Abstract
Most prions in yeast form amyloid fibrils that must be severed by the protein disaggregase Hsp104 to be propagated and transmitted efficiently to newly formed buds. Only one yeast prion, [PSI+], is cured by Hsp104 overexpression. We investigated the interaction between Hsp104 and Sup35, the priongenic protein in yeast that forms the [PSI+] prion.1 We found that a 20-amino acid segment within the highly-charged, unstructured middle domain of Sup35 contributes to the physical interaction between the middle domain and Hsp104. When this segment was deleted from Sup35, the efficiency of [PSI+] severing was substantially reduced, resulting in larger Sup35 particles and weakening of the [PSI+] phenotype. Furthermore, [PSI+] in these cells was completely resistant to Hsp104 curing. The affinity of Hsp104 was considerably weaker than that of model Hsp104-binding proteins and peptides, implying that Sup35 prions are not ideal substrates for Hsp104-mediated remodeling. In light of this finding, we present a modified model of Hsp104-mediated [PSI+] propagation and curing that requires only partial remodeling of Sup35 assembled into amyloid fibrils.
Keywords: molecular chaperone, prion, protein aggregation, protein folding, yeast
Influence of Hsp104 on [PSI+]
The biological implications of Stanley Prusiner’s prion hypothesis,2 originally framed to explain the biology of transmissible spongiform encephalopathies, underwent a startling expansion in the mid-1990s when Reed Wickner first proposed that a prion-like phenomenon could explain the curious inheritance pattern and the appearance and disappearance of certain metastable phenotypes in the yeast S. cerevisiae.3 One of these, an omnipotent suppressor, was called [PSI+], which Wickner proposed was a self-replicating aggregate of the translation termination factor Sup35.
At the time, Yury Chernoff and colleagues had been looking for genes that influenced [PSI+] when overexpressed, and had repeatedly found that additional copies of the gene encoding Hsp104 significantly elevated the loss (“curing”) of [PSI+].4 Furthermore, the deletion of the HSP104 gene also converted [PSI+] yeast to [psi-]. These findings were particularly intriguing as work in the lab of Susan Lindquist, then at the University of Chicago, had just established that Hsp104 restores solubility and activity to aggregated proteins that had been thermally denatured in vivo.5
Wickner’s extension of the prion hypothesis, the genetic interaction between HSP104 and [PSI+], and the finding that Hsp104 could remodel misfolded, aggregated proteins, focused attention on the physical state of Sup35. Papers emerged demonstrating Sup35 in [PSI+] cells was largely insoluble, while in [psi-] cells, Sup35 was soluble.6,7 One of these7 included the first proposal that Hsp104 could play a role in propagating [PSI+] by breaking up large aggregates into smaller seeds. Subsequently, contemporaneous investigations in the labs of Lindquist,8 Wüthrich,9 and Ter-Avanesyan10 established that Sup35 could form amyloid fibrils, and that these seeded the polymerization of soluble Sup35 in vitro. Ultimate proof of the “protein-only” nature of the [PSI+] element was subsequently provided by the lab of Jonathan Weissman, who successfully used fibrils produced from a purified recombinant fragment of Sup35 to transform [psi-] cells to [PSI+].11
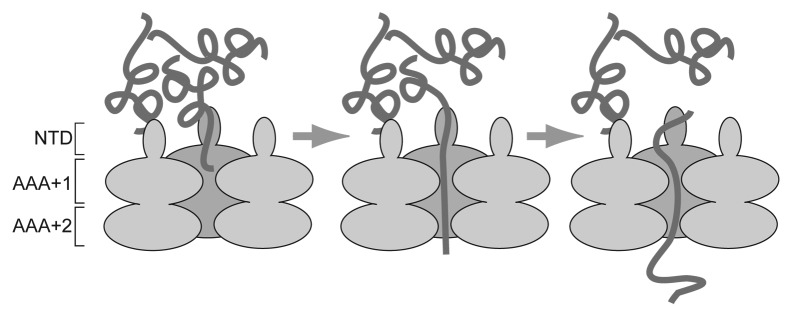
Around same time, as the first details of the prion-like nature of [PSI+] were emerging, the protein disaggregation and refolding activity of Hsp104 was reconstituted with purified proteins.12 The most widely accepted model of Hsp104-mediated disaggregation13 is that Hsp104 engages misfolded polypeptide substrate trapped in aggregates at the N-terminal proximal entrance of the axial channel formed by the Hsp104 hexamer (Fig. 1). ATP hydrolysis is coupled to the progressive unfolding and threading of the substrate polypeptide through the axial channel of Hsp104 until it is completely freed from the aggregate and can be released for refolding.
Figure 1. Model of Hsp104-mediated disaggregation. In a simplified model of the mechanism of protein disaggregation, a single polypeptide trapped in an aggregate binds to Hsp104 near or within the entrance of the axial channel formed by the N-terminal proximal AAA+ domain (AAA+1) of the Hsp104 hexamer (viewed as a cutaway in this cartoon). ATP-driven conformational changes in Hsp104 draw the polypeptide deeper into the axial channel where it comes into contact with AAA+2. This results in the progressive disruption of the interactions that trap the polypeptide in the aggregate. The polypeptide is finally completely extracted from the aggregate and can be released from the axial channel for refolding. NTD = N-terminal domain.
It was not until several years later that Hsp104, either alone or in combination with Hsp70 and 40, was shown to stimulate polymerization of a Sup35 fragment or, at higher concentrations, completely disperse preformed fibers.14,15 When combined, the experimental results suggest a model of Hsp104’s role in [PSI+] propagation and curing (Fig. 2). Prior to cytokinesis, a number of amyloid particles are transmitted from the mother to the newly formed daughter cell and these undergo growth or elongation by recruitment of newly synthesized soluble Sup35 molecules. These extended amyloid fibrils are severed by Hs104 extraction of individual Sup35 molecules from the amyloid core. Transmission of some of these “propagons” to new buds reinitiates the cycle of recruitment and propagation. Loss of Hsp104 rapidly diminishes the transmissibility of [PSI+] by blocking the severing step required to maintain the number and size of prion particles necessary to ensure stable inheritance. Overexpression of Hsp104 amplifies the frequency of protomer extraction and cures [PSI+] by completely dissolving the amyloid.
Figure 2. Propagation and curing of the [PSI+] prion by Hsp104. The [PSI+] prion has a core fibril formed by the assembly the N-domain of Sup35 into amyloid-like fibrils. The M-domain connects the globular C-domain as a flexible disordered linker. [PSI+] prions grow by recruitment of soluble Sup35 N-domains onto the ends of preexisting fibrils. Amyloid fibrils are severed by the Hsp104-mediated extraction of single Sup35 molecules to propagate heritable seeds or propagons. Some of these propagons are inherited by transmission to yeast buds in during transfer of cytosol from the mother cell. Overexpression of Hsp104 cures cells of [PSI+] possibly by completely solubilizing most or all of the Sup35 in amyloid fibrils. (C-domains are omitted from parts of the figure for simplicity.)
Probing the Hsp104/Sup35 Interaction
Our lab is interested in how Hsp104 recognizes substrates. Because the curing of prions by Hsp104 overexpression is particular to [PSI+], we hypothesized that Sup35 may differ from other prionogenic proteins in yeast by displaying determinants that makes it specifically more susceptible to Hsp104-mediated remodeling. The structure of the amyloid fibril formed by Sup35 is analogous to a pipe cleaner. The wire core of the pipe cleaner is analogous to the amyloid fibril composed of the N-domain of Sup35 (amino acids 1–123; see Fig. 2). The bristles of the pipe cleaner correspond to the unstructured M-domain (amino acids 124–253). Electron micrographs of freshly prepared fibrils of full-length Sup35 show that the M-domain is in a largely extended conformation with the globular C-domain of Sup35 located on the periphery.16 This topological arrangement suggests that the M-domain is accessible in Sup35 assembled into amyloid and a likely site for Hsp104 binding.
Using peptide arrays, we had previously reported that Hsp104-binding peptides are enriched in hydrophobic residues and generally shielded from the solvent in folded protein domains.17 The model soluble peptide we characterized most thoroughly, p370 (KLSFDDVFEREYA), binds to Hsp104 with high affinity (about 17 nM with respect to Hsp104 hexamers), stimulates the ATPase activity of Hsp104, and competes for the binding of the model unfolded protein reduced carboxymethylated α-lactalbumin [RCMLA; RCMLA binds to Hsp104 heeamers with a Kd of 35 nM (JRG, M. Michalowska, R. Lum, unpublished)]. In experiments that probed the interaction between Hsp104 and Sup35,1 we found that the full-length M-domain (we used Sup35 amino acids 105–253 as the full-length M-domain) also has these properties. We found that the binding affinity between M-domain and Hsp104 (about 540 nM) is substantially weaker than that of p370 or RCMLA. Nonetheless, the M-domain competes for RCMLA binding to Hsp104 and stimulates the ATPase activity of Hsp104.
Using biochemical approaches and we determined that a region of the M-domain, specifically amino acids 129–148 (FQKQQKQAAPKPKKTLKLVS), makes a considerable contribution to the biochemical interaction between the M-domain and Hsp104. Semi-denaturing agarose gel electrophoresis showed that Sup35Δ129–148 in [PSI+] cells exists SDS-resistant oligomers that are substantially larger than those formed by full-length Sup35, suggesting that the intact M-domain enhances the frequency of fibril severing in vivo. Even so, Sup35Δ129–148 prions are still dependent on Hsp104 for propagation. Importantly, [PSI+] maintained by Sup35Δ129–148 is completely resistant to curing by Hsp104 overexpression. Thus, attenuating the interaction between Sup35 and Hsp104 by the elimination of residues 129–148 converts [PSI+] to a prion that, like other yeast prions, is dependent on Hsp104 for propagation, but resistant to curing by Hsp104 overexpression.
When we compared direct binding of Hsp104 to the intact M-domain and the M-domain lacking residues 129–148, we found that the interaction was only modestly diminished (< 2-fold). Although we fit the M-domain/Hsp104 binding curves using a single site model, the data deviate from the fitted curve suggesting that, other than the contribution of the 129–148 segment to binding, there exists another complex and less specific mode of binding. This is consistent with the reduction but not complete loss of either ATPase stimulation or RCMLa competition conferred by eliminating the 129–148 segment from the M-domain. We concluded from these observations that the frequency of fibril severing and whether [PSI+] is curable by Hsp104 can be attributed, at least in part, to the attenuation of the Sup35/Hsp104 interaction. The residual interactions are apparently sufficient for propagation but at a much reduced frequency.
Toward a Modified Model of [PSI+] Propagation and Curing
To gain perspective on the propagation and curing of [PSI+], we can speculate on how frequently Sup35 fibrils may be remodeled by Hsp104. The estimated mean number of Sup35 molecules per cell 8 x 104.18 Based on biochemical evidence, the average Sup35 particle in [PSI+] cells exhibiting a strong suppression phenotype consists of 20 protomers.19 If nearly all the Sup35 was sequestered in such particles, we would expect there to be about 4000 oligomers/cell. However, using the rate of prion loss after the shut-off of Hsp104 with guanidinium hydrochloride, estimates of the number of functional [PSI+] propagons present in each cell go as high as 1000, about a third of which are transferred to the daughter cell.20 During the cell cycle each propagon needs to be severed once or twice to restore the population of propagons before the next bud is formed. For example, a daughter cell starting out with 300 propagons would need to create 700 new propagons via severing to maintain the mean population. Even using the larger estimate of particles, only 1–4% of Sup35 molecules need to be remodeled to restore the average size and number of particles.
In unstressed cells, the estimated number of Hsp104 protomers is 3 x 104 (about 5 x 103 hexamers). It is currently impossible to reliably estimate the length of time an Hsp104 hexamer might be occupied in each remodeling event, but at least with respect to Sup35, there is likely excess Hsp104-remodeling capacity in the cell compared with the number of severing events required for maintenance of a suitable population of propagons.
One possible explanation for why Hsp104 only sparingly fragments prions is that Sup35 and other priongenic proteins in yeast evade the surveillance of Hsp104 because they do not display hydrophobic peptide segments that are the signature characteristic of misfolded globular proteins. In ongoing experiments we have found that the aromatic residues in the model Hsp104-binding peptide p370 are crucial for its ability to bind to Hsp104 and regulate its activity (JRG, M. Michalowska, R. Lum, unpublished). Although aromatic residues are not sole determinant of Hsp104-binding, the M-domain (amino acids 124–253) is notably free of them (with the exception of F129).
Disordered aggregates and amyloid fibrils likely differ with respect to other physical properties as well. The resistance to extraction of a protein sequestered within an aggregate will be roughly the sum of all the intermolecular interactions in which it participates. In amyloids, this could be the sum of all interstrand hydrogen bonds. Depending on the length of the strands participating in β-sheet formation, the mechanical energy required for Hsp104 to liberate even a single strand from some amyloid oligomers could be substantial. In contrast, aggregates induced by thermal denaturation are thought to assemble primarily through the association of hydrophobic residues that are solvent-exposed on the surface of misfolded proteins. The non-specific side chain packing in these associations likely precludes the complete exclusion of water molecules from hydrophobic interfaces, making the interactions inherently weak. Compared with disintegrating β-strands from an amyloid, one-by-one extraction of polypeptide segments from a disordered aggregate might require less mechanical force. Thus, it is possible that Hsp104 remodeling of Sup35 protomers is initiated frequently but only occasionally does this lead to particle severing.
The difference in physical stability between disordered aggregates and amyloid fibrils could explain Hsp104 variants that are capable of providing thermotolerance in yeast but are unable to propagate prions.21 This discrepancy has been attributed to the possibility that Hsp104 remodels prions and thermally induced aggregates by distinct mechanisms perhaps involving alternate threading pathways. However, it seems more likely that many amino acid substitutions simply attenuate some aspect of Hsp104 function such as coupling of ATPase hydrolysis to substrate unfolding. Some variants of Hsp104 could generate sufficient mechanical force to remodel less challenging disordered aggregates, yet fall below a threshold required to remodel proteins integrated into amyloid fibrils.
The idea that overexpressed Hsp104 completely solubilizes Sup35 amyloid tallies well with in vitro experiments in which NM fibrils are totally dissolved by Hsp104 working alone.14 However, we and others22 have been unable to detect robust disassembly of preformed Sup35 fibrils in vitro. Furthermore, SDS-resistant particle size analysis indicates that during Hsp104 overexpression19 and following induction of Hsp104 by heat shock,23 prion particles actually enlarge rather than diminish in size. Given that Hsp104 only intermittently severs prions during propagation and that tight integration of Sup35 protomers into amyloid fibrils make them resistant to extraction, prompted us to re-evaluate the model of Hsp104-mediated propagation and curing presented in Figure 2.
How could the remodeling activity of Hsp104 lead to particle severing and particle growth? In the light of the idea that prions are relatively poor substrates for Hsp104, we speculate that Hsp104 is capable of only partially remodeling Sup35 protomers integrated into amyloid fibrils. Hsp104-mediated extraction of only a single β-strand would result in the formation of a less stable intermediate particle with a weakened amyloid core (Fig. 3). After release from Hsp104, some of these destabilized intermediates may revert by reintegration of the liberated segment into the fibril while some fibrils may fall apart spontaneously, the end product being two smaller particles. When Hsp104 is overexpressed the abundance of such intermediates would be enhanced increasing the possibility that strand-swapping between amyloid fibrils could occur. Such bundled structures would have the same stability (SDS-resistance for example) as unbundled amyloids. Ultimately, assembly of larger particles would deplete the number of functional propagons and those remaining may be too large to be transmitted to daughter cells efficiently. Importantly the same destabilized intermediate formed by the action of Hsp104 is on-pathway for both propagation and curing.
Figure 3. Alternative hypothesis for propagation and curing by partial remodeling of Sup35. Hsp104 infrequently extracts a β-strand from the amyoid core that leads to the formation of an unstable intermediate that can spontaneously revert or fall apart. When Hsp104 is overexpressed, the accumulation of partially remodeled intermediates is enhanced increasing the probability of strand swapping between amyloid fibrils to promote particle growth. Factors that stabilize the intermediate will favor severing and antagonize curing (see text).
This modified model permits reinterpretation of other experimental observations. Molecular chaperones other than Hsp104 are known to modulate both propagation and curing of of [PSI+]. If Hsp70 (Ssa1, Ssa2, Ssa3, and Ssa4 in yeast) were to bind to the strand released by Hsp104, the reverse reaction— reintegration of the liberated strand into its original position in the amyloid— would be delayed, thereby favoring the formation of completely severed particles. With Hsp104 overexpression, simultaneous overexpression of Hsp70 would antagonize the formation of strand-swapped oligomers and inhibit bundling of fibrils and curing of [PSI+]. Indeed, Ssa1 has been implicated both in assisting [PSI+] propagation24,25 and antagonizing Hsp104-mediated curing.26,27 The ability of Ssa1 to transiently bind a segment of Sup35 involved in β-strand formation could also explain why Ssa1 inhibits Sup35 assembly into fibrils in vitro.1,22,28
Incomplete extraction of Sup35 protomers could also result in Hsp104 becoming stalled with partially processed substrates stuck in the axial channel. However, Hsp104, like its bacterial ortholog ClpB, may release partially threaded substrates when it encounters an intractably stably-folded domain.29 The model we suggest predicts that Hsp104 variants with impaired release of incompletely processed substrates would show a reduced requirement for Hsp70 in propagation and fail to efficiently cure [PSI+] by favoring severing over strand swapping. Hsp104 with mutations in the N-terminal domain (NTD) has such properties.30 As polypeptides destined for unfolding enter the axial channel of Hsp104 through the pore formed by the first AAA+ domain (see Fig. 1), the NTD is well situated to detect the presence of protein domains that are resistant to unfolding during Hsp104-mediated extraction.
Concluding Remarks
Our work1 has demonstrated that by attenuating the interaction with Hsp104 it is possible to inhibit the ability of the [PSI+] prion to be cured by Hsp104 and reduce the efficiency with [PSI+] oligomers can be severed and form new propagons. We speculate that most yeast prions do not display peptide segments that make them good targets for aggressive remodeling by Hsp104, even during Hsp104 overexpression. Indeed, many yeast proteins that are capable of undergoing amyloid assembly may for oligomers that are severed so infrequently that it is not possible to establish a self-sustaining heritable population of seeds. Furthermore, other amyloid-forming proteins that by chance display more ideal Hsp104 binding sites, or whose components are integrated into amyloids with relatively lower stability, will be routinely solubilized in the early stages of aggregation.
Ever since the convergence of Hsp104-mediated disaggregation and prion remodeling, there has been much speculation about whether Hsp104 could be used to modulate aggregation associated with human disease.31 Understanding the molecular basis of the interaction between Hsp104 and yeast prions can play an important role in predicting the promise and limitations of chaperone-mediated protein disaggregation as a therapeutic approach to diseases of protein aggregation. The effectiveness of Hsp104 deployed in specific instances will depend the degree to which target proteins display sites that could be efficiently engaged by Hsp104 and other molecular chaperones, whether the physical stability of target aggregates is low enough to render them susceptible to action of Hsp104, and ultimately whether the remodeled products— soluble, potentially toxic monomers or small aggregates that could exacerbate rather than alleviate the propagation of misfolding— will benefit or further jeopardize the survival of cells.
Funding
This work is funded by Canadian Institutes for Health Research (operating grant MOP-86754).
Footnotes
Previously published online: www.landesbioscience.com/journals/prion/article/19913
References
- 1.Helsen CW, Glover JR. Insight into molecular basis of curing of [PSI+] prion by overexpression of 104-kDa heat shock protein (Hsp104) J Biol Chem. 2012;287:542–56. doi: 10.1074/jbc.M111.302869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–83. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–9. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 4.Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+] Science. 1995;268:880–4. doi: 10.1126/science.7754373. [DOI] [PubMed] [Google Scholar]
- 5.Parsell DA, Kowal AS, Singer MA, Lindquist S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature. 1994;372:475–8. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- 6.Patino MM, Liu JJ, Glover JR, Lindquist S. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science. 1996;273:622–6. doi: 10.1126/science.273.5275.622. [DOI] [PubMed] [Google Scholar]
- 7.Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD. Propagation of the yeast prion-like [psi+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. EMBO J. 1996;15:3127–34. [PMC free article] [PubMed] [Google Scholar]
- 8.Glover JR, Kowal AS, Schirmer EC, Patino MM, Liu JJ, Lindquist S. Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell. 1997;89:811–9. doi: 10.1016/S0092-8674(00)80264-0. [DOI] [PubMed] [Google Scholar]
- 9.King CY, Tittmann P, Gross H, Gebert R, Aebi M, Wüthrich K. Prion-inducing domain 2-114 of yeast Sup35 protein transforms in vitro into amyloid-like filaments. Proc Natl Acad Sci U S A. 1997;94:6618–22. doi: 10.1073/pnas.94.13.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD. In vitro propagation of the prion-like state of yeast Sup35 protein. Science. 1997;277:381–3. doi: 10.1126/science.277.5324.381. [DOI] [PubMed] [Google Scholar]
- 11.Sparrer HE, Santoso A, Szoka FC, Jr., Weissman JS. Evidence for the prion hypothesis: induction of the yeast [PSI+] factor by in vitro- converted Sup35 protein. Science. 2000;289:595–9. doi: 10.1126/science.289.5479.595. [DOI] [PubMed] [Google Scholar]
- 12.Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/S0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- 13.Glover JR, Lum R. Remodeling of protein aggregates by Hsp104. Protein Pept Lett. 2009;16:587–97. doi: 10.2174/092986609788490087. [DOI] [PubMed] [Google Scholar]
- 14.Shorter J, Lindquist S. Hsp104 catalyzes formation and elimination of self-replicating Sup35 prion conformers. Science. 2004;304:1793–7. doi: 10.1126/science.1098007. [DOI] [PubMed] [Google Scholar]
- 15.Shorter J, Lindquist S. Destruction or potentiation of different prions catalyzed by similar Hsp104 remodeling activities. Mol Cell. 2006;23:425–38. doi: 10.1016/j.molcel.2006.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baxa U, Keller PW, Cheng N, Wall JS, Steven AC. In Sup35p filaments (the [PSI+] prion), the globular C-terminal domains are widely offset from the amyloid fibril backbone. Mol Microbiol. 2011;79:523–32. doi: 10.1111/j.1365-2958.2010.07466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lum R, Niggemann M, Glover JR. Peptide and protein binding in the axial channel of Hsp104. Insights into the mechanism of protein unfolding. J Biol Chem. 2008;283:30139–50. doi: 10.1074/jbc.M804849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, et al. Global analysis of protein expression in yeast. Nature. 2003;425:737–41. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 19.Kryndushkin DS, Alexandrov IM, Ter-Avanesyan MD, Kushnirov VV. Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J Biol Chem. 2003;278:49636–43. doi: 10.1074/jbc.M307996200. [DOI] [PubMed] [Google Scholar]
- 20.Byrne LJ, Cole DJ, Cox BS, Ridout MS, Morgan BJ, Tuite MF. The number and transmission of [PSI] prion seeds (Propagons) in the yeast Saccharomyces cerevisiae. PLoS One. 2009;4:e4670. doi: 10.1371/journal.pone.0004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurahashi H, Nakamura Y. Channel mutations in Hsp104 hexamer distinctively affect thermotolerance and prion-specific propagation. Mol Microbiol. 2007;63:1669–83. doi: 10.1111/j.1365-2958.2007.05629.x. [DOI] [PubMed] [Google Scholar]
- 22.Krzewska J, Melki R. Molecular chaperones and the assembly of the prion Sup35p, an in vitro study. EMBO J. 2006;25:822–33. doi: 10.1038/sj.emboj.7600985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newnam GP, Birchmore JL, Chernoff YO. Destabilization and recovery of a yeast prion after mild heat shock. J Mol Biol. 2011;408:432–48. doi: 10.1016/j.jmb.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones G, Song Y, Chung S, Masison DC. Propagation of Saccharomyces cerevisiae [PSI+] prion is impaired by factors that regulate Hsp70 substrate binding. Mol Cell Biol. 2004;24:3928–37. doi: 10.1128/MCB.24.9.3928-3937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones GW, Masison DC. Saccharomyces cerevisiae Hsp70 mutations affect [PSI+] prion propagation and cell growth differently and implicate Hsp40 and tetratricopeptide repeat cochaperones in impairment of [PSI+] Genetics. 2003;163:495–506. doi: 10.1093/genetics/163.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen KD, Wegrzyn RD, Chernova TA, Müller S, Newnam GP, Winslett PA, et al. Hsp70 chaperones as modulators of prion life cycle: novel effects of Ssa and Ssb on the Saccharomyces cerevisiae prion [PSI+] Genetics. 2005;169:1227–42. doi: 10.1534/genetics.104.037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newnam GP, Wegrzyn RD, Lindquist SL, Chernoff YO. Antagonistic interactions between yeast chaperones Hsp104 and Hsp70 in prion curing. Mol Cell Biol. 1999;19:1325–33. doi: 10.1128/mcb.19.2.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shorter J, Lindquist S. Hsp104, Hsp70 and Hsp40 interplay regulates formation, growth and elimination of Sup35 prions. EMBO J. 2008;27:2712–24. doi: 10.1038/emboj.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haslberger T, Zdanowicz A, Brand I, Kirstein J, Turgay K, Mogk A, et al. Protein disaggregation by the AAA+ chaperone ClpB involves partial threading of looped polypeptide segments. Nat Struct Mol Biol. 2008;15:641–50. doi: 10.1038/nsmb.1425. [DOI] [PubMed] [Google Scholar]
- 30.Hung GC, Masison DC. N-terminal domain of yeast Hsp104 chaperone is dispensable for thermotolerance and prion propagation but necessary for curing prions by Hsp104 overexpression. Genetics. 2006;173:611–20. doi: 10.1534/genetics.106.056820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vashist S, Cushman M, Shorter J. Applying Hsp104 to protein-misfolding disorders. Biochem Cell Biol. 2010;88:1–13. doi: 10.1139/O09-121. [DOI] [PMC free article] [PubMed] [Google Scholar]