as the ICD that are specifically targeted toward SCD prevention. In addition, SCD is often the first manifestation of cardiovascular disease, and risk stratification in high risk patients will not address the majority of SCDs that occur in the population. Therefore, a more thorough understanding regarding risk factors for SCD in the general population is also needed.
CHD Risk Factors
Since approximately 80% of men who suffer SCD have underlying CHD, it follows that the standard CHD risk factors are predictive of SCD in the general population. Modifiable CHD risk factors that have been demonstrated to predict SCD in diverse cohorts include hypertension, hypercholesterolemia, diabetes,67-69 kidney dysfunction,70, 71 obesity, and smoking.27,24, 72, 73 Although the prevalence of CHD among female SCD victims may be lower than their male counterparts,29, 30 conventional CHD risk factors still appear to predict SCD in women.24, 28, 65 Smoking, in particular, is an important risk factor for SCD with risk elevations in the general population similar to that conferred by MI.24, 43, 44 Continued smoking increases the risk of recurrent cardiac arrest,74 and smoking cessation is associated with a prompt reduction in SCD risk.26, 75, 76 Diabetes and hypertension are also strong risk factors for SCD,67-69 and recent evidence has highlighted the potential importance of diabetes as a potential risk stratifier for SCD even in high risk populations.77 Serum cholesterol appears to be more strongly related to SCD at younger ages.24, 28
All of the risk factors discussed above predict CHD in general and are not specific for SCD, and with the exception of diabetes,65, 77 kidney disease,65, 71 and smoking,75 do not appear to predict SCD risk once overt CHD has been established.52 However, modification of traditional CHD risk factors will impact on SCD incidence at the population level. Reduced incidence rates of all manifestations of CHD including SCD since the mid-1960s provide indirect evidence of the success of CHD risk factor modification.
Electrocardiographic Measures of Risk
Standard 12-lead electrocardiographic measures including heart rate, QRS duration, QT interval and early repolarization have been assessed as risk factors for SCD. Population based studies have demonstrated that an elevated resting heart rate78 and prolonged QT interval increase SCD risk in the general population.79, 80 Similarly, a prolonged QRS duration has also been associated with SCD.81, 82 Recent interest has focused on early repolarization (ER) as a novel risk factor for SCD and cardiovascular death. ER is defined as an elevation of the junction between the end of the QRS complex and the beginning of the ST segment (J point), and its presence in the inferior or lateral ECG leads has been associated with a history of SCA and idiopathic VF in case-control studies.83-85 In a population based study from Finland, ER patterns associated with > 0.2mV elevations in the inferior leads were associated with marked elevations in the risk of death from cardiac causes or from arrhythmia.86 In a follow-up analysis from this same cohort, ER was associated with arrhythmic death only when horizontal or descending ST segments were present.85 Individuals with ER and rapidly ascending/upsloping ST segment were not at elevated risk.
Nutritional Risk Factors
Dietary intake and blood-based measures of selected nutrients have been specifically associated with SCD in observational studies (Table 1).87-102 Several epidemiologic studies suggest that increased consumption of n-3 polyunsaturated fatty acids (PUFAs) is inversely associated with SCD to a greater extent than non-fatal MI.103-107 In 4 observational studies, consuming fish approximately 1-2 times per week was associated with 42-50% reductions in SCD risk.103-106 Alpha-linolenic acid (ALA), which is an intermediate chain n-3 PUFA found in foods of plant origin, has also been associated with a reduced risk of SCD in one observational study of women.107 These data from relatively healthy observational cohorts support experimental data demonstrating a protective effect of these nutrients on arrhythmia susceptibility.108 Data from randomized clinical trials, however, have not consistently supported this hypothesis. The GISSI- Prevenzione trial, which tested supplementation with n-3 PUFAs (combination of 850 mg eicosapentanoic acid and docosahexanoic acid daily) in an open-label fashion among 11,324 patients with recent MI, found a significant 45% reduction in SCD without any benefit on non-fatal MI or stroke.109 More recently, however, two randomized, blinded trials of n-3 PUFAs performed in post-MI populations were unable to confirm these benefits on SCD.110, 111 The SCD event rates in both of these post-MI populations were much lower than expected and the studies were likely underpowered. As a result, it will be challenging to test whether interventions reduce SCD rates in lower risk populations.
Table 1.
Biological Markers and Sudden Cardiac Death in Prospective Studies
| Biomarker | Mechanism | Study | Findings |
|---|---|---|---|
| Dietary Markers | |||
| Long chain n-3 fatty acids | Ionic channel stabilization, inflammation | Physicians’ Health Study87 (n=278) | Baseline level of long-chain n-3 fatty acids were inversely related to the risk of SCD. |
| Magnesium | Repolarization, membrane stabilization | Nurses’ Health Study88 (n=88,735) | Higher plasma concentrations and dietary magnesium intake were associated with lower risks of SCD. |
| ARIC study89 (n=14,232) | Participants in the highest quartile of serum Mg were at a significantly lower risk of SCD compared to those in the lowest one. | ||
| Nonesterified fatty acids | Membrane stabilization | Paris Prospective Study90 (n=5250 men) | Fasting plasma NEFA measurements at baseline were independently associated with SCD after a 22 year follow-up period. |
| Trans-fatty acids | Inflammation, endothelial dysfunction | Cardiovascular Health Study91 (n=428) | Higher plasma phospholipid trans-18:2 fatty acids were associated with higher risk of SCD. Higher trans-18:1 levels were associated with lower SCD risk. |
|
| |||
| Inflammatory Markers | |||
| CRP, IL6, Fibrinogen | Inflammation, oxidative stress, insulin resistance | PRIME Study92 (n=9771 men) | Baseline concentrations of interleukin 6, but not CRP or fibrinogen, were an independent risk factor for SCD after 10 years of follow-up. |
| CRP | Inflammation, oxidative stress | Nurses’ Health Study93 (n=121,700 women) | Baseline concentrations of CRP were not associated with SCD events after 16 years of follow-up. |
| CRP | Inflammation, oxidative stress, apoptosis | Physicians’ Health Study94 (n=22,071 men) | Baseline CRP levels were associated with an increased risk of SCD over a 17-year follow-up period. |
| ST2 | Interleukin-1 receptor, myocardial fibrosis | MUSIC Registry,95 ambulatory heart failure patients (n=99) | Over a 3 year follow-up period, elevated soluble ST2 concentrations at baseline were independently associated with SCD. |
|
| |||
| Metabolic Markers | |||
| Aldosterone | Myocardial tension, fibrosis, electrical remodeling | STEMI population96 (n=356) | Among patients referred for primary PCI for STEMI, high aldosterone levels at admission were associated with death or resuscitated cardiac arrest during a 6-month follow-up period. |
| Cystatin C | Marker of glomerular filtration rate | Cardiovascular Health Study,70 excluded participants with prevalent cardiac disease (n=4,482) | Over a median follow-up of 11.2 years, elevated cystatin C concentrations at baseline had an independent association with SCD in elderly people without prevalent cardiovascular disease. |
| Renin | Fibrosis and electrical remodeling | LURIC study,97 patients referred for coronary angiography (n=3303) | Baseline plasma renin is associated with long-term cardiovascular mortality including both SCD and death due to heart failure. |
| Vitamin D and Parathyroid Hormone | Fibrosis, Electrical remodeling, metabolic effects | Cardiovascular Health Study, 98 excluded participants with prevalent cardiac disease (n=2,312) | The combination of lower vitamin D and higher PTH concentrations was an independent risk factor for SCD among older adults without cardiovascular disease. |
| Vitamin D | Fibrosis and electrical remodeling | German Diabetes and Dialysis Study99 (n=1108) | Over a median follow-up of 4 years in this dialysis cohort with diabetes, severe vitamin D deficiency was associated with SCD. |
|
| |||
| Neurohormonal Markers | |||
| BNP | Increased myocardial tension | Nurses’ Health Study93 (n=121,700 women) | Increased baseline NT-pro-BNP concentrations were independently associated with SCD events after 16 years of follow-up. |
| NT-pro-BNP | |||
| Cardiovascular Health Study100 (n=5,447) | Elevated baseline NT-pro-BNP levels were associated with SCD after a median 12.5 year follow-up period. | ||
| Vienna Heart Failure cohort (LVEF<35%)101 (n=452) | After 3 years of follow-up, elevated BNP levels at baseline were an independent risk factor for SCD in patients with CHF. | ||
| Multiple Risk Factor Analysis Trial (MRFAT, post-MI population)102 (n=521) | During a 3.5 year follow-up period, elevated baseline BNP levels were associated with SCD after adjustment for clinical risk factors and LVEF. | ||
Alcohol and magnesium intake may also have a selective effect on SCD risk. Heavy alcohol consumption (> 5 drinks/day) is associated with an increased risk of SCD73 but not non-fatal MI.112 In contrast, light-to-moderate levels of alcohol consumption (approximately ½ to 1 drink per day) may be associated with reduced risks of SCD.113-115 Magnesium intake may also be related to SCD rates. In the Nurses’ Health Study, the relative risk of SCD was significantly lower among women in the highest quartile of dietary magnesium intake. In addition each 0.25 mg/dL (1-SD) increment in plasma magnesium was associated with a 41% reduced risk of SCD.88 A similar inverse association between serum magnesium and SCD was also found in the Atherosclerosis Risk in Communities study; however, a single measure of dietary magnesium intake was not associated with SCD risk.89
Finally, there is some evidence that certain dietary patterns, which account for additive and interactive effects of multiple nutrients,116 are associated with lower SCD risk. A Mediterranean-style diet consisting of higher intake of vegetables, fruits, nuts, whole grains, fish, moderate intake of alcohol, and low intake of red/processed meat, has been associated with lower risks of cardiovascular disease in clinical trials117 and observational studies.118 The association appears stronger for fatal as compared to nonfatal events, and may be driven partially through protection against ventricular arrhythmias and SCD.119 Recent data from the Nurses’ Health Study suggest that women whose dietary habits most resemble the Mediterranean dietary pattern have a significantly lower risk of SCD.120
Biological markers
In addition to the nutrient biomarkers described above, multiple epidemiologic investigations have evaluated dysregulation in inflammatory, metabolic and neurohormonal pathways as predisposing factors for SCD (Table 1). Several epidemiologic studies have assessed biomarkers at a time when the majority of participants are free of significant clinical cardiovascular disease. As a result, abnormal concentrations may reflect subclinical changes in cardiovascular processes that eventually predispose individuals to SCD risk. The early stages of hemodynamic stress, atherosclerotic plaque instability and cardiac remodeling may only be detectable with biomarkers that are associated with inflammatory processes, metabolic factors, and neurohormonal regulation. Experimental evidence suggest that these markers regulate pathophysiologic mechanisms implicated in CHD, heart failure and cardiac arrhythmias. Although many of the prospective epidemiologic studies on which these inferences are based have enrolled many participants, they contain only a limited number of SCD events. Future studies will require larger samples of SCD cases with prospectively collected blood samples in order to validate these findings and to determine whether biomarkers have a diagnostic role121 in identifying high risk individuals in the general population.
Triggers
SCD risk in the population is not only a function of the underlying substrate and its vulnerability to arrhythmias but also the frequency of exposure to acute precipitants or triggers (Figure 4). These triggers tend to increase sympathetic activity, which in turn may precipitate arrhythmias and SCD.
Diurnal/Seasonal Variation
Several studies have demonstrated a circadian pattern to the occurrence of SCD and out of hospital cardiac arrest.122 The peak incidence occurs in the morning hours from 6 AM to noon123 with a smaller peak in the late afternoon for out-of-hospital VF arrests.124, 125 This morning peak in SCD is blunted by beta-blockers,126 supporting the concept that excessive activation of the sympathetic nervous system in the morning hours may be responsible. Weekly and seasonal patterns to SCD onset have also been appreciated. The risk of out-of-hospital cardiac arrest127 and SCD128 appears to be highest on Monday with a nadir over the weekend.127 These patterns of onset suggest that activity and psychological exposures play roles in triggering SCD. There have also been reports of seasonal variation in SCD rates with lower rates in the summer and higher rates in winter months in both hemispheres.128, 129 SCD may be associated with endogenous rhythms and environmental factors including temperature,129, 130 sunlight exposure, and other climatic conditions.
Physical Activity
Physical activity has both beneficial and adverse effects on SCD risk. Most studies,65, 73, 131-134 but not all,135, 136 have found inverse associations between increasing regular physical activity and SCD or SCA. Results are most consistent for moderate levels of exertion,65, 73, 132-134 where the majority of studies have documented favorable associations. Despite the long-term benefits of exercise, it is also well known that SCD occurs with a higher than average frequency during or shortly after vigorous exertion.137 Case-control and case-crossover studies performed among men have demonstrated that vigorous exertion can trigger cardiac arrest131 and SCD.136 Regular vigorous exertion diminishes the magnitude of this excess risk; however, the risk remains significantly elevated even in the most habitually active men.138 The magnitude of the risk associated with exertion appears to be lower among women134 where exertion-related SCD is much less common.138 (Figure 6) The effect of exertion on plaque vulnerability139 and the sympathetic nervous system could account for both the transiently increased risk of SCD during a bout of exertion and the ability of habitual vigorous exercise to modify this excess risk.140, 141 Acute bouts of exercise decrease vagal activity leading to an acute increase in susceptibility to ventricular fibrillation,140 whereas habitual exertion increases basal vagal tone resulting in increased cardiac electrical stability.
Figure 6.
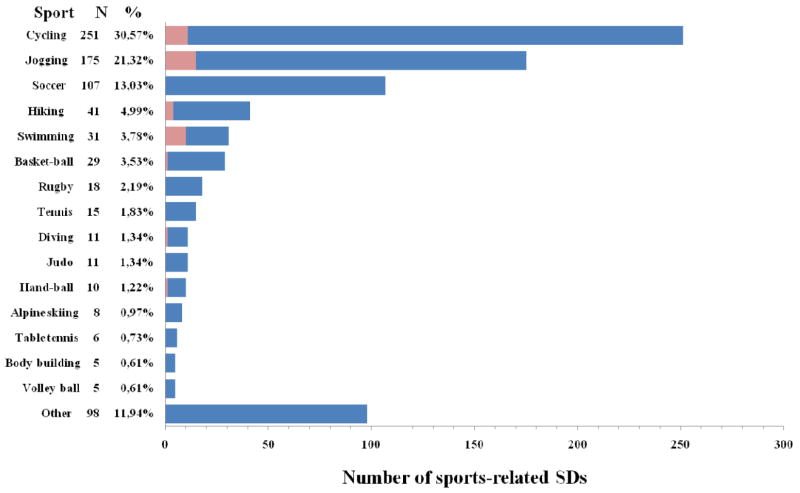
Sports engaged in at the time of the SCD events. There were a total of 820 SCD events evaluated in this study. N refers to the absolute number of SCD events that occurred during the specified sport. The percentage refers to the percent of deaths engaged in the specific activity. The pink shaded region represents the number of women.138 SDs indicate sudden deaths.
Despite these transiently elevated relative risks, the absolute risk of SCD during any particular episode of exertion is extremely low in most series142 and exertion-related SCDs are felt to be relatively rare outcomes. A recent national survey in France estimated that the incidence of exertion-related SCD in the population may be as high as 17 cases per million population per year.138 In this study, the absolute number of SCDs associated with exertion in the general population (n=770) far exceeded that observed among young competitive athletes (n=50), where the majority of the public attention has been directed.
Psychosocial Determinants
Lower socioeconomic status, depression, anxiety, social isolation, and psychological stress have all been linked to an increase in cardiovascular mortality in diverse populations.143,144 Although arrhythmic mechanisms have been postulated to partly underlie these associations, there are few if any studies that have prospectively examined associations with SCD. The incidence of SCD is higher in regions with lower SES, and this gradient in risk is more exaggerated below age 65.145 Chronic psychological stressors such as anxiety disorders and depression have also been associated with SCD in population based studies. Phobic anxiety has been directly associated with SCD but not non-fatal MI risk in three separate populations of men146 and women.147 Depression has also been associated with elevated risks of cardiac arrest148 and SCD among women without CHD.149 In addition to the chronic effects of psychosocial stress, it appears that acute mental stress can trigger SCD as well. Acute increases in the incidence of SCD have been documented in populations suffering disasters such as earthquakes or wars.150, 151 In addition to disasters, life stresses such as death of a spouse and loss of job have been associated with an increase in total mortality152 and SCD153 in healthy populations.
Genetic Predisposition to SCD
Over the past decade, investigations focused on the genetic bases of rare, inherited arrhythmic diseases (IADS) have provided insight into understanding the heritability of vulnerability to ventricular arrhythmias.154 The discovery of novel genes implicated in IADS and the effects of mutant alleles on basic electrophysiology raised the possibility that common genetic variants or polymorphisms in these same regions may account for part of the familial component of SCD risk observed in epidemiologic studies. Subsequently, completion of the Human Genome Project provided the foundation to identify novel genes and biological pathways implicated in conduction system disease, cardiac arrhythmias and SCD.
Familial Studies
Several studies have demonstrated a familial predisposition to SCD.72, 155-157 SCD events and fatal arrhythmias such as ventricular fibrillation (VF) are often the initial manifestation of an acute myocardial infarction and appear to cluster in families. Two case-control studies demonstrate that a history of SCD among a first-degree relative is an independent risk factor for VF156 or SCD157 in the setting of an acute myocardial infarction (AMI). Similar results have been documented in a prospective population-based study, where parental history of SCD was ascertained prior to death. Over a 20 plus year follow-up period,72 parental history of SCD was an independent risk factor for SCD (RR = 1.80; 95% CI 1.11 to 2.88) but not for fatal MI. Conversely, a parental history of fatal MI was only associated with an increased risk of fatal MI and had no effect on risk of SCD. These data in aggregate suggest that the familial aggregation of SCD or ischemic VF may be distinct from the familial risk pattern of MI or CHD. The consistent associations implicating a family history of arrhythmic death as an independent risk factor for SCD in the general population has led to several studies focused on identifying genetic variants that may influence vulnerability to ventricular arrhythmias and SCD in the population.
Intermediate Phenotypes for SCD: ECG variables
As discussed previously, quantitative measures obtained from ECGs, including those for heart rate, QRS duration and QT interval, have been associated with SCD. These measures are heritable and have multiple environmental and genetic contributors.158-162 As a result, genetic working groups across the world have partnered to identify common genetic variation associated with these quantitative traits through genome-wide association studies (GWAS). These genetic variants, which usually confer modest effects, may provide further insight not only into the cardiac conduction system but also into arrhythmic diseases including SCD. Novel variants identified through this mechanism may also eventually serve as susceptibility alleles for SCD in the population.
QT interval
Three GWA studies focused on variation in the QT interval among individuals of European ancestry have been completed.163-165 In total, these studies evaluated almost 30,000 individuals.164, 165 Approximately half the loci identified in these unbiased analyses map near the monogenic long-QT syndrome genes (KCNQ1, KCNH2, KCNE1 and SCN5A) (Table 2). The strongest and most consistent signal is within the NOS1AP gene, which encodes a nitric oxide synthase 1 adaptor protein.163 This gene has been demonstrated to be a modulator of myocardial repolarization in translational models,166 and variants in NOS1AP also modulate risk in the long QT syndrome.167, 168 Approximately half the genetic variants identified in these GWA studies were in loci not previously implicated in cardiac electrophysiology or recognized to regulate myocardial repolarization. In combination, these variants explain approximately 5-6% of variation in QT interval.
Table 2.
Genetic Variants of ECG Traits Identified through Genome-wide Association Studies
| Chr | Gene/Region | SNP | Coded Allele Freq. | Eff. (ms) | Findings/Notes |
|---|---|---|---|---|---|
|
QT Interval
| |||||
| 1p36 | RNF207 | rs846111 | 0.29 | 1.8 | The function of this locus is unknown. |
| 1q24 | ATP1B1 | rs10919071 | 0.87 | 2.0 | ATP1B1 encodes a transmembrane protein that maintains Na+ and K+ gradients across the membranes. |
| 1q23 | NOS1AP | rs12143842 | 0.24 | 3.2 | Neuronal nitric oxide synthase 1 regulates calcium cycling in the sarcoplasmic reticulum. |
| 3p22 | SCN5A | rs12053903 | 0.34 | -1.2 | Rare variants in SCN5A result in long-QT syndrome type 3 and the Brugada syndrome. |
| 6q22 | c6orf204, SLC35F1, PLN | rs11756438 | 0.47 | 1.4 | Phospholamban (PLN) inhibits cardiac sarcoplasmic reticulum Ca2+-ATPase. Increased PLN activity is linked to cardiomyopathy and ventricular tachycardia. |
| 7q36 | KCNH2 | rs2968864 | 0.25 | -1.4 | Rare variants in KCNH2 are associated with congenital long-QT syndrome type 2 and short-QT syndrome type 1. |
| 7q36 | KCNH2 | rs4725982 | 0.22 | 1.6 | |
| 11p15 | KCNQ1 | rs2074238 | 0.06 | -7.9 | Rare variants in KCNQ1 are associated with long-QT syndrome type 1 and short-QT syndrome type 2. |
| 11p15 | KCNQ1 | rs12576239 | 0.13 | 1.8 | |
| 16p13 | LITAF | rs8049607 | 0.49 | 1.2 | This gene has no known association with myocardial repolarization. |
| 16q21 | NDRG4 | rs7188697 | 0.74 | 1.7 | Novel locus associated with myocardial repolarization. |
| 17q12 | LIG3 | rs2074518 | 0.46 | -1.1 | LIG3 encodes DNA ligase III repair; the mechanism of modulating repolarization is unknown. |
| 17q24 | KCNJ2 | rs17779747 | 0.35 | -1.2 | Rare variants are associated with Anderson syndrome, which is characterized by periodic paralysis, dysmorphic features and ventricular arrhythmias. |
| 21q22 | KCNE1 | rs1805128 | 0.01 | 0.88 | Rare variants in KCNE1 result in long-QT syndrome type 5. |
|
| |||||
|
QRS Interval
| |||||
| 1p31 | NFIA | rs9436640 | 0.46 | -0.59 | The association of Nuclear Factor One with QRS duration is unclear. |
| 1p32 | CDKN2C | rs17391905 | 0.05 | -1.35 | This gene is a cyclin dependent kinase inhibitor and regulates cell growth. |
| 1p13 | CASQ2 | rs4074536 | 0.29 | -0.42 | Calsequestrin 2 is a calcium-handling protein that regulates opening of the ryanodine receptor. Mutations in CASQ2 have been implicated in CPVT. |
| 2p22 | HEATR5B, STRN | rs17020136 | 0.21 | 0.51 | Striatin is a calmodulin binding protein. It has recently been implicated in a dog model of ARVC. |
| 2p21 | CRIM1 | rs7562790 | 0.40 | 0.39 | CRIM1 is expressed in cardiac tissues and encodes a transmembrane protein that may bind to various members of the TGF-beta superfamily of ligands. |
| 3p22 | SCN10A, SCN5A | rs9851724 | 0.33 | -0.66 | Both genes encode voltage-gated Na channels and are important in cardiac conduction. SCN5A is also associated with the QTc interval. |
| 3p14 | TKT, PRKCD, CACNA1D | rs4687718 | 0.14 | -0.63 | Transketolase (TKT) is an enzyme used in multiple metabolic pathways. |
| 3p14 | LRIG1, SLC25A26 | rs2242285 | 0.42 | 0.37 | LRIG1 is upregulated in malignancies. |
| 5q33 | HAND1, SAP30L | rs13165478 | 0.36 | -0.55 | HAND1 encodes a transcription factor essential to cardiac development. Mutations have been associated with septal defects and ventricular arrhythmias. |
| 6p21 | CDKN1A | rs9470361 | 0.25 | 0.87 | CDKN1A is a cyclin dependent kinase inhibitor that is important for cardiac conduction system development. It can also aid with gap junction assembly. |
| 6q22 | C6orf204, SLC35F1, PLN | rs11153730 | 0.49 | 0.59 | Cardiac phospholamban (PLN) regulates calcium uptake into the sarcoplasmic reticulum by SERCA2a. This locus is also associated with the QTc and left ventricular end diastolic dimension. |
| 7p14 | TBX20 | rs1362212 | 0.18 | 0.69 | TBX20 demarcates the left and right ventricles. |
| 7p13 | IGFBP3 | rs7784776 | 0.43 | 0.39 | The function of this locus is unknown. |
| 10q25 | VTI1A | rs7342028 | 0.27 | 0.48 | The function of this locus is unknown. |
| 10q11 | DKK1 | rs1733724 | 0.25 | 0.49 | DKK1 is involved with axial development during embryological development. It also inhibits the Wnt signaling pathway, which is an important modulator of connexin43 activity. |
| 12q24 | TBX5 | rs883079 | 0.29 | 0.49 | TBX3 and TBX5 encode transcription factors found in the cardiac conduction system. TBX5 (activator) competes with TBX3 (repressor) for the regulation of myocardial genes such as GJA1. Mutations in TBX3 and TBX5 have been associated with rare inherited syndromes manifested by structural and conduction defects. |
| 12q24 | TBX3 | rs10850409 | 0.27 | -0.49 | |
| 13q22 | KLF12 | rs1886512 | 0.37 | -0.40 | The association of this transcription factor with QRS duration is unclear. |
| 14q24 | SIPA1L1 | rs11848785 | 0.27 | -0.50 | It contributes to Wnt signaling and cardiac development. |
| 17q22 | PRKCA | rs9912468 | 0.43 | 0.39 | Protein kinase C alters sarcoplasmic reticulum Ca2+ loading. |
| 17q21 | GOSR2 | rs17608766 | 0.16 | 0.53 | The function of this locus is unknown. |
| 18q21 | SETBP | rs991014 | 0.42 | 0.42 | The function of this locus is unknown. |
|
| |||||
|
RR Interval
| |||||
| 1q32 | CD46, LOC148696 | rs12731740 | 0.03 | -5.9 | This locus has an unclear association with heart rate. It has been observed in both Caucasian and non-Caucasian studies. |
| 6q22 | GJA1 | rs9398652 | 0.49 | -12.7 | GJA1 encodes Cx43, a connexin family protein and component of cardiac gap junctions. It is responsible for synchronized cardiac contractions. Mutations in GJA1 have been implicated in hypoplastic left heart syndrome. |
| 6q22 | SLC35F1, PLA | rs281868 | 0.44 | 1.50 | This locus is > 3Mb away from GJA1. It is also associated with QTc. Phospholamban is also involved in excitation-contraction coupling and intracellular calcium signaling. |
| 7q22 | SLC12A9 | rs314370 | 0.94 | 9.65 | It encodes a Cl- co-transporter interacting protein. |
| 7q22 | UfSp1 | rs12666989 | 0.07 | -9.31 | The function of this locus is unknown. |
| 11q12 | FADS1 | rs174547 | 0.91 | 4.20 | FADS1 was previously associated with cholesterol levels. |
| 12q24 | GPR133 | rs885389 | 0.30 | -14 | This locus encodes a G-protein coupled receptor and is expressed in both the atria and ventricles. |
| 14q12 | MYH6 | rs452036 | 0.62 | -9.65 | Two different sarcomeric myosin heavy chain (MYHC) isoforms are present: α-MYHC (encoded by MYH6) and β-MYHC (encoded by MYH7). Mutations in these genes have been implicated in various cardiomyopathies. |
| 14q12 | MYH6 | rs365990 | 0.38 | 9.80 | |
| 14q12 | MYH7 | rs223116 | 0.77 | -4.47 | |
QRS Interval
A recent genome wide meta-analysis of 14 studies including a total 40,407 individuals of European descent has identified 22 loci associated with QRS duration (Table 2).169 Some of these loci map within or near genes implicated in ventricular conduction such as sodium channels, transcription factors and calcium-handling proteins. In addition, several loci are associated with previously unidentified biologic processes. Several of these loci also exhibit associations with PR interval and QT interval but most often in the inverse direction for the latter. Overall, these loci in combination explain approximately 5.7% of the observed variance in QRS duration. The strongest association signal mapped in or near two genes, SCN5A and SCN10A, which encode the alpha subunit of the Nav1.5 and Nav1.8 sodium channels respectively. The SCN5A locus is well established as a susceptibility locus for a variety of IADS, but the involvement of SCN10A in cardiac conduction was previously unrecognized until an initial GWA study identified associations with PR interval and QRS duration.170, 171 Experimental models suggest that the SCN10A transcript and product are expressed in mouse and human hearts170 and localize to the mouse His-Purkinje system.169
RR interval
GWA studies have identified 9 loci associated with heart rate in populations of European ancestry171, 172 (Table 2). Two of these loci have been identified in participants of East Asian ancestry.173 One of the variants described in both Europeans and East Asians is located on chromosome 6q22 and is located near the GJA1 gene. GJA1 encodes gap junction protein and is critical for synchronized contraction of the heart. It is a major component of cardiac gap junctions174 and is known to play a role in arrhythmogenesis.175, 176
Genetic Determinants of ECG Phenotypes as Susceptibility Alleles for SCD
Several of the single nucleotide polymorphisms (SNPs) and related loci associated with variations in ECG phenotypes have been evaluated for specific associations with SCD. NOS1AP variation has been associated with SCD risk in 3 separate studies.177-179 In a combined analysis of 334 SCDs among white individuals participating in the Atherosclerosis Risk In Communities Study and Cardiovascular Health Study, a tagging SNP approach identified two intronic variants in NOS1AP that were associated with SCD even after controlling for QT interval. Interestingly, the variant with the strongest association (rs12567209) was not associated with QT interval duration. A follow-up study in the Rotterdam cohort found evidence for replication for this latter variant in analyses limited to witnessed SCDs;178 however, a case-control study from Oregon did not.179 The latter study reported another variant, which was correlated with the rs12567209 SNP, to be nominally significant. A recent study examined 49 independent loci, including NOS1AP, associated with intermediate ECG traits of QT interval, QRS duration, and heart rate in 1,283 SCD cases.180 Only one locus, TKT/CACNA1D/PRKCD, which had been previously associated with QRS duration, was associated with SCD after adjustment for multiple testing. However, the QRS prolonging allele was associated with a reduction in risk, which was opposite to that predicted based upon associations between QRS duration and SCD.
All of the above common variants individually confer relatively modest effect sizes on ECG characteristics, and thus, may not display detectable associations with SCD even with large sample sizes. Therefore, attempts have been made to combine variants into a genetic risk score to increase the power to detect associations. Recently, all genome-wide significant SNPs associated with the QT interval were entered into a QT genotype score, which was then evaluated for an association with SCD in two Finnish cohort studies.181 The QT genotype score was linearly associated with QT interval and explained 8.6% of the variance in the QT interval within these populations. A linear relationship between the genotype score and SCD risk, however, was not detected for the combined 116 SCD cases within these cohorts, which may have been underpowered. From these data, it has become clear, that genetic variants identified in genome-wide studies on ECG markers can provide important information for future translational and experimental work but will not be sufficient to explain the heritability of SCD.
Intermediate Phenotypes for SCD: Coronary Heart Disease
Given the high prevalence of CHD, often undiagnosed in SCD victims, genetic variants that are associated with CHD may also serve as susceptibility alleles for SCD in the general population. Shared variants for both traits may further our understanding regarding biologic processes that predispose to SCD in the setting of CHD. International consortiums have meta-analyzed GWA studies to enhance the power of identifying loci associated with CHD in European, African American and South Asian populations.182-184 The most recent meta-analysis included over 22,000 cases of CHD in both the discovery and replication phase and identified 10 previously recognized and 13 novel loci associated with CHD.182 The majority of these loci reside in gene regions that were not previously suspected in the pathogenesis of coronary disease. The strongest association signal remains a region on chromosome 9p21, which has been documented to regulate expression of two cyclin dependent kinase inhibitor genes CDKN2A and CDKN2B,185 known to have critical roles in cell proliferation, aging, senescence, and apoptosis.186 SNPs which tag the 9p21 region have been specifically associated with SCD in a meta-analysis involving 492 SCDs among Caucasian individuals from 6 prospective cohort studies.187 None of the other loci associated with CHD in GWAS have been reported to be associated with SCD.
Candidate Genes Analyses of SCD
The above examinations of genetic variation associated with intermediate phenotypes have been complemented by studies using a candidate gene approach to identify susceptibility alleles for SCD. This hypothesis-driven approach has focused on several biologic pathways implicated in the monogenic arrhythmia disorders and SCD within the population.
Common Variants
Polymorphisms in genes fundamental to electrical propagation, cardiac conduction, sympathetic activation, thrombosis, atherogenesis, and the renin-angiotensin-aldosterone system have been assessed for associations with SCD in isolated studies using a variety of designs and definitions (Table 3). 179, 188-201 The prevalence of allelic variants in these studies is at least 5% and often extends to 50-60% of the control population. As a result, it is expected that these variants will have a modest effect on SCD risk since a particularly deleterious variant would evolve over time to a rare variant/mutation in the human gene pool (Figure 7). The vast majority of these associations have not been independently replicated. Of the candidates studied, genetic variants encoding for amino acid polymorphisms in the β2-adrenergic receptor (Gln27Glu in β2AR) in Caucasians192, 194 and the α-subunit of the Nav1.5 cardiac sodium channel (Y1102A in SCN5A) in African Americans190, 191, 202 have been associated with SCD or arrhythmic events in more than one study; however, results have not been entirely consistent.193
Table 3.
Candidate Genes for SCD in the General Population
| Study | Gene | Frequency of Variant Allele | Population | N (SCD cases / controls) | Findings/Notes |
|---|---|---|---|---|---|
|
Ion Channels
| |||||
| Westaway, et al. 2011179 | CASQ2 GPD1L | 29-45% | Americans of European ancestry, general population | 670/299 | Polymorphisms in these genes are associated with SCD. |
| Albert, et al. 2010188 | KCNQ1 KCNH2 SCN5A KCNE1 KCNE2 | 60-70% | Americans of European ancestry, general population | 516/1522 | 2 intronic variants (1 in KCNQ1 and 1 in SCN5A) were associated with SCD. |
| Stecker, et al. 2006189 | SCN5A | 1-4%* | Americans of European ancestry with coronary disease | 67/91 | No association was observed between SCN5A polymorphisms or mutations with SCD. |
| Burke, et al. 2005190 | SCN5A (Y1102A) | 9% | African American, general population | 182/107 | Y1102A was associated with unexplained arrhythmic death and with SCA with ventricular hypertrophy compared with non-cardiac deaths. |
| Splawski, et al. 2002191 | SCN5A (Y1102A) | 13% | African American, general population | 23/100 | Variant is associated with an increased risk of SCD or medication induced QTc prolongation. |
|
| |||||
|
Autonomic Nervous System
| |||||
| Gavin, et al. 2011192 | β2AR (Gln27Glu) | 40-60% | Americans of European ancestry, general population | 492/1388 | When combined with the 2 analyses below, the β2AR polymorphism is associated with SCD. |
| Tseng, et al. 2008193 | β2AR and β1AR | 30-40% | Aborted SCD and history of MI/CAD, 75% Americans of European ancestry | 107/388 | No association was observed between any of the variants and SCD. |
| Sotoodehnia, et al. 2006194 | β2AR (Gln27Glu) | 57% whites, 81% African Americans | American cohort (4441 European ancestry, 808 African Americans) | 195/5249 | The β2AR variant is associated with SCD in whites but not blacks. |
| Snapir, et al. 2003195 | Alpha2B-AR | 51% | Finnish, population based | 278/405 | The deletion/deletion genotype of the alpha2B-adrenoceptor gene increased the risk for SCD in middle-aged men. |
|
| |||||
|
Thrombotic and Atherogenic Factors
| |||||
| Hernesniemi, et al. 2008196 | IL-18 | 40% | Finnish, population based | 275/388 | The IL-18 polymorphism is associated with SCD. |
| Mikkelsson, et al. 2002197 | GPIa | 50-60% | Finnish, population based | 275/369 | Polymorphisms on the glycoprotein Ia receptor are not associated with SCD. |
| Reiner, et al. 2002198 | Factor V Leiden and PT 20210A | 6-9% | American cohort (93% European ancestry) | 145/592 | Mutations in these genes are not associated with SCD. |
| Mikkelsson, et al. 2001199 | GPIbα | 23% | Finnish | 196/289 | The variant was associated with coronary thrombosis, fatal MI and SCD in middle age men. |
| Mikkelsson, et al. 2000200 | GPIIIa | 30-40% | Finnish, population based | 281/385 | The PlA1/A2 polymorphism of GPIIIa is a risk factor for coronary thrombosis and SCD in middle age. |
|
| |||||
|
Angiotensin-Converting Enzyme Pathway
| |||||
| Sotoodehnia, et al. 2009201 | REN | 15% | Americans of European ancestry, population based | 211/730 | Variations in AGTR1 and AGTR2 are associated with SCA risk in a population-based case-control study. |
| AGTR1 | |||||
| AGTR2 | |||||
| ACE2 | |||||
| BDRK2 | |||||
| AGT | |||||
| ACE | |||||
| KNG1 | |||||
Figure 7.
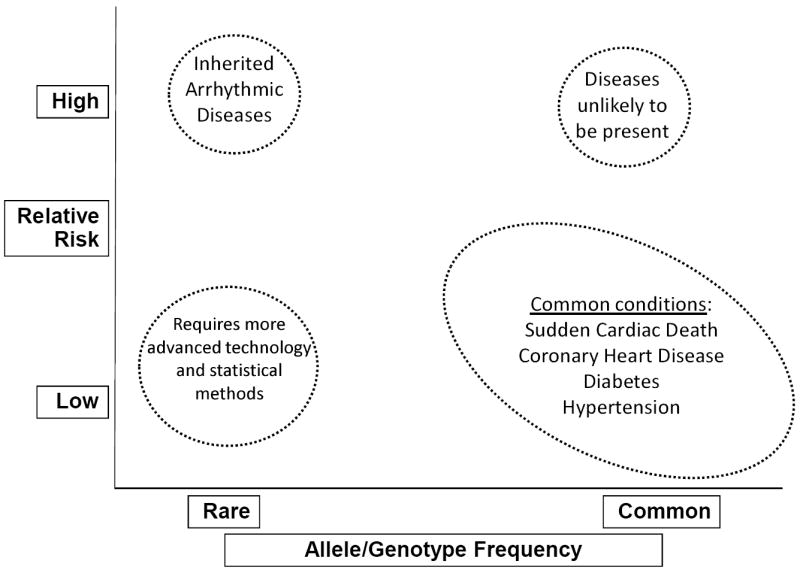
Overview of genetic studies. Genome-wide association studies aim to identify common allelic variants that have a low relative risk of disease. Evolution will select for variants that carry a high relative risk of disease; as a result, they will be rare.
Rare Variants
Given the high lethality of SCD, it is possible that the genetic architecture might be more similar to that underlying the rare IADS, which is characterized by rare alleles associated with variable penetrance. Such rare alleles are best detected by direct sequencing, which is rapidly becoming more accessible due to the development of next generation sequencing technologies. To our knowledge, only one study has utilized sequencing to examine rare variation in unselected SCD cases from adult populations.189, 203 The entire coding sequence and splice junctions of five ion channel genes associated with IADS, SCN5A, KCNE1, KCNE2, KCNQ1 and KCNH2, were directly sequenced in 113 cases of SCD.203 No unique or rare coding sequence variants were identified in any of the ion channel genes in 53 men.189 In 60 women with SCD, 6 rare missense variants (10%) were identified in the cardiac sodium channel gene (SCN5A).203 The overall frequency of these rare variants in SCN5A was significantly higher in the SCD cases compared to 733 controls from the same population (1.6%; P=0.001), and subtle alterations in ion channel function were observed for 4 of the 5 variants. Although not a common cause of SCD, these data suggest that functionally significant mutations and rare variants in SCN5A may contribute to SCD risk among women where the prevalence of structural heart disease is lower.29, 39
GWAS of SCD
In addition to the above candidate gene studies for SCD, GWA studies have been performed directly on SCD cases to identify novel genetic variants associated with SCD risk. This unbiased approach has the potential to discover previously unsuspected genetic variants and novel biologic pathways involved in the genesis of lethal ventricular arrhythmias. The number of validated loci achieving genome-wide significance for SCD, however, is much smaller than for other complex diseases. This finding is likely due, in large part, to the smaller numbers of SCD cases available for genetic analyses and greater heterogeneity with respect to underlying pathology and case definitions in comparison to other complex phenotypes.
One recent study sought to minimize heterogeneity by focusing on a highly specific arrhythmic phenotype. In the AGNES case-control study,204 a GWAS was performed among 505 cases of VF and 457 controls all presenting with a first ST-elevation MI. SNPs on chromosome 21q21 were associated with VF at a level of genome-wide significance. The strongest signal, which was found at rs2824292, remained significantly associated with VF (OR=1.51; 95% CI, 0.30-0.76. P=0.005) after adjustment for baseline characteristics and was replicated in another 156 cases of VF arrest in the setting of an acute MI from the ARREST study. The genetic locus is situated near the CXADR gene, which encodes the coxsackie virus and adenovirus receptor (CAR) protein.205, 206 These proteins have a recognized role in the pathogenesis of viral myocarditis207 and may also be involved in connexin localization at intercalated discs of AV nodal myocytes.208
Another recently published GWAS utilized a broader spectrum of SCD cases from case-control and cohort studies.180 A genome-wide approach was implemented to identify variations among 1,283 SCD cases from 5 separate studies and 20,000 controls, all of European ancestry. The most significant SNPs in this discovery phase were then genotyped in an additional 1,730 SCD and VF cases and 10,530 controls of European ancestry. The combined meta-analyses of all discovery and replication populations resulted in the discovery of a novel marker at the BAZ2B locus (bromodomain adjacent zinc finger domain 2B) which reached genome-wide significance with a relatively strong effect size (OR=1.92; 95% CI=1.57-2.34). The putative risk allele was rare (minor allele frequency 1.4%) and in strong linkage disequilibrium with genes critical in cardiogenesis and formation of the autonomic nervous system. This finding of a rare variant, which is unusual for GWA studies, highlights the potential role that rare variants may play in SCD risk.
It should be noted that an unbiased evaluation of variants associated with SCD in these two genome-wide studies did not identify the same variants. This lack of replication, which is commonly seen in genetic studies related to SCD, likely relates to heterogeneity in the case definition. Although the case definition utilized in the AGNES study is highly specific, it is also quite selective and would not apply to the majority of SCDs in the community.40-42 In contrast, the majority of cases in the population-based samples were out-of-hospital SCD events defined broadly. However, the heterogeneity both within and between studies likely limited the power to detect associations, even with a larger number of cases. Phenotypic homogeneity, therefore, is critical across studies especially when pooling results in a genome-wide analysis to detect variants with small effects. Larger sample sizes and a greater effort toward establishing homogenous sub-phenotypes will be needed to identify and replicate additional genetic variants associated with SCD.
Future Directions
Although much is known regarding risk factors for SCD, there is still a paucity of data for important subgroups within the population, and established racial and sex differences are poorly understood. In addition, our ability to accurately identify individuals most at risk for SCD within the population remains poor. Unlike global CHD risk prediction, where there are widely accepted predictive models, there are no similar models for SCD risk prediction among the general population despite multiple studies reporting on individual risk factors. Risk stratification algorithms based upon findings from epidemiologic studies which evaluate traditional cardiovascular risk factors, lifestyle and dietary habits, biological markers and genetic variants in combination may aid in the identification of susceptible subgroups within the population. It will also be critical to determine whether novel markers associate with SCD to a greater extent than other manifestations of heart disease. Such markers will not only improve risk stratification but will also provide insights into arrhythmic mechanisms within the population which could lead to novel preventive and therapeutic strategies.
The heritability of SCD remains poorly understood with the current data. Although candidate gene and genome-wide analyses have enlightened our appreciation for the intricacies of cardiac electrophysiology, arrhythmias and SCD, many questions remain. Very few of the SNPs identified or assessed in these studies have been replicated and many do not have clear functional implications as of yet. Due to the rapid development of next-generation sequencing technologies, large scale sequencing projects are becoming possible which will allow the examination of rare genetic variation as a component of SCD risk. It is also possible that structural variations, including copy-number variants, inversions, and translocations may contribute to SCD risk and will not be identified with standard GWAS and sequencing techniques. In order to address this potential complexity of the genetic architecture, large scale collaborations involving populations with synchronized definitions of SCD will be necessary.
SCD is a complex disorder that has been a research and clinical focus for several decades. As our understanding of this condition continues to improve with epidemiologic studies, experimental investigations, and clinical trials, strategies to reduce the incidence and lethality of SCD across the population remain important priorities. Low-risk interventions and therapies that are directed toward cardiovascular disease in general and SCD specifically will likely help reduce the burden of SCD in the population. In addition, continued campaigns in SCD education and awareness among the population remain important steps in reducing the impact of this condition.
Acknowledgments
Sources of Funding
RD is supported by K23DK089118 from the National Institutes of Health. CA is supported by an Established Investigator Award from the American Heart Association.
Footnotes
Disclosures
Dr. Albert is the Principal Investigator on research grants received from National Heart, Lung, and Blood Institute as well as St. Jude Medical Inc to study genetic and biologic markers as risk predictors of sudden cardiac death. Dr. Albert has previously received funding from National Heart, Lung, and Blood Institute as well as Boston Scientific, to study triggers and predictors of ventricular arrhythmias in ICD patients, and from Siemens Healthcare Diagnostics to study biomarkers and prediction of sudden cardiac death.
References
- 1.Lopshire JC, Zipes DP. Sudden cardiac death: Better understanding of risks, mechanisms, and treatment. Circulation. 2006;114:1134–1136. doi: 10.1161/CIRCULATIONAHA.106.647933. [DOI] [PubMed] [Google Scholar]
- 2.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association task force and the European Society of Cardiology committee for practice guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death): Developed in collaboration with the European Heart Rhythm association and the Heart Rhythm Society. Circulation. 2006;114:e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 3.Fishman GI, Chugh S, DiMarco JP, Albert CM, Anderson ME, Bonow, Buxton AE, Chen P-S, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O’Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng Z-J. Sudden cardiac death prediction and prevention report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 5.Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, Thomas KL, Hayes DL, Al-Khatib SM. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794–801. doi: 10.1016/j.jacc.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: Multiple source surveillance versus retrospective death certificate-based review in a large U.S. Community J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 8.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, Wellens HJ. Out-of-hospital cardiac arrest in the 1990’s: A population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–1505. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 9.Byrne R, Constant O, Smyth Y, Callagy G, Nash P, Daly K, Crowley J. Multiple source surveillance incidence and aetiology of out-of-hospital sudden cardiac death in a rural population in the west of Ireland. Eur Heart J. 2008;29:1418–1423. doi: 10.1093/eurheartj/ehn155. [DOI] [PubMed] [Google Scholar]
- 10.Hua W, Zhang LF, Wu YF, Liu XQ, Guo DS, Zhou HL, Gou ZP, Zhao LC, Niu HX, Chen KP, Mai JZ, Chu LN, Zhang S. Incidence of sudden cardiac death in China: Analysis of 4 regional populations. J Am Coll Cardiol. 2009;54:1110–1118. doi: 10.1016/j.jacc.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. Deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 12.Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Clegg L, Wang CH, Heiss G. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861–867. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 13.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: The Framingham Heart Study. Circulation. 2004;110:522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 14.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 15.Dudas K, Lappas G, Stewart S, Rosengren A. Trends in out-of-hospital deaths due to coronary heart disease in Sweden (1991 to 2006) Circulation. 2011;123:46–52. doi: 10.1161/CIRCULATIONAHA.110.964999. [DOI] [PubMed] [Google Scholar]
- 16.Gerber Y, Jacobsen SJ, Frye RL, Weston SA, Killian JM, Roger VL. Secular trends in deaths from cardiovascular diseases: A 25-year community study. Circulation. 2006;113:2285–2292. doi: 10.1161/CIRCULATIONAHA.105.590463. [DOI] [PubMed] [Google Scholar]
- 17.Gillum RF. Geographic variation in sudden coronary death. Am Heart J. 1990;119:380–389. doi: 10.1016/s0002-8703(05)80031-6. [DOI] [PubMed] [Google Scholar]
- 18.Myerburg RJ, Interian A, Simmons J, Castellanos A. Sudden cardiac death. In: Zipes DP, editor. Cardiac electrophysiology: From cell to bedside. Philadelphia: WB Saunders; 2004. pp. 720–731. [Google Scholar]
- 19.Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980-2000. JAMA. 2002;288:3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 20.Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, Samson RA, Kattwinkel J, Berg RA, Bhanji F, Cave DM, Jauch EC, Kudenchuk PJ, Neumar RW, Peberdy MA, Perlman JM, Sinz E, Travers AH, Berg MD, Billi JE, Eigel B, Hickey RW, Kleinman ME, Link MS, Morrison LJ, O’Connor RE, Shuster M, Callaway CW, Cucchiara B, Ferguson JD, Rea TD, Vanden Hoek TL. Part 1: Executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S640–656. doi: 10.1161/CIRCULATIONAHA.110.970889. [DOI] [PubMed] [Google Scholar]
- 21.Hallstrom AP, Ornato JP, Weisfeldt M, Travers A, Christenson J, McBurnie MA, Zalenski R, Becker LB, Schron EB, Proschan M. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004;351:637–646. doi: 10.1056/NEJMoa040566. [DOI] [PubMed] [Google Scholar]
- 22.Straus SM, Bleumink GS, Dieleman JP, van der Lei J, Stricker BH, Sturkenboom MC. The incidence of sudden cardiac death in the general population. J Clin Epidemiol. 2004;57:98–102. doi: 10.1016/S0895-4356(03)00210-5. [DOI] [PubMed] [Google Scholar]
- 23.Becker LB, Han BH, Meyer PM, Wright FA, Rhodes KV, Smith DW, Barrett J. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago Project. N Engl J Med. 1993;329:600–606. doi: 10.1056/NEJM199308263290902. [DOI] [PubMed] [Google Scholar]
- 24.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 25.Krahn AD, Connolly SJ, Roberts RS, Gent M. Diminishing proportional risk of sudden death with advancing age: Implications for prevention of sudden death. Am Heart J. 2004;147:837–840. doi: 10.1016/j.ahj.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Kannel WB, Schatzkin A. Sudden death: Lessons from subsets in population studies. J Am Coll Cardiol. 1985;5:141B–149B. doi: 10.1016/s0735-1097(85)80545-3. [DOI] [PubMed] [Google Scholar]
- 27.Cupples LA, Gagnon DR, Kannel WB. Long- and short-term risk of sudden coronary death. Circulation. 1992;85:I11–18. [PubMed] [Google Scholar]
- 28.Kannel WB, Wilson PW, D’Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–212. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 29.Albert CM, McGovern BA, Newell JB, Ruskin JN. Sex differences in cardiac arrest survivors. Circulation. 1996;93:1170–1176. doi: 10.1161/01.cir.93.6.1170. [DOI] [PubMed] [Google Scholar]
- 30.Chugh SS, Uy-Evanado A, Teodorescu C, Reinier K, Mariani R, Gunson K, Jui J. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The ORE-SUDS (Oregon Sudden Unexpected Death Study) J Am Coll Cardiol. 2009;54:2006–2011. doi: 10.1016/j.jacc.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowie MR, Fahrenbruch CE, Cobb LA, Hallstrom AP. Out-of-hospital cardiac arrest: Racial differences in outcome in Seattle. Am J Public Health. 1993;83:955–959. doi: 10.2105/ajph.83.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillum RF. Sudden cardiac death in Hispanic Americans and African Americans. Am J Public Health. 1997;87:1461–1466. doi: 10.2105/ajph.87.9.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan PS, Birkmeyer JD, Krumholz HM, Spertus JA, Nallamothu BK. Racial and gender trends in the use of implantable cardioverter-defibrillators among medicare beneficiaries between 1997 and 2003. Congest Heart Fail. 2009;15:51–57. doi: 10.1111/j.1751-7133.2009.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teodorescu C, Reinier K, Dervan C, Uy-Evanado A, Samara M, Mariani R, Gunson K, Jui J, Chugh SS. Factors associated with pulseless electric activity versus ventricular fibrillation: The Oregon Sudden Unexpected Death Study. Circulation. 2010;122:2116–2122. doi: 10.1161/CIRCULATIONAHA.110.966333. [DOI] [PubMed] [Google Scholar]
- 35.Chan PS, Nichol G, Krumholz HM, Spertus JA, Jones PG, Peterson ED, Rathore SS, Nallamothu BK. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009;302:1195–1201. doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spain DM, Bradess VA, Mohr C. Coronary atherosclerosis as a cause of unexpected and unexplained death. An autopsy study from 1949-1959. JAMA. 1960;174:384–388. doi: 10.1001/jama.1960.03030040038010. [DOI] [PubMed] [Google Scholar]
- 37.Manfredini R, Portaluppi F, Grandi E, Fersini C, Gallerani M. Out-of-hospital sudden death referring to an emergency department. J Clin Epidemiol. 1996;49:865–868. doi: 10.1016/0895-4356(96)00114-x. [DOI] [PubMed] [Google Scholar]
- 38.Priori SG, Borggrefe M, Camm AJ, Hauer RN, Klein H, Kuck KH, Schwartz PJ, Touboul P, Wellens HJ. Unexplained cardiac arrest. The need for a prospective registry. Eur Heart J. 1992;13:1445–1446. doi: 10.1093/oxfordjournals.eurheartj.a060083. [DOI] [PubMed] [Google Scholar]
- 39.Chugh SS, Kelly KL, Titus JL. Sudden cardiac death with apparently normal heart. Circulation. 2000;102:649–654. doi: 10.1161/01.cir.102.6.649. [DOI] [PubMed] [Google Scholar]
- 40.Adelson L, Hoffman W. Sudden death from coronary disease related to a lethal mechanism arising independently of vascular occlusion or myocardial damage. JAMA. 1961;176:129–135. doi: 10.1001/jama.1961.03040150045011. [DOI] [PubMed] [Google Scholar]
- 41.Farb A, Tang AL, Burke AP, Sessums L, Liang Y, Virmani R. Sudden coronary death. Frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation. 1995;92:1701–1709. doi: 10.1161/01.cir.92.7.1701. [DOI] [PubMed] [Google Scholar]
- 42.Greene HL. Sudden arrhythmic cardiac death--mechanisms, resuscitation and classification: The Seattle perspective. Am J Cardiol. 1990;65:4B–12B. doi: 10.1016/0002-9149(90)91285-e. [DOI] [PubMed] [Google Scholar]
- 43.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 44.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–2116. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 45.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 46.Bayes de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: Mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117:151–159. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- 47.Gang UJ, Jons C, Jorgensen RM, Abildstrom SZ, Haarbo J, Messier MD, Huikuri HV, Thomsen PE. Heart rhythm at the time of death documented by an implantable loop recorder. Europace. 2010;12:254–260. doi: 10.1093/europace/eup383. [DOI] [PubMed] [Google Scholar]
- 48.Liberthson RR, Nagel EL, Hirschman JC, Nussenfeld SR, Blackbourne BD, Davis JH. Pathophysiologic observations in prehospital ventricular fibrillation and sudden cardiac death. Circulation. 1974;49:790–798. doi: 10.1161/01.cir.49.5.790. [DOI] [PubMed] [Google Scholar]
- 49.Eisenberg MS, Copass MK, Hallstrom AP, Blake B, Bergner L, Short FA, Cobb LA. Treatment of out-of-hospital cardiac arrests with rapid defibrillation by emergency medical technicians. N Engl J Med. 1980;302:1379–1383. doi: 10.1056/NEJM198006193022502. [DOI] [PubMed] [Google Scholar]
- 50.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 51.Polentini MS, Pirrallo RG, McGill W. The changing incidence of ventricular fibrillation in Milwaukee, Wisconsin (1992-2002) Prehosp Emerg Care. 2006;10:52–60. doi: 10.1080/10903120500366961. [DOI] [PubMed] [Google Scholar]
- 52.Kannel WB, Cupples LA, D’Agostino RB. Sudden death risk in overt coronary heart disease: The Framingham Study. Am Heart J. 1987;113:799–804. doi: 10.1016/0002-8703(87)90722-8. [DOI] [PubMed] [Google Scholar]
- 53.Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioni A, Kober L, White H, Van de Werf F, Pieper K, Califf RM, Pfeffer MA. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 54.Adabag AS, Therneau TM, Gersh BJ, Weston SA, Roger VL. Sudden death after myocardial infarction. JAMA. 2008;300:2022–2029. doi: 10.1001/jama.2008.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huikuri HV, Tapanainen JM, Lindgren K, Raatikainen P, Makikallio TH, Juhani Airaksinen KE, Myerburg RJ. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2003;42:652–658. doi: 10.1016/s0735-1097(03)00783-6. [DOI] [PubMed] [Google Scholar]
- 56.Makikallio TH, Barthel P, Schneider R, Bauer A, Tapanainen JM, Tulppo MP, Perkiomaki JS, Schmidt G, Huikuri HV. Frequency of sudden cardiac death among acute myocardial infarction survivors with optimized medical and revascularization therapy. Am J Cardiol. 2006;97:480–484. doi: 10.1016/j.amjcard.2005.09.077. [DOI] [PubMed] [Google Scholar]
- 57.Stevenson WG, Stevenson LW, Middlekauff HR, Saxon LA. Sudden death prevention in patients with advanced ventricular dysfunction. Circulation. 1993;88:2953–2961. doi: 10.1161/01.cir.88.6.2953. [DOI] [PubMed] [Google Scholar]
- 58.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 59.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 60.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 61.Reinier K, Dervan C, Singh T, Uy-Evanado A, Lai S, Gunson K, Jui J, Chugh SS. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm. 2011;8:1177–1182. doi: 10.1016/j.hrthm.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 63.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 64.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: Two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 65.Deo R, Vittinghoff E, Lin F, Tseng ZH, Hulley SB, Shlipak MG. Risk factor and prediction modeling for sudden cardiac death in women with coronary artery disease. Arch Intern Med. 2011 doi: 10.1001/archinternmed.2011.328. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buxton AE. Should everyone with an ejection fraction less than or equal to 30% receive an implantable cardioverter-defibrillator? Not everyone with an ejection fraction < or = 30% should receive an implantable cardioverter-defibrillator. Circulation. 2005;111:2537–2549. doi: 10.1161/01.CIR.0000165057.88551.2C. discussion 2537-2549. [DOI] [PubMed] [Google Scholar]
- 67.Balkau B, Jouven X, Ducimetiere P, Eschwege E. Diabetes as a risk factor for sudden death. Lancet. 1999;354:1968–1969. doi: 10.1016/S0140-6736(99)04383-4. [DOI] [PubMed] [Google Scholar]
- 68.Jouven X, Lemaitre RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26:2142–2147. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 69.Kucharska-Newton AM, Couper DJ, Pankow JS, Prineas RJ, Rea TD, Sotoodehnia N, Chakravarti A, Folsom AR, Siscovick DS, Rosamond WD. Diabetes and the risk of sudden cardiac death, the Atherosclerosis Risk in Communities study. Acta Diabetol. 2010;47:161–168. doi: 10.1007/s00592-009-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deo R, Sotoodehnia N, Katz R, Sarnak MJ, Fried LF, Chonchol M, Kestenbaum B, Psaty BM, Siscovick DS, Shlipak MG. Cystatin C and sudden cardiac death risk in the elderly. Circ Cardiovasc Qual Outcomes. 2010;3:159–164. doi: 10.1161/CIRCOUTCOMES.109.875369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deo R, Lin F, Vittinghoff E, Tseng ZH, Hulley SB, Shlipak MG. Kidney dysfunction and sudden cardiac death among women with coronary heart disease. Hypertension. 2008;51:1578–1582. doi: 10.1161/HYPERTENSIONAHA.107.103804. [DOI] [PubMed] [Google Scholar]
- 72.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: The Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 73.Wannamethee G, Shaper AG, Macfarlane PW, Walker M. Risk factors for sudden cardiac death in middle-aged British men. Circulation. 1995;91:1749–1756. doi: 10.1161/01.cir.91.6.1749. [DOI] [PubMed] [Google Scholar]
- 74.Hallstrom AP, Cobb LA, Ray R. Smoking as a risk factor for recurrence of sudden cardiac arrest. N Engl J Med. 1986;314:271–275. doi: 10.1056/NEJM198601303140502. [DOI] [PubMed] [Google Scholar]
- 75.Vlietstra RE, Kronmal RA, Oberman A, Frye RL, Killip T., 3rd Effect of cigarette smoking on survival of patients with angiographically documented coronary artery disease. Report from the CASS registry. JAMA. 1986;255:1023–1027. [PubMed] [Google Scholar]
- 76.Goldenberg I, Jonas M, Tenenbaum A, Boyko V, Matetzky S, Shotan A, Behar S, Reicher-Reiss H. Current smoking, smoking cessation, and the risk of sudden cardiac death in patients with coronary artery disease. Arch Intern Med. 2003;163:2301–2305. doi: 10.1001/archinte.163.19.2301. [DOI] [PubMed] [Google Scholar]
- 77.Junttila MJ, Barthel P, Myerburg RJ, Makikallio TH, Bauer A, Ulm K, Kiviniemi A, Tulppo M, Perkiomaki JS, Schmidt G, Huikuri HV. Sudden cardiac death after myocardial infarction in patients with type 2 diabetes. Heart Rhythm. 2010;7:1396–1403. doi: 10.1016/j.hrthm.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 78.Jouven X, Zureik M, Desnos M, Guerot C, Ducimetiere P. Resting heart rate as a predictive risk factor for sudden death in middle-aged men. Cardiovasc Res. 2001;50:373–378. doi: 10.1016/s0008-6363(01)00230-9. [DOI] [PubMed] [Google Scholar]
- 79.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 80.Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 81.Teodorescu C, Reinier K, Uy-Evanado A, Navarro J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged QRS duration on the resting ECG is associated with sudden death risk in coronary disease, independent of prolonged ventricular repolarization. Heart Rhythm. 2011 doi: 10.1016/j.hrthm.2011.06.011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morin DP, Oikarinen L, Viitasalo M, Toivonen L, Nieminen MS, Kjeldsen SE, Dahlof B, John M, Devereux RB, Okin PM. QRS duration predicts sudden cardiac death in hypertensive patients undergoing intensive medical therapy: The life study. Eur Heart J. 2009;30:2908–2914. doi: 10.1093/eurheartj/ehp321. [DOI] [PubMed] [Google Scholar]
- 83.Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquie JL, Nogami A, Babuty D, Yli-Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Lacroix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O’Neill M, Hocini M, Lim KT, Knecht S, Veenhuyzen GD, Bordachar P, Chauvin M, Jais P, Coureau G, Chene G, Klein GJ, Clementy J. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 84.Nam GB, Kim YH, Antzelevitch C. Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med. 2008;358:2078–2079. doi: 10.1056/NEJMc0708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, Halkin A, Steinvil A, Heller K, Glikson M, Katz A, Viskin S. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: Incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–1238. doi: 10.1016/j.jacc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 86.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 87.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 88.Chiuve SE, Korngold EC, Januzzi JL, Jr, Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr. 2011;93:253–260. doi: 10.3945/ajcn.110.002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2010;160:464–470. doi: 10.1016/j.ahj.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jouven X, Charles MA, Desnos M, Ducimetiere P. Circulating nonesterified fatty acid level as a predictive risk factor for sudden death in the population. Circulation. 2001;104:756–761. doi: 10.1161/hc3201.094151. [DOI] [PubMed] [Google Scholar]
- 91.Lemaitre RN, King IB, Mozaffarian D, Sotoodehnia N, Siscovick DS. Trans-fatty acids and sudden cardiac death. Atheroscler Suppl. 2006;7:13–15. doi: 10.1016/j.atherosclerosissup.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 92.Empana JP, Jouven X, Canoui-Poitrine F, Luc G, Tafflet M, Haas B, Arveiler D, Ferrieres J, Ruidavets JB, Montaye M, Yarnell J, Morange P, Kee F, Evans A, Amouyel P, Ducimetiere P. C-reactive protein, interleukin 6, fibrinogen and risk of sudden death in European middle-aged men: The Prime Study. Arterioscler Thromb Vasc Biol. 2010;30:2047–2052. doi: 10.1161/ATVBAHA.110.208785. [DOI] [PubMed] [Google Scholar]
- 93.Korngold EC, Januzzi JL, Jr, Gantzer ML, Moorthy MV, Cook NR, Albert CM. Amino-terminal pro-B-type natriuretic peptide and high-sensitivity C-reactive protein as predictors of sudden cardiac death among women. Circulation. 2009;119:2868–2876. doi: 10.1161/CIRCULATIONAHA.108.832576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 95.Pascual-Figal DA, Ordonez-Llanos J, Tornel PL, Vazquez R, Puig T, Valdes M, Cinca J, de Luna AB, Bayes-Genis A. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol. 2009;54:2174–2179. doi: 10.1016/j.jacc.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 96.Beygui F, Collet JP, Benoliel JJ, Vignolles N, Dumaine R, Barthelemy O, Montalescot G. High plasma aldosterone levels on admission are associated with death in patients presenting with acute ST-elevation myocardial infarction. Circulation. 2006;114:2604–2610. doi: 10.1161/CIRCULATIONAHA.106.634626. [DOI] [PubMed] [Google Scholar]
- 97.Tomaschitz A, Pilz S, Ritz E, Morganti A, Grammer T, Amrein K, Boehm BO, Marz W. Associations of plasma renin with 10-year cardiovascular mortality, sudden cardiac death, and death due to heart failure. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr150. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 98.Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, Fried L, Chonchol M, de Boer IH, Enquobahrie D, Siscovick D, Kestenbaum B. Vitamin D, parathyroid hormone and sudden cardiac death: Results from the cardiovascular health study. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.111.179135. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, Espe K, Dekker F, Brandenburg V, Marz W, Ritz E, Wanner C. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31:2253–2261. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patton KK, Sotoodehnia N, DeFilippi C, Siscovick DS, Gottdiener JS, Kronmal RA. N-terminal pro-B-type natriuretic peptide is associated with sudden cardiac death risk: The Cardiovascular Health Study. Heart Rhythm. 2011;8:228–233. doi: 10.1016/j.hrthm.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–2397. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 102.Tapanainen JM, Lindgren KS, Makikallio TH, Vuolteenaho O, Leppaluoto J, Huikuri HV. Natriuretic peptides as predictors of non-sudden and sudden cardiac death after acute myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2004;43:757–763. doi: 10.1016/j.jacc.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 103.Albert CM, Hennekens CH, O’Donnell CJ, Ajani UA, Carey VJ, Willett WC, Ruskin JN, Manson JE. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–28. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 104.Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111:157–164. doi: 10.1161/01.CIR.0000152099.87287.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: The Cardiovascular Health Study. Circulation. 2003;107:1372–1377. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- 106.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 107.Albert CM, Oh K, Whang W, Manson JE, Chae CU, Stampfer MJ, Willett WC, Hu FB. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 2005;112:3232–3238. doi: 10.1161/CIRCULATIONAHA.105.572008. [DOI] [PubMed] [Google Scholar]
- 108.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 109.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the Gissi-Prevenzione trial. Gruppo Italiano per Lo Studio della Sopravvivenza Nell’infarto Miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 110.Kromhout D, Giltay EJ, Geleijnse JM. N-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 111.Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J. Omega, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 112.Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, Willett W, Hennekens CH. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med. 1993;329:1829–1834. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]
- 113.Siscovick DS, Weiss NS, Fox N. Moderate alcohol consumption and primary cardiac arrest. Am J Epidemiol. 1986;123:499–503. doi: 10.1093/oxfordjournals.aje.a114265. [DOI] [PubMed] [Google Scholar]
- 114.Albert CM, Manson JE, Cook NR, Ajani UA, Gaziano JM, Hennekens CH. Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation. 1999;100:944–950. doi: 10.1161/01.cir.100.9.944. [DOI] [PubMed] [Google Scholar]
- 115.Chiuve SE, Rimm EB, Mukamal KJ, Rexrode KM, Stampfer MJ, Manson JE, Albert CM. Light-to-moderate alcohol consumption and risk of sudden cardiac death in women. Heart Rhythm. 2010;7:1374–1380. doi: 10.1016/j.hrthm.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zarraga IG, Schwarz ER. Impact of dietary patterns and interventions on cardiovascular health. Circulation. 2006;114:961–973. doi: 10.1161/CIRCULATIONAHA.105.603910. [DOI] [PubMed] [Google Scholar]
- 117.Sebbag L, Forrat R, Canet E, Renaud S, Delaye J, de Lorgeril M. Effects of dietary supplementation with alpha-tocopherol on myocardial infarct size and ventricular arrhythmias in a dog model of ischemia-reperfusion. J Am Coll Cardiol. 1994;24:1580–1585. doi: 10.1016/0735-1097(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 118.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 119.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119:1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chiuve SE, Fung TT, Rexrode KM, Spiegelman D, Manson JE, Stampfer MJ, Albert CM. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306:62–69. doi: 10.1001/jama.2011.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE, Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O’Donnell CJ, Smith SC, Jr, Wilson PW. Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- 123.Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512–1516. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- 124.Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Circadian variations in the occurrence of cardiac arrests: Initial and repeat episodes. Circulation. 1998;98:31–39. doi: 10.1161/01.cir.98.1.31. [DOI] [PubMed] [Google Scholar]
- 125.Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol. 1992;70:65–68. doi: 10.1016/0002-9149(92)91391-g. [DOI] [PubMed] [Google Scholar]
- 126.Peters RW. Propranolol and the morning increase in sudden cardiac death: (the beta-blocker heart attack trial experience) Am J Cardiol. 1990;66:57G–59G. doi: 10.1016/0002-9149(90)90398-k. [DOI] [PubMed] [Google Scholar]
- 127.Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Weekly and seasonal variation in the incidence of cardiac arrests. Am Heart J. 1999;137:512–515. doi: 10.1016/s0002-8703(99)70507-7. [DOI] [PubMed] [Google Scholar]
- 128.Arntz HR, Willich SN, Schreiber C, Bruggemann T, Stern R, Schultheiss HP. Diurnal, weekly and seasonal variation of sudden death. Population-based analysis of 24,061 consecutive cases. Eur Heart J. 2000;21:315–320. doi: 10.1053/euhj.1999.1739. [DOI] [PubMed] [Google Scholar]
- 129.Gerber Y, Jacobsen SJ, Killian JM, Weston SA, Roger VL. Seasonality and daily weather conditions in relation to myocardial infarction and sudden cardiac death in Olmsted county, Minnesota, 1979 to 2002. J Am Coll Cardiol. 2006;48:287–292. doi: 10.1016/j.jacc.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 130.Empana JP, Sauval P, Ducimetiere P, Tafflet M, Carli P, Jouven X. Increase in out-of-hospital cardiac arrest attended by the medical mobile intensive care units, but not myocardial infarction, during the 2003 heat wave in Paris, France. Crit Care Med. 2009;37:3079–3084. doi: 10.1097/CCM.0b013e3181b0868f. [DOI] [PubMed] [Google Scholar]
- 131.Siscovick DS, Weiss NS, Fletcher RH, Lasky T. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311:874–877. doi: 10.1056/NEJM198410043111402. [DOI] [PubMed] [Google Scholar]
- 132.Lemaitre RN, Siscovick DS, Raghunathan TE, Weinmann S, Arbogast P, Lin DY. Leisure-time physical activity and the risk of primary cardiac arrest. Arch Intern Med. 1999;159:686–690. doi: 10.1001/archinte.159.7.686. [DOI] [PubMed] [Google Scholar]
- 133.Group MRFITR. Multiple risk factor intervention trial. Risk factor changes and mortality results. JAMA. 1982;248:1465–1477. [PubMed] [Google Scholar]
- 134.Whang W, Manson JE, Hu FB, Chae CU, Rexrode KM, Willett WC, Stampfer MJ, Albert CM. Physical exertion, exercise, and sudden cardiac death in women. JAMA. 2006;295:1399–1403. doi: 10.1001/jama.295.12.1399. [DOI] [PubMed] [Google Scholar]
- 135.Kannel WB. Exercise and sudden death. JAMA. 1982;248:3143–3144. [PubMed] [Google Scholar]
- 136.Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343:1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 137.Kohl HW, 3rd, Powell KE, Gordon NF, Blair SN, Paffenbarger RS., Jr Physical activity, physical fitness, and sudden cardiac death. Epidemiol Rev. 1992;14:37–58. doi: 10.1093/oxfordjournals.epirev.a036091. [DOI] [PubMed] [Google Scholar]
- 138.Marijon E, Tafflet M, Celermajer DS, Dumas F, Perier MC, Mustafic H, Toussaint JF, Desnos M, Rieu M, Benameur N, Le Heuzey JY, Empana JP, Jouven X. Sports-related sudden death in the general population. Circulation. 2011;124:672–681. doi: 10.1161/CIRCULATIONAHA.110.008979. [DOI] [PubMed] [Google Scholar]
- 139.Burke AP, Farb A, Malcom GT, Liang Y, Smialek JE, Virmani R. Plaque rupture and sudden death related to exertion in men with coronary artery disease. JAMA. 1999;281:921–926. doi: 10.1001/jama.281.10.921. [DOI] [PubMed] [Google Scholar]
- 140.Peronnet F, Cleroux J, Perrault H, Cousineau D, de Champlain J, Nadeau R. Plasma norepinephrine response to exercise before and after training in humans. J Appl Physiol. 1981;51:812–815. doi: 10.1152/jappl.1981.51.4.812. [DOI] [PubMed] [Google Scholar]
- 141.Hull SS, Jr, Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation. 1994;89:548–552. doi: 10.1161/01.cir.89.2.548. [DOI] [PubMed] [Google Scholar]
- 142.Dahabreh IJ, Paulus JK. Association of episodic physical and sexual activity with triggering of acute cardiac events: Systematic review and meta-analysis. JAMA. 2011;305:1225–1233. doi: 10.1001/jama.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 144.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 145.Reinier K, Stecker EC, Vickers C, Gunson K, Jui J, Chugh SS. Incidence of sudden cardiac arrest is higher in areas of low socioeconomic status: A prospective two year study in a large United States community. Resuscitation. 2006;70:186–192. doi: 10.1016/j.resuscitation.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 146.Kawachi I, Colditz GA, Ascherio A, Rimm EB, Giovannucci E, Stampfer MJ, Willett WC. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994;89:1992–1997. doi: 10.1161/01.cir.89.5.1992. [DOI] [PubMed] [Google Scholar]
- 147.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111:480–487. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 148.Empana JP, Jouven X, Lemaitre RN, Sotoodehnia N, Rea T, Raghunathan TE, Simon G, Siscovick DS. Clinical depression and risk of out-of-hospital cardiac arrest. Arch Intern Med. 2006;166:195–200. doi: 10.1001/archinte.166.2.195. [DOI] [PubMed] [Google Scholar]
- 149.Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, Glynn RJ, Garan H, Albert CM. Depression and risk of sudden cardiac death and coronary heart disease in women: Results from the Nurses’ Health Study. J Am Coll Cardiol. 2009;53:950–958. doi: 10.1016/j.jacc.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334:413–419. doi: 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- 151.Meisel SR, Kutz I, Dayan KI, Pauzner H, Chetboun I, Arbel Y, David D. Effect of Iraqi missile war on incidence of acute myocardial infarction and sudden death in Israeli civilians. Lancet. 1991;338:660–661. doi: 10.1016/0140-6736(91)91234-l. [DOI] [PubMed] [Google Scholar]
- 152.Rosengren A, Orth-Gomer K, Wedel H, Wilhelmsen L. Stressful life events, social support, and mortality in men born in 1933. BMJ. 1993;307:1102–1105. doi: 10.1136/bmj.307.6912.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rahe RH, Romo M, Bennett L, Siltanen P. Recent life changes, myocardial infarction, and abrupt coronary death. Studies in Helsinki. Arch Intern Med. 1974;133:221–228. [PubMed] [Google Scholar]
- 154.Cerrone M, Priori SG. Genetics of sudden death: Focus on inherited channelopathies. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr082. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 155.Friedlander Y, Siscovick DS, Weinmann S, Austin MA, Psaty BM, Lemaitre RN, Arbogast P, Raghunathan TE, Cobb LA. Family history as a risk factor for primary cardiac arrest. Circulation. 1998;97:155–160. doi: 10.1161/01.cir.97.2.155. [DOI] [PubMed] [Google Scholar]
- 156.Dekker LR, Bezzina CR, Henriques JP, Tanck MW, Koch KT, Alings MW, Arnold AE, de Boer MJ, Gorgels AP, Michels HR, Verkerk A, Verheugt FW, Zijlstra F, Wilde AA. Familial sudden death is an important risk factor for primary ventricular fibrillation: A case-control study in acute myocardial infarction patients. Circulation. 2006;114:1140–1145. doi: 10.1161/CIRCULATIONAHA.105.606145. [DOI] [PubMed] [Google Scholar]
- 157.Kaikkonen KS, Kortelainen ML, Linna E, Huikuri HV. Family history and the risk of sudden cardiac death as a manifestation of an acute coronary event. Circulation. 2006;114:1462–1467. doi: 10.1161/CIRCULATIONAHA.106.624593. [DOI] [PubMed] [Google Scholar]
- 158.Newton-Cheh C, Larson MG, Corey DC, Benjamin EJ, Herbert AG, Levy D, D’Agostino RB, O’Donnell CJ. QT interval is a heritable quantitative trait with evidence of linkage to chromosome 3 in a genome-wide linkage analysis: The Framingham Heart Study. Heart Rhythm. 2005;2:277–284. doi: 10.1016/j.hrthm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 159.Hanson B, Tuna N, Bouchard T, Heston L, Eckert E, Lykken D, Segal N, Rich S. Genetic factors in the electrocardiogram and heart rate of twins reared apart and together. The American Journal of Cardiology. 1989;63:606–609. doi: 10.1016/0002-9149(89)90907-7. [DOI] [PubMed] [Google Scholar]
- 160.Russell MW, Law I, Sholinsky P, Fabsitz RR. Heritability of ECG measurements in adult male twins. J Electrocardiol. 1998;30(Suppl):64–68. doi: 10.1016/s0022-0736(98)80034-4. [DOI] [PubMed] [Google Scholar]
- 161.Singh JP, Larson MG, O’Donnell CJ, Tsuji H, Evans JC, Levy D. Heritability of heart rate variability: The Framingham Heart Study. Circulation. 1999;99:2251–2254. doi: 10.1161/01.cir.99.17.2251. [DOI] [PubMed] [Google Scholar]
- 162.Martin LJ, Comuzzie AG, Sonnenberg GE, Myklebust J, James R, Marks J, Blangero J, Kissebah AH. Major quantitative trait locus for resting heart rate maps to a region on chromosome 4. Hypertension. 2004;43:1146–1151. doi: 10.1161/01.HYP.0000122873.42047.17. [DOI] [PubMed] [Google Scholar]
- 163.Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M, West K, Kashuk C, Akyol M, Perz S, Jalilzadeh S, Illig T, Gieger C, Guo CY, Larson MG, Wichmann HE, Marban E, O’Donnell CJ, Hirschhorn JN, Kaab S, Spooner PM, Meitinger T, Chakravarti A. A common genetic variant in the nos1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–651. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 164.Pfeufer A, Sanna S, Arking DE, Muller M, Gateva V, Fuchsberger C, Ehret GB, Orru M, Pattaro C, Kottgen A, Perz S, Usala G, Barbalic M, Li M, Putz B, Scuteri A, Prineas RJ, Sinner MF, Gieger C, Najjar SS, Kao WH, Muhleisen TW, Dei M, Happle C, Mohlenkamp S, Crisponi L, Erbel R, Jockel KH, Naitza S, Steinbeck G, Marroni F, Hicks AA, Lakatta E, Muller-Myhsok B, Pramstaller PP, Wichmann HE, Schlessinger D, Boerwinkle E, Meitinger T, Uda M, Coresh J, Kaab S, Abecasis GR, Chakravarti A. Common variants at ten loci modulate the QT interval duration in the QTSCD study. Nat Genet. 2009;41:407–414. doi: 10.1038/ng.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Newton-Cheh C, Eijgelsheim M, Rice KM, de Bakker PI, Yin X, Estrada K, Bis JC, Marciante K, Rivadeneira F, Noseworthy PA, Sotoodehnia N, Smith NL, Rotter JI, Kors JA, Witteman JC, Hofman A, Heckbert SR, O’Donnell CJ, Uitterlinden AG, Psaty BM, Lumley T, Larson MG, Stricker BH. Common variants at ten loci influence QT interval duration in the QTGEN study. Nat Genet. 2009;41:399–406. doi: 10.1038/ng.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chang KC, Barth AS, Sasano T, Kizana E, Kashiwakura Y, Zhang Y, Foster DB, Marban E. Capon modulates cardiac repolarization via neuronal nitric oxide synthase signaling in the heart. Proc Natl Acad Sci USA. 2008;105:4477–4482. doi: 10.1073/pnas.0709118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tomas M, Napolitano C, De Giuli L, Bloise R, Subirana I, Malovini A, Bellazzi R, Arking DE, Marban E, Chakravarti A, Spooner PM, Priori SG. Polymorphisms in the NOS1AP gene modulate QT interval duration and risk of arrhythmias in the long QT syndrome. J Am Coll Cardiol. 2010;55:2745–2752. doi: 10.1016/j.jacc.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 168.Crotti L, Monti MC, Insolia R, Peljto A, Goosen A, Brink PA, Greenberg DA, Schwartz PJ, George AL., Jr NOS1AP is a genetic modifier of the long-QT syndrome. Circulation. 2009;120:1657–1663. doi: 10.1161/CIRCULATIONAHA.109.879643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sotoodehnia N, Isaacs A, de Bakker PI, Dorr M, Newton-Cheh C, Nolte IM, van der Harst P, Muller M, Eijgelsheim M, Alonso A, Hicks AA, Padmanabhan S, Hayward C, Smith AV, Polasek O, Giovannone S, Fu J, Magnani JW, Marciante KD, Pfeufer A, Gharib SA, Teumer A, Li M, Bis JC, Rivadeneira F, Aspelund T, Kottgen A, Johnson T, Rice K, Sie MP, Wang YA, Klopp N, Fuchsberger C, Wild SH, Mateo Leach I, Estrada K, Volker U, Wright AF, Asselbergs FW, Qu J, Chakravarti A, Sinner MF, Kors JA, Petersmann A, Harris TB, Soliman EZ, Munroe PB, Psaty BM, Oostra BA, Cupples LA, Perz S, de Boer RA, Uitterlinden AG, Volzke H, Spector TD, Liu FY, Boerwinkle E, Dominiczak AF, Rotter JI, van Herpen G, Levy D, Wichmann HE, van Gilst WH, Witteman JC, Kroemer HK, Kao WH, Heckbert SR, Meitinger T, Hofman A, Campbell H, Folsom AR, van Veldhuisen DJ, Schwienbacher C, O’Donnell CJ, Volpato CB, Caulfield MJ, Connell JM, Launer L, Lu X, Franke L, Fehrmann RS, te Meerman G, Groen HJ, Weersma RK, van den Berg LH, Wijmenga C, Ophoff RA, Navis G, Rudan I, Snieder H, Wilson JF, Pramstaller PP, Siscovick DS, Wang TJ, Gudnason V, van Duijn CM, Felix SB, Fishman GI, Jamshidi Y, Stricker BH, Samani NJ, Kaab S, Arking DE. Common variants in 22 loci are associated with QRS duration and cardiac ventricular conduction. Nat Genet. 2010;42:1068–1076. doi: 10.1038/ng.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chambers JC, Zhao J, Terracciano CM, Bezzina CR, Zhang W, Kaba R, Navaratnarajah M, Lotlikar A, Sehmi JS, Kooner MK, Deng G, Siedlecka U, Parasramka S, El-Hamamsy I, Wass MN, Dekker LR, de Jong JS, Sternberg MJ, McKenna W, Severs NJ, de Silva R, Wilde AA, Anand P, Yacoub M, Scott J, Elliott P, Wood JN, Kooner JS. Genetic variation in SCN10A influences cardiac conduction. Nat Genet. 2010;42:149–152. doi: 10.1038/ng.516. [DOI] [PubMed] [Google Scholar]
- 171.Holm H, Gudbjartsson DF, Arnar DO, Thorleifsson G, Thorgeirsson G, Stefansdottir H, Gudjonsson SA, Jonasdottir A, Mathiesen EB, Njolstad I, Nyrnes A, Wilsgaard T, Hald EM, Hveem K, Stoltenberg C, Lochen ML, Kong A, Thorsteinsdottir U, Stefansson K. Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet. 2010;42:117–122. doi: 10.1038/ng.511. [DOI] [PubMed] [Google Scholar]
- 172.Eijgelsheim M, Newton-Cheh C, Sotoodehnia N, de Bakker PI, Muller M, Morrison AC, Smith AV, Isaacs A, Sanna S, Dorr M, Navarro P, Fuchsberger C, Nolte IM, de Geus EJ, Estrada K, Hwang SJ, Bis JC, Ruckert IM, Alonso A, Launer LJ, Hottenga JJ, Rivadeneira F, Noseworthy PA, Rice KM, Perz S, Arking DE, Spector TD, Kors JA, Aulchenko YS, Tarasov KV, Homuth G, Wild SH, Marroni F, Gieger C, Licht CM, Prineas RJ, Hofman A, Rotter JI, Hicks AA, Ernst F, Najjar SS, Wright AF, Peters A, Fox ER, Oostra BA, Kroemer HK, Couper D, Volzke H, Campbell H, Meitinger T, Uda M, Witteman JC, Psaty BM, Wichmann HE, Harris TB, Kaab S, Siscovick DS, Jamshidi Y, Uitterlinden AG, Folsom AR, Larson MG, Wilson JF, Penninx BW, Snieder H, Pramstaller PP, van Duijn CM, Lakatta EG, Felix SB, Gudnason V, Pfeufer A, Heckbert SR, Stricker BH, Boerwinkle E, O’Donnell CJ. Genome-wide association analysis identifies multiple loci related to resting heart rate. Hum Mol Genet. 2010;19:3885–3894. doi: 10.1093/hmg/ddq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, Yoon D, Lee MH, Kim DJ, Park M, Cha SH, Kim JW, Han BG, Min H, Ahn Y, Park MS, Han HR, Jang HY, Cho EY, Lee JE, Cho NH, Shin C, Park T, Park JW, Lee JK, Cardon L, Clarke G, McCarthy MI, Lee JY, Oh B, Kim HL. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–534. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- 174.Peters NS. New insights into myocardial arrhythmogenesis: Distribution of gap-junctional coupling in normal, ischaemic and hypertrophied human hearts. Clinical Science. 1996;90:447–452. doi: 10.1042/cs0900447. [DOI] [PubMed] [Google Scholar]
- 175.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nature Medicine. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 176.Roell W, Lewalter T, Sasse P, Tallini YN, Choi BR, Breitbach M, Doran R, Becher UM, Hwang SM, Bostani T, von Maltzahn J, Hofmann A, Reining S, Eiberger B, Gabris B, Pfeifer A, Welz A, Willecke K, Salama G, Schrickel JW, Kotlikoff MI, Fleischmann BK. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 177.Kao WH, Arking DE, Post W, Rea TD, Sotoodehnia N, Prineas RJ, Bishe B, Doan BQ, Boerwinkle E, Psaty BM, Tomaselli GF, Coresh J, Siscovick DS, Marban E, Spooner PM, Burke GL, Chakravarti A. Genetic variations in nitric oxide synthase 1 adaptor protein are associated with sudden cardiac death in US white community-based populations. Circulation. 2009;119:940–951. doi: 10.1161/CIRCULATIONAHA.108.791723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Eijgelsheim M, Newton-Cheh C, Aarnoudse AL, van Noord C, Witteman JC, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in NOS1AP is associated with sudden cardiac death: Evidence from the Rotterdam study. Hum Mol Genet. 2009;18:4213–4218. doi: 10.1093/hmg/ddp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Westaway SK, Reinier K, Huertas-Vazquez A, Evanado A, Teodorescu C, Navarro J, Sinner MF, Gunson K, Jui J, Spooner P, Kaab S, Chugh SS. Common variants in CASQ2, GPD1L and NOS1AP are significantly associated with risk of sudden death in patients with coronary artery disease. Circ Cardiovasc Genet. 2011 doi: 10.1161/CIRCGENETICS.111.959916. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Arking DE, Junttila MJ, Goyette P, Huertas-Vazquez A, Eijgelsheim M, Blom MT, Newton-Cheh C, Reinier K, Teodorescu C, Uy-Evanado A, Carter-Monroe N, Kaikkonen KS, Kortelainen ML, Boucher G, Lagace C, Moes A, Zhao X, Kolodgie F, Rivadeneira F, Hofman A, Witteman JC, Uitterlinden AG, Marsman RF, Pazoki R, Bardai A, Koster RW, Dehghan A, Hwang SJ, Bhatnagar P, Post W, Hilton G, Prineas RJ, Li M, Kottgen A, Ehret G, Boerwinkle E, Coresh J, Kao WH, Psaty BM, Tomaselli GF, Sotoodehnia N, Siscovick DS, Burke GL, Marban E, Spooner PM, Cupples LA, Jui J, Gunson K, Kesaniemi YA, Wilde AA, Tardif JC, O’Donnell CJ, Bezzina CR, Virmani R, Stricker BH, Tan HL, Albert CM, Chakravarti A, Rioux JD, Huikuri HV, Chugh SS. Identification of a sudden cardiac death susceptibility locus at 2q24.2 through genome-wide association in European ancestry individuals. PLoS Genet. 2011;7:e1002158. doi: 10.1371/journal.pgen.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Noseworthy PA, Havulinna AS, Porthan K, Lahtinen AM, Jula A, Karhunen PJ, Perola M, Oikarinen L, Kontula KK, Salomaa V, Newton-Cheh C. Common genetic variants, QT interval, and sudden cardiac death in a Finnish population-based study. Circ Cardiovasc Genet. 2011;4:305–311. doi: 10.1161/CIRCGENETICS.110.959049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, Absher D, Aherrahrou Z, Allayee H, Altshuler D, Anand SS, Andersen K, Anderson JL, Ardissino D, Ball SG, Balmforth AJ, Barnes TA, Becker DM, Becker LC, Berger K, Bis JC, Boekholdt SM, Boerwinkle E, Braund PS, Brown MJ, Burnett MS, Buysschaert I, Carlquist JF, Chen L, Cichon S, Codd V, Davies RW, Dedoussis G, Dehghan A, Demissie S, Devaney JM, Diemert P, Do R, Doering A, Eifert S, Mokhtari NE, Ellis SG, Elosua R, Engert JC, Epstein SE, de Faire U, Fischer M, Folsom AR, Freyer J, Gigante B, Girelli D, Gretarsdottir S, Gudnason V, Gulcher JR, Halperin E, Hammond N, Hazen SL, Hofman A, Horne BD, Illig T, Iribarren C, Jones GT, Jukema JW, Kaiser MA, Kaplan LM, Kastelein JJ, Khaw KT, Knowles JW, Kolovou G, Kong A, Laaksonen R, Lambrechts D, Leander K, Lettre G, Li M, Lieb W, Loley C, Lotery AJ, Mannucci PM, Maouche S, Martinelli N, McKeown PP, Meisinger C, Meitinger T, Melander O, Merlini PA, Mooser V, Morgan T, Muhleisen TW, Muhlestein JB, Munzel T, Musunuru K, Nahrstaedt J, Nelson CP, Nothen MM, Olivieri O, Patel RS, Patterson CC, Peters A, Peyvandi F, Qu L, Quyyumi AA, Rader DJ, Rallidis LS, Rice C, Rosendaal FR, Rubin D, Salomaa V, Sampietro ML, Sandhu MS, Schadt E, Schafer A, Schillert A, Schreiber S, Schrezenmeir J, Schwartz SM, Siscovick DS, Sivananthan M, Sivapalaratnam S, Smith A, Smith TB, Snoep JD, Soranzo N, Spertus JA, Stark K, Stirrups K, Stoll M, Tang WH, Tennstedt S, Thorgeirsson G, Thorleifsson G, Tomaszewski M, Uitterlinden AG, van Rij AM, Voight BF, Wareham NJ, Wells GA, Wichmann HE, Wild PS, Willenborg C, Witteman JC, Wright BJ, Ye S, Zeller T, Ziegler A, Cambien F, Goodall AH, Cupples LA, Quertermous T, Marz W, Hengstenberg C, Blankenberg S, Ouwehand WH, Hall AS, Deloukas P, Thompson JR, Stefansson K, Roberts R, Thorsteinsdottir U, O’Donnell CJ, McPherson R, Erdmann J, Samani NJ. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339–344. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 184.Lettre G, Palmer CD, Young T, Ejebe KG, Allayee H, Benjamin EJ, Bennett F, Bowden DW, Chakravarti A, Dreisbach A, Farlow DN, Folsom AR, Fornage M, Forrester T, Fox E, Haiman CA, Hartiala J, Harris TB, Hazen SL, Heckbert SR, Henderson BE, Hirschhorn JN, Keating BJ, Kritchevsky SB, Larkin E, Li M, Rudock ME, McKenzie CA, Meigs JB, Meng YA, Mosley TH, Newman AB, Newton-Cheh CH, Paltoo DN, Papanicolaou GJ, Patterson N, Post WS, Psaty BM, Qasim AN, Qu L, Rader DJ, Redline S, Reilly MP, Reiner AP, Rich SS, Rotter JI, Liu Y, Shrader P, Siscovick DS, Tang WH, Taylor HA, Tracy RP, Vasan RS, Waters KM, Wilks R, Wilson JG, Fabsitz RR, Gabriel SB, Kathiresan S, Boerwinkle E. Genome-wide association study of coronary heart disease and its risk factors in 8,090 African Americans: The NHLBI CARe project. PLoS Genet. 2011;7:e1001300. doi: 10.1371/journal.pgen.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Visel A, Zhu Y, May D, Afzal V, Gong E, Attanasio C, Blow MJ, Cohen JC, Rubin EM, Pennacchio LA. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature. 2010;464:409–412. doi: 10.1038/nature08801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 187.Newton-Cheh C, Cook NR, VanDenburgh M, Rimm EB, Ridker PM, Albert CM. A common variant at 9p21 is associated with sudden and arrhythmic cardiac death. Circulation. 2009;120:2062–2068. doi: 10.1161/CIRCULATIONAHA.109.879049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Albert CM, MacRae CA, Chasman DI, VanDenburgh M, Buring JE, Manson JE, Cook NR, Newton-Cheh C. Common variants in cardiac ion channel genes are associated with sudden cardiac death. Circ Arrhythm Electrophysiol. 2010;3:222–229. doi: 10.1161/CIRCEP.110.944934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Stecker EC, Sono M, Wallace E, Gunson K, Jui J, Chugh SS. Allelic variants of SCN5A and risk of sudden cardiac arrest in patients with coronary artery disease. Heart Rhythm. 2006;3:697–700. doi: 10.1016/j.hrthm.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 190.Burke A, Creighton W, Mont E, Li L, Hogan S, Kutys R, Fowler D, Virmani R. Role of SCN5A Y1102 polymorphism in sudden cardiac death in blacks. Circulation. 2005;112:798–802. doi: 10.1161/CIRCULATIONAHA.104.482760. [DOI] [PubMed] [Google Scholar]
- 191.Splawski I, Timothy KW, Tateyama M, Clancy CE, Malhotra A, Beggs AH, Cappuccio FP, Sagnella GA, Kass RS, Keating MT. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science. 2002;297:1333–1336. doi: 10.1126/science.1073569. [DOI] [PubMed] [Google Scholar]
- 192.Gavin MC, Newton-Cheh C, Gaziano JM, Cook NR, VanDenburgh M, Albert CM. A common variant in the beta2-adrenergic receptor and risk of sudden cardiac death. Heart Rhythm. 2011;8:704–710. doi: 10.1016/j.hrthm.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tseng ZH, Aouizerat BE, Pawlikowska L, Vittinghoff E, Lin F, Whiteman D, Poon A, Herrington D, Howard TD, Varosy PD, Hulley SB, Malloy M, Kane J, Kwok PY, Olgin JE. Common beta-adrenergic receptor polymorphisms are not associated with risk of sudden cardiac death in patients with coronary artery disease. Heart Rhythm. 2008;5:814–821. doi: 10.1016/j.hrthm.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sotoodehnia N, Siscovick DS, Vatta M, Psaty BM, Tracy RP, Towbin JA, Lemaitre RN, Rea TD, Durda JP, Chang JM, Lumley TS, Kuller LH, Burke GL, Heckbert SR. Beta2-adrenergic receptor genetic variants and risk of sudden cardiac death. Circulation. 2006;113:1842–1848. doi: 10.1161/CIRCULATIONAHA.105.582833. [DOI] [PubMed] [Google Scholar]
- 195.Snapir A, Mikkelsson J, Perola M, Penttila A, Scheinin M, Karhunen PJ. Variation in the alpha2b-adrenoceptor gene as a risk factor for prehospital fatal myocardial infarction and sudden cardiac death. J Am Coll Cardiol. 2003;41:190–194. doi: 10.1016/s0735-1097(02)02702-x. [DOI] [PubMed] [Google Scholar]
- 196.Hernesniemi JA, Karhunen PJ, Rontu R, Ilveskoski E, Kajander O, Goebeler S, Viiri LE, Pessi T, Hurme M, Lehtimaki T. Interleukin-18 promoter polymorphism associates with the occurrence of sudden cardiac death among caucasian males: The Helsinki Sudden Death Study. Atherosclerosis. 2008;196:643–649. doi: 10.1016/j.atherosclerosis.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 197.Mikkelsson J, Perola M, Penttila A, Karhunen PJ. Platelet collagen receptor GPIa (C807T/HPA-5) haplotype is not associated with an increased risk of fatal coronary events in middle-aged men. Atherosclerosis. 2002;165:111–118. doi: 10.1016/s0021-9150(02)00110-7. [DOI] [PubMed] [Google Scholar]
- 198.Reiner AP, Rosendaal FR, Reitsma PH, Lemaitre RN, Pearce RM, Friedlander Y, Raghunathan TE, Psaty BM, Siscovick DS. Factor V Leiden, Prothrombin G20210A, and risk of sudden coronary death in apparently healthy persons. The American Journal of Cardiology. 2002;90:66–68. doi: 10.1016/s0002-9149(02)02391-3. [DOI] [PubMed] [Google Scholar]
- 199.Mikkelsson J, Perola M, Penttila A, Karhunen PJ. Platelet glycoprotein Ibalpha HPA-2 Met/VNTR B haplotype as a genetic predictor of myocardial infarction and sudden cardiac death. Circulation. 2001;104:876–880. doi: 10.1161/hc3301.094907. [DOI] [PubMed] [Google Scholar]
- 200.Mikkelsson J, Perola M, Laippala P, Penttila A, Karhunen PJ. Glycoprotein IIIa Pl(A1/A2) polymorphism and sudden cardiac death. J Am Coll Cardiol. 2000;36:1317–1323. doi: 10.1016/s0735-1097(00)00871-8. [DOI] [PubMed] [Google Scholar]
- 201.Sotoodehnia N, Li G, Johnson CO, Lemaitre RN, Rice KM, Rea TD, Siscovick DS. Genetic variation in angiotensin-converting enzyme-related pathways associated with sudden cardiac arrest risk. Heart Rhythm. 2009;6:1306–1314. doi: 10.1016/j.hrthm.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sun AY, Koontz JI, Shah SH, Piccini JP, Nilsson KR, Jr, Craig D, Haynes C, Gregory SG, Hranitzky PM, Pitt GS. The S1103Y cardiac sodium channel variant is associated with implantable cardioverter-defibrillator events in blacks with heart failure and reduced ejection fraction. Circ Cardiovasc Genet. 2011;4:163–168. doi: 10.1161/CIRCGENETICS.110.958652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Albert CM, Nam EG, Rimm EB, Jin HW, Hajjar RJ, Hunter DJ, MacRae CA, Ellinor PT. Cardiac sodium channel gene variants and sudden cardiac death in women. Circulation. 2008;117:16–23. doi: 10.1161/CIRCULATIONAHA.107.736330. [DOI] [PubMed] [Google Scholar]
- 204.Bezzina CR, Pazoki R, Bardai A, Marsman RF, de Jong JS, Blom MT, Scicluna BP, Jukema JW, Bindraban NR, Lichtner P, Pfeufer A, Bishopric NH, Roden DM, Meitinger T, Chugh SS, Myerburg RJ, Jouven X, Kaab S, Dekker LR, Tan HL, Tanck MW, Wilde AA. Genome-wide association study identifies a susceptibility locus at 21q21 for ventricular fibrillation in acute myocardial infarction. Nat Genet. 2010;42:688–691. doi: 10.1038/ng.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 206.Tomko RP, Xu R, Philipson L. HCAR and MCAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bowles NE, Richardson PJ, Olsen EG, Archard LC. Detection of coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986;1:1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- 208.Lim BK, Xiong D, Dorner A, Youn TJ, Yung A, Liu TI, Gu Y, Dalton ND, Wright AT, Evans SM, Chen J, Peterson KL, McCulloch AD, Yajima T, Knowlton KU. Coxsackievirus and adenovirus receptor (CAR) mediates atrioventricular-node function and connexin 45 localization in the murine heart. J Clin Invest. 2008;118:2758–2770. doi: 10.1172/JCI34777. [DOI] [PMC free article] [PubMed] [Google Scholar]


