Abstract
Ovine scrapie and cervid chronic wasting disease can be transmitted in the absence of animal-to-animal contact, and environmental reservoirs of infectivity have been implicated in their spread and persistence. Investigating environmental factors that influence the interaction of disease-associated PrP with soils is imperative to understanding what is likely to be the complex role of soil in disease transmission. Here, we describe the effects of soil temperature on the binding/desorption and persistence of both ovine scrapie- and bovine BSE-PrPTSE. Binding of PrPTSE to a sandy loam soil at temperatures of 4°C, 8–12°C and 25–30°C demonstrated that an increase in temperature resulted in (1) a decrease in the amount of PrPTSE recovered after 24 h of interaction with soil, (2) an increase in the amount of N-terminal cleavage of the prion protein over 11 d and (3) a decrease in the persistence of PrPTSE on soil over an 18 mo period.
Keywords: BSE, environment, prion, scrapie, soil, transmission
Introduction
Transmissible spongiform encephalopathies (TSEs or prion diseases) are incurable, degenerative neurological disorders that affect both humans and animal species. Prion diseases include Creutzfeldt-Jakob disease (CJD) in humans, bovine spongiform encephalopathy (BSE) in cattle, scrapie in sheep/goats, and chronic wasting disease in deer/elk. The central event in these diseases is the conversion of the cellular prion protein (PrPC) into a disease-associated conformation known as PrPTSE.1 PrPTSE accumulates in affected individuals, particularly within the central nervous system, over long, asymptomatic incubation periods. PrPTSE is the only validated marker for prion diseases and is the likely disease agent.
Most prion diseases have a very limited natural host range with the notable exception of BSE. This disease was first recorded in cattle in the mid-1980s and has subsequently been established as the causative agent for variant CJD (vCJD) in humans, feline spongiform encephalopathy, and BSE in goats; with disease being caused by ingestion of contaminated foodstuff.2
Scrapie in small ruminants and cervid CWD are the only prion diseases that are known to readily undergo horizontal transmission, leading to endemic infections within susceptible populations.3,4 It has been shown that horizontal transmission is not only facilitated by animal-to-animal contact but also involves environmental reservoirs of infectivity.5,6 The PrPTSE agent is shed from scrapie and/or CWD infected animals via milk, saliva, blood, urine, faeces, skin and placental tissue.7-13 In addition, prion could enter the environment via the deposition of infected wild animal carcasses or the burial of farmed animals. The prion agent is highly resistant to enzymatic and chemical degradation, and can persist within the environment, including the soil, for years.14,15 The location of environmental sources of prion is still not clear; however PrPTSE has been reported on a range of farm surfaces and within a water sample.16,17 It is also proposed that the TSE agent persists bound to soil particles.18 Within an experimental setting, infectivity and/or PrPTSE have been shown to bind rapidly, and mostly irreversibly, to a range of soils with distinct textures and mineral compositions.18-20 The detailed understanding of how prions interact with soil is of key importance in understanding the risks involved in scrapie/CWD transmission in farmed and wild-life populations. The interaction of prions with soils is dictated by not only the soil type but also the prion strain/host20,21 indicating that studies detailing the interaction of prions with soil should ideally be performed using environmentally relevant prion sources; that is ovine scrapie, bovine BSE and/or cervid CWD. Here, we examine the interaction of bovine BSE and ovine scrapie with a complex soil matrix and demonstrate that temperature has an effect on the interaction of PrPSc with soil.
Results
Effect of temperature on prion interaction with soil
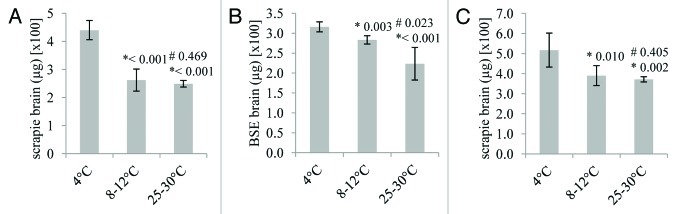
The soil used within the present study was classified as a sandy loam. Distinct temperatures were used which represented the seasonal variation seen within UK soils, 25–30°C, 12–18°C and 4°C. After 24 h, the desorption of full-length scrapie PrPTSE from soil at 4°C was significantly higher than that at 12–18°C and 25–30°C (Fig. 1A). The same trend was observed when analyzing soil columns spiked with full length BSE PrPTSE (Fig. 1B). No PrPTSE spike could be detected in soil washes after 24 h incubation under the temperatures tested (data not shown) demonstrating that over 99% of the PrPTSE spike binds to soil, as previously reported.20 This indicates that temperature does not affect the adsorption of PrPTSE to soil after 24 h interaction. Extraction of PrPTSE by boiling in SDS resulted in approximately 20 to 26% of the protease-resistant PrPTSE spike being recovered from the soil indicating that the remainder was irreversibly bound and/or degraded.
Figure 1. The effect of temperature on the desorption of ruminant PrPTSE bound to a sandy loam soil for up to 4 d. Scrapie (A and C) and BSE (B) prions were analyzed. PrPTSE was bound to soil either at 25–30°C, 8–12°C or 4°C as indicated. For individual soil columns, thermolysin-resistant prion was extracted in triplicate and the eluates from 100 mg of soil analyzed, each in duplicate, either by ELISA detecting full length PrPTSE (A and B) or by ELISA against an epitope within the 'core' of PrP and therefore measuring both full length and truncated PrPTSE (C). Standard deviations of the six replicate analyses of each column are shown. Each set of six replicates was compared by an unpaired, two-tailed Student's t-test. *p values derived by comparison of each data set to the data for prion recovery at 4°C; #p values derived by comparison of each data set to the data for prion recovery at 8–12°C.
These experiments investigated the recovery of full length PrPTSE by thermolysin digestion of prion to remove PrPC and detection of full length PrPTSE using a combination of antibodies specific for the N-terminus and 'core' of the protein. Scrapie samples from the soil columns kept at distinct temperatures were also analyzed by thermolysin-digestion and detection of PrPTSE using a commercial ELISA that recognizes only the 'core' of the protein. This test therefore detects both full length and N-terminally truncated species of PrPTSE (Fig. 1C). Temperature affected total PrPTSE recovery; soil columns kept at 4°C yielded more PrPTSE than soil columns at the higher temperatures.
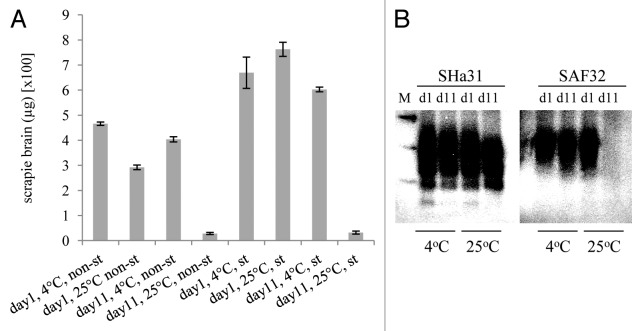
To further examine the effects of temperature on the binding and desorption of PrPTSE to soil, the recoveries of scrapie-PrPTSE were determined after batch binding to soil and 1 or 11 d of incubation at 4°C or 25°C. Again, after incubation for 24 h the levels of recoverable PrPTSE were significantly greater at the lower temperature (p < 0.001, unpaired 2-tailed t-test, n = 3 for each data set). In addition, after 11 d it was clear that the vast majority of full length PrPTSE (92% +/− 1.6) became unrecoverable from soil at the higher temperature but not at the lower temperature although, even at the lower temperature, there was a significant ‘loss' of full length prion over the 10 d period (p < 0.001, unpaired 2-tailed t-test, n = 3 for each data set). This experiment was repeated for sterile soil (after autoclaving) and produced distinct results (Fig. 2A): at day 1 the temperature did not have a significant effect on the recovery of PrPTSE, with the lower temperature yielding less recoverable PrPTSE (p = 0.08, unpaired 2-tailed t-test, n = 3 for each data set). In addition, the recovery of prion was higher from sterile soil compared with non-sterile soil. However, the dramatic drop in the recovery of full-length prion after 11 d incubation at an elevated temperature was not affected by autoclaving of the soil. When prion was analyzed by western blotting it was clear that the large drop in the recovery of full length PrPTSE between days 1 and 11 is due primarily to a “loss” of the N-terminus of the protein while the 'core' of the protein is still recovered at relatively high levels after 11 d incubation on soil at 25°C (Fig. 2B). Brain homogenate incubated at 4°C for 1 or 11 d did not yield significantly higher levels of full length PrPTSE than when incubated for the same periods at 25°C, indicating that endogenous protease activity does not account for the observed effects of temperature on prion-soil interaction (data not shown).
Figure 2. The effect of temperature on the desorption of scrapie PrPTSE bound to a sandy loam soil for 1 or 11 d. (A): PrPTSE was bound at 25°C or 4°C to soil that was either non-sterile (non-st) or sterile (st; autoclaved), as indicated. On day 1 and 11 thermolysin-resistant prion was extracted and the eluates from 100 mg of soil analyzed, each in triplicate, by ELISA detecting full length PrPTSE. Standard deviations of the three replicate analyses of each experimental condition are shown. The experiment was repeated twice more and gave equivalent results. (B) Representative samples were analyzed by western blot (10µl per lane, equivalent of an extract from 100 mg of soil), prion was detected with monoclonal antibody SHa31 (detecting a 'core' epitope) or SAF32 (detecting an N-terminal epitope) as indicated, and molecular mass markers are shown (M; 20, 30 and 40kDa). Both non-autoclaved and autoclaved samples gave equivalent results.
Overall, the data indicate that the efficiency of recovery of PrPTSE from soil increases with a decrease in soil temperature only for non-sterile soils and that desorption of PrPTSE from soils is more efficient following autoclaving. In addition, between 1 and 11 d incubation, the persistence of recoverable, full length PrPTSE on soil is dramatically affected by temperature, with the N-terminus of the prion being truncated far more at higher temperatures, irrespective of microbial activity within the soil.
Effect of temperature on prion persistence on soil over 18 mo
The effect of temperature on the persistence of PrPTSE on soil over prolonged incubation periods was investigated. Multiple soil columns were kept at three distinct temperatures: 25–30°C, 8–12°C and 4°C; soil columns kept at 4°C were frozen and thawed once a month to represent winter freezing events. Individual columns kept under each treatment regime were sampled periodically throughout an 18-mo timeframe and recovered PrPTSE analyzed by ELISA to determine the persistence of full length PrPTSE (Fig. 3A-D), or ELISA and/or western blot to determine the persistence of total PrPTSE (Figs. 3E-F and 4). Soil columns spiked with scrapie- and BSE-PrPTSE gave equivalent results. For ELISA analysis, in order to determine the persistence of PrPTSE under different conditions, recoveries at later time points were expressed as a percentage of those at days 1–4, thereby adjusting recoveries at these time-points for the effects of the different temperatures on the efficiency of desorption of the prion from the soil. The recovery and persistence of PrPTSE decreased during the 18-mo incubation period for all temperatures. Data demonstrated that at every time-point the recovery and persistence of full length scrapie PrPTSE decreased systematically as temperature increased from 4°C to 8–12°C to 25–30°C and that these differences in prion persistence and recovery were significant at each time point (Fig. 3A, B). This effect of temperature on the persistence and recovery of PrPTSE was also seen for full length BSE-PrPTSE (Fig. 3C, D). In addition, the same trend was also observed when determining the persistence of total PrPTSE (full length and truncated PrPTSE) for both scrapie and BSE prions (Figs. 3E, F and 4).
Figure 3. The effect of temperature on the desorption and persistence of PrPTSE on a sandy loam soil over 18 mo. Scrapie (A, B, E, F) or BSE (C, D) prion was incubated on soil at distinct temperatures as indicated. Samples were taken for analysis at 1–4 d, day 188, 411 and 566. Thermolysin-resistant prion was extracted in triplicate (A-D) or singles (E,F) and the eluates from 100 mg of soil analyzed by ELISA, each in triplicate (A-D) or singles (E,F). Analyses detected full length PrPTSE (A-D) or both full length and truncated conformers (E,F). In order to determine the persistence of PrPTSE under different conditions, recoveries at later time points are expressed as a percentage of that extracted at the earliest time point (1- 4 d); therefore adjusting recoveries at these time-points for the effects of temperature on the efficiency of desorption of the prion from the soil (A, C, E). The actual recoveries of PrPTSE for each time-point under the distinct temperatures are also shown (B, D, F). Standard deviations of the nine replicate analyses of each column are shown (A-D). Each set of nine replicates were compared by an unpaired, two-tailed Student's t-test: comparison of each data set to the recovery of PrPTSE at 4°C (* indicates p values < 0.001 or the value is given), or comparison of each data set to PrPTSE recovery at 8–12°C (# indicates p values < 0.001 or the value is given).
Figure 4. The effect of temperature on the desorption and persistence of PrPTSE on a sandy loam soil over 18 mo. Scrapie or BSE (as indicated) prion was incubated on soil and samples were analyzed at days1–4, 49, 188, 411 and 566 (Lanes 1 to 5, respectively). Thermolysin-resistant prion was extracted and 10µl analyzed (equivalent of an extract from 100 mg of soil) by western blot. Prion was detected with monoclonal antibody SHa31 (detecting a 'core' epitope) and molecular mass markers are shown (M; 20, 30 and 40kDa).
Discussion
Scrapie in sheep and CWD in deer appear to be transmissible via animal-to-animal contact as well as via environmental reservoirs of infectivity,5,6,16 with soil being proposed as one such potential reservoir. Previous studies have indicated that prions bind rapidly, and largely irreversibly to soils, do not migrate through soil columns and can persist for years.20,22,23 Furthermore, the interaction of prions with soil does not remove infectivity.24,25 This interaction of prions with soil is influenced not only by the soil type but also by the prion source.20,21
Here, we determine the effects of temperature on the binding of bovine BSE and ovine scrapie to a complex soil matrix. PrPTSE binding and desorption from soil at a low temperature (4°C) correlated with increased prion desorption after interaction for up to 96 h. This was consistent for two distinct prion strains, BSE and scrapie, when measuring either full length PrPTSE or total PrPTSE. There are no reports in the literature on the effects of temperature on PrPTSE interaction with soils. However, Bai and coworkers have examined the effects of temperature on the degradation kinetics of Bt toxin, which binds rapidly to soil particles and retains its biological activity. Their study concluded that as temperatures increased from 15 to 35°C the degradation of the protein increased and the authors speculated that this effect is likely to be associated with an increase in microbial degradation of the protein.26
With regard to the persistence of PrPTSE on soil, higher temperatures (25–30°C and 8–12°C) reduced the amount of recoverable prion at all time points over an 18-mo period (compared with that at 4°C). In addition, these higher temperatures also resulted in accelerated N-terminal cleavage of the prion upon binding/desorption between days 1 and 11 of soil-prion interaction, with almost all of the N-terminus removed after 11 d.
The loss of the N-terminus of PrPTSE over 11 d incubation on sandy-loam soil at 25°C was observed not only with non-sterile soils but also with soil which had been autoclaved. The introduction of proteins into a soil matrix can result in their degradation via several mechanisms including microbial digestion or abiotic degradation.27,28 Autoclaving is known to kill microbes and reduce protease activity.29 The data presented here indicate that the temperature-dependent cleavage of the N-terminal region of PrPTSE that is observed within the first 11 d of incubation is due to abiotic rather than microbial degradation. However, autoclaving soil did result in an increased recovery of PrPTSE at both temperatures indicating microbial activity reduced prion recovery. Furthermore, at day 1, while non-sterile soil displayed a decrease in PrPTSE recovery at the higher temperatures, this effect was not seen with autoclaved soil. For non-sterile soils, microbial activity will be relatively elevated at the higher temperatures used in the study, again indicating that microbial activity reduced PrPTSE recovery. Microbial activity may exert this effect possibly by proteolytic degradation of the PrPTSE.
It has been previously reported that the N-terminal region of the prion protein influences its binding to soil particles. It has been suggested that the presence of this region may increase adsorption to clay-rich soils but decrease adsorption to sand-rich soils.21 In addition, the N-terminal region is cleaved during adsorption/desorption in a range of clay-rich soils and clay minerals18,20,30,31 and when using distinct chemical desorption conditions.31 The interaction of proteins with soils will be influenced by electrostatic attraction and repulsion, van der Waals forces and hydrophobic interactions. Both the N-terminal and 'core' regions of the PrP protein will be involved in electrostatic interaction with soil particles as they both contain numerous charged amino acid residues. In addition, the PrPTSE conformation is highly aggregated and insoluble and as such will promote hydrophobic interactions. Indeed, previous studies have shown that prion strain can influence PrPTSE-soil interaction indicating that the conformation and/or aggregation state of PrPTSE may contributed to these complex molecular interactions.21
Here, there were high levels of abiotic truncation of the N-terminal region of PrPTSE after 11 d interaction with sandy loam soil, but only at the higher temperatures tested. Synthetic peptides of sequences within the N-terminal region of PrP have previously been shown to possess conformations that are influenced by changes in temperature within the range studied here.32 It is possible that temperature-induced changes in the conformation of the N-terminal region of PrPTSE may influence its interaction with soil, with the higher temperatures producing conformations that are more prone to abiotic truncation upon binding/desorption.
In the present study we investigated the effects of soil temperature in the range 4°C to 30°C on the interaction of BSE- and scrapie-PrPTSE with a complex soil matrix. Lower soil temperatures resulted in increased levels of PrPTSE recovery and persistence over an 18-mo incubation period. A low soil temperature also resulted in less cleavage of the N-terminal domain of PrPTSE after an 11-d interaction. These effects of temperature on PrPTSE-soil interaction were likely to be exerted through both microbial activity and abiotic cleavage mechanisms. Together, the data indicate that for the recoverable fraction of PrPTSE, soils at lower temperature may release increased levels of PrPTSE.
A recent study using transmissible mink encephalopathy reported a correlation between the level of desorption of PrPTSE from soil and the infectivity titer of the sample.25 If such a correlation is also true for ovine scrapie and bovine BSE, the data presented here indicate that the bioavailability of prions in soil for the environmental transmission of scrapie or BSE may be influenced by the temperature of the soil. However, it remains to be seen whether the reported influence of temperature on prion interaction with a sandy-loam soil is consistent with other soil types. Of course, it should also be considered that temperature would be just one of a range of factors influencing the bioavailability of prions from soil; other factors would likely include soil type, prion strain and the biological matrix of the prion source. Scrapie and CWD are known to be spread by environmental routes and therefore understanding the range of factors that influence the persistence of environmental prions is vital in developing eradication programmes.
Materials and Methods
Samples
All TSE and healthy brain material was obtained from the Animal Health Veterinary Laboratories Agency TSE-archive (AHVLA, Addlestone, Surrey, UK). Samples were pools of hind brain from BSE infected cattle (n = 10) and BSE-free controls (n = 10), scrapie infected sheep (n = 9) and genotype matched scrapie-free controls (n = 20). Samples were homogenized in deionised water containing 0.5% (w/v) sodium deoxycholate and 0.5% (v/v) Nonidet NP40 as previously described.33 The use of detergent is a standard methodology to release cell-associated PrPTSE and it is known that the presence of detergents does not prevent PrPTSE adsorption to soil particles.20 While any effects of the detergents on prion-soil interactions have not been defined, previous data using this exact methodology demonstrated strong correlations with data from numerous studies that had used prion-infected brain homogenates within PBS, or partially purified PrPTSE.20
Soil columns
This soil was sampled from 53° 13′26.55˝N 001°07′00.59˝W (Nottinghamshire, UK). The soil was classified as sandy loam, it had sand, silt and clay contents of 62, 27 and 11% respectively, a porosity of 45%, a pH of 5.9, an organic matter content of 3% and a soil respiration rate of 2.3 mg CO2-C per kg per day. The principal physical, chemical and biological characteristics of this soil have been reported previously.20
The soil was packed into 11cm x 1.3cm (internal diameter) columns at a bulk density of 1.26 g/cm3 dry weight as previously described.21 Soil was packed at the moisture content found within the field (13% water per dry weight of soil). PrPTSE-positive brain homogenate from either BSE or scrapie affected ruminants was applied to the soil surface at 97 µl 20% (w/v) homogenate g−1 soil. Soil columns were maintained within temperature controlled illuminated cabinets (Snijders Scientific) and watered from the top every 7–14 d to replace measured evaporative losses. When determining the effects of temperature on PrPTSE interaction with soil, columns were kept at either 25–30°C, 8–12°C, or 4°C. During analysis of PrPTSE persistence over an 18-mo time-period, the columns incubated at 4°C were subjected to a freeze-thaw cycle once every month. The incubator was set to -5°C and left for 16 h to equilibrate soils to this temperature, after this time period the settings were returned to 4°C. The air temperature changed from 4°C to -5°C at an average rate of 0.27°C/min, and increased from -5°C to -4°C at an average rate of 0.41°C/min. Soil columns were sampled at various time-points throughout an 18-mo period. Sampling involved removing all soil from a column and homogenizing the sample by mixing manually for up to 10 min. PrPTSE was isolated from whole soil using a modification of the method described by Johnson et al.18 This involved resuspending 0.5 g soil as a 20% (w/v) slurry in 5mM calcium chloride for 5 min. Supernatant (soil wash) was removed following centrifugation of the slurry for 10 min at 800 g, and the pellet fraction was resuspended for 1 h in 5 mM calcium chloride prior to being overlaid on a 750 mM sucrose cushion (10 ml). After centrifugation as above the pellet was resuspended in 250 µl of 100 µg/ml thermolysin and digested for 1 h at 70°C. Samples were boiled for 10 min after the addition of an equal volume of 20% (w/v) SDS in 5 mM calcium chloride. Supernatant was collected following centrifugation as above and PrPTSE was precipitated by the addition of 5 volumes of methanol and incubation at -20°C for 16 h. PrPTSE was recovered by centrifuging at 12,100 g for 30 min, washed with cold methanol and air-dried. For western blot analysis, extracts from 0.5 g of soil were resuspended in 50 µl NuPage 2x LDS sample buffer containing 5% β-mercaptoethanol. For ELISA analysis, soil extracts were resuspended by boiling in 100 µl PBS containing 4% (w/v) SDS. This method digests all PrPC as determined in parallel experiments using brain homogenate from healthy animals (data not shown). In addition, in the absence of soil no PrPTSE was recovered using the method described, indicating that only soil-associated PrPTSE is being measured within the experiments. All experiments with columns spiked with BSE or scrapie and kept at distinct temperatures for 1 to 4 d were performed twice and gave comparable results. Experiments measuring scrapie or BSE recovery from soil at later time-points were performed once each.
Batch binding of prions to soils
The effects of distinct temperatures on the interaction of PrPTSE with soil were tested in batch binding experiments. Soil was kept at either 25°C or 4°C. Scrapie brain homogenate spike was applied to 1g of soil within 7 ml tubes at 100 µl 20% (w/v) homogenate g−1 soil and incubated static for 1 or 11 d. PrPTSE was isolated from whole soil as described for soil columns with the following modifications: all centrifugations were performed at 50 g rather than 800 g and the sample was passed twice through a sucrose cushion. A parallel experiment was performed using soil which had been autoclaved prior to the experiment at 121°C for 15 min. By measuring ATP levels it was confirmed that the autoclaving of soil removed any microbial activity for the duration of the experiment (data not shown). Both experiments were repeated twice more and gave equivalent results.
Detection of PrPTSE by western blotting and ELISA
Samples were analyzed by western blot as previously described,33 PrP was detected with monoclonal antibodies SHa3134 and horseradish peroxidase conjugated mouse-specific secondary antibodies (Dako), and EZ-ECL substrate (Biological Industries).
ELISA detection of full length PrPTSE: Nunc Maxisorp plates were coated with anti-PrP antibody SAF34 (1:8000) diluted in PBS for 16 h then blocked for 1 h with 0.5% (w/v) ovalbumin in PBS supplemented with 0.05% (v/v) Tween20 (PBST). Soil extracts (60µl) digested with thermolysin were added to 540µl PBST and 200µl applied in triplicate to the SAF34 coated wells for 1 h. After washing 5 times with 400µl PBST, 100µl of anti-PrP antibody SHa31 (1:8,000) in PBST was added to the plate for 1 h and washed as previously. SHa31 was specifically detected with rabbit anti-mouse IgG1-specific alkaline phosphatase conjugated secondary antibody diluted 1:2000 (Invitrogen). Signals were detected at 405nm using PNPP substrate. PrPTSE recovery from soil was quantified by comparison of signals obtained against a standard curve of brain homogenate spiked into a soil extract obtained by processing unspiked soil, digested with thermolysin, methanol precipitated and resuspended in buffer.
ELISA detection of total PrPTSE (N-terminally truncated and full length PrPTSE): soil extracts (60µl) digested with thermolysin were added to 540µl buffer R6 and 200µl applied in triplicate to the Bio-Rad TeSeE® Sheep/Goat ELISA. PrPTSE was detected following the manufacturer's instructions.
Statistical analyses
PrPTSE levels recovered from individual soil samples kept under distinct temperatures were compared by Student's t-test as unpaired data sets. In addition, the recoveries of prion over a time course of 18 mo under distinct temperatures were expressed as percentages of the recoveries at the start of the experiment (days 1–4); these recoveries at each time-point were again compared between individual soil columns by Student's t-test as unpaired data sets. The null hypothesis tested was that soil columns kept under distinct temperatures would yield the same levels of PrPTSE.
Acknowledgments
We thank the TSE Archive at the AHVLA (Addlestone, Surrey, UK) for the provision of ruminant brain material. This work was funded by the Department for Environment, Food and Rural Affairs, UK (Defra project SE1858).
Glossary
Abbreviations:
- TSE
transmissible spongiform encephalopathy
- BSE
bovine spongiform encephalopathy
- CJD
Creutzfeldt-Jakob disease
- PrP
prion protein
- PrPC
cellular prion protein
- PrPTSE
disease-associated prion protein
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/prion/article/20025
References
- 1.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–83. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature. 1996;383:685–90. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 3.Truscott JE, Ferguson NM. Transmission dynamics and mechanisms of endemicity of scrapie in the UK sheep population. Epidemiol Infect. 2009;137:762–74. doi: 10.1017/S0950268808001052. [DOI] [PubMed] [Google Scholar]
- 4.Kahn S, Dubé C, Bates L, Balachandran A. Chronic wasting disease in Canada: Part 1. Can Vet J. 2004;45:397–404. [PMC free article] [PubMed] [Google Scholar]
- 5.Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerg Infect Dis. 2004;10:1003–6. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, et al. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One. 2009;4:e5916. doi: 10.1371/journal.pone.0005916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddison BC, Baker C, Rees HC, Terry LA, Thorne L, Bellworthy SJ, et al. Prions are secreted in milk from clinically normal scrapie-exposed sheep. J Virol. 2009;83:8293–6. doi: 10.1128/JVI.00051-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maddison BC, Rees HC, Baker CA, Taema M, Bellworthy SJ, Thorne L, et al. Prions are secreted into the oral cavity in sheep with preclinical scrapie. J Infect Dis. 2010;201:1672–6. doi: 10.1086/652457. [DOI] [PubMed] [Google Scholar]
- 9.Hunter N, Foster J, Chong A, McCutcheon S, Parnham D, Eaton S, et al. Transmission of prion diseases by blood transfusion. J Gen Virol. 2002;83:2897–905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- 10.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One. 2009;4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terry LA, Howells L, Bishop K, Baker CA, Everest S, Thorne L, et al. Detection of prions in the faeces of sheep naturally infected with classical scrapie. Vet Res. 2011;42:65. doi: 10.1186/1297-9716-42-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomzig A, Schulz-Schaeffer W, Wrede A, Wemheuer W, Brenig B, Kratzel C, et al. Accumulation of pathological prion protein PrPSc in the skin of animals with experimental and natural scrapie. PLoS Pathog. 2007;3:e66. doi: 10.1371/journal.ppat.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andréoletti O, Lacroux C, Chabert A, Monnereau L, Tabouret G, Lantier F, et al. PrP(Sc) accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J Gen Virol. 2002;83:2607–16. doi: 10.1099/0022-1317-83-10-2607. [DOI] [PubMed] [Google Scholar]
- 14.Georgsson G, Sigurdarson S, Brown P. Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J Gen Virol. 2006;87:3737–40. doi: 10.1099/vir.0.82011-0. [DOI] [PubMed] [Google Scholar]
- 15.Brown P, Gajdusek DC. Survival of scrapie virus after 3 years’ interment. Lancet. 1991;337:269–70. doi: 10.1016/0140-6736(91)90873-N. [DOI] [PubMed] [Google Scholar]
- 16.Maddison BC, Baker CA, Terry LA, Bellworthy SJ, Thorne L, Rees HC, et al. Environmental sources of scrapie prions. J Virol. 2010;84:11560–2. doi: 10.1128/JVI.01133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols TA, Pulford B, Wyckoff AC, Meyerett C, Michel B, Gertig K, et al. Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion. 2009;3:171–83. doi: 10.4161/pri.3.3.9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Prions adhere to soil minerals and remain infectious. PLoS Pathog. 2006;2:e32. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leita L, Fornasier F, De Noblili M, Bertoli A, Genovesi S, Sequi P. Interactions of prion proteins with soil. Soil Biol Biochem. 2006;38:1638–44. doi: 10.1016/j.soilbio.2005.11.018. [DOI] [Google Scholar]
- 20.Maddison BC, Owen JP, Bishop K, Shaw G, Rees HC, Gough KC. The interaction of ruminant PrP(Sc) with soils is influenced by prion source and soil type. Environ Sci Technol. 2010;44:8503–8. doi: 10.1021/es101591a. [DOI] [PubMed] [Google Scholar]
- 21.Saunders SE, Bartz JC, Bartelt-Hunt SL. Influence of prion strain on prion protein adsorption to soil in a competitive matrix. Environ Sci Technol. 2009;43:5242–8. doi: 10.1021/es900502f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson KH, Lee S, McKenzie D, Benson CH, Pedersen JA. Transport of the pathogenic prion protein through landfill materials. Environ Sci Technol. 2009;43:2022–8. doi: 10.1021/es802632d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson KH, Lee S, Somerville RA, McKenzie D, Benson CH, Pedersen JA. Transport of the pathogenic prion protein through soils. J Environ Qual. 2010;39:1145–52. doi: 10.2134/jeq2009.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog. 2007;3:e93. doi: 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders SE, Shikiya RA, Langenfeld K, Bartelt-Hunt SL, Bartz JC. Replication efficiency of soil-bound prions varies with soil type. J Virol. 2011;85:5476–82. doi: 10.1128/JVI.00282-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai Y-y, Jiang M-x, Cheng J-a. Impacts of environmental factors on degradation of Cry1Ab insecticidal protein in leaf blade powders of transgenic Bt rice. Agric Sci China. 2007;6:167–74. doi: 10.1016/S1671-2927(07)60031-5. [DOI] [Google Scholar]
- 27.Saunders SE, Bartelt-Hunt SL, Bartz JC. Prions in the environment: occurrence, fate and mitigation. Prion. 2008;2:162–9. doi: 10.4161/pri.2.4.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stotzky G. Persistence and biological activity in soil of the insecticidal proteins from Bacillus thuringiensis, especially from transgenic plants. Plant Soil. 2004;266:77–89. doi: 10.1007/s11104-005-5945-6. [DOI] [Google Scholar]
- 29.Carter DO, Yellowlees D, Tibbet M. Autoclaving kills soil microbes yet soil enzymes remain active. Pedobiologia (Jena) 2007;51:295–9. doi: 10.1016/j.pedobi.2007.05.002. [DOI] [Google Scholar]
- 30.Rees HC, Maddison BC, Owen JP, Whitelam GC, Gough KC. Concentration of disease-associated prion protein with silicon dioxide. Mol Biotechnol. 2009;41:254–62. doi: 10.1007/s12033-008-9129-5. [DOI] [PubMed] [Google Scholar]
- 31.Davies P, Brown DR. Manganese enhances prion protein survival in model soils and increases prion infectivity to cells. PLoS One. 2009;4:e7518. doi: 10.1371/journal.pone.0007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill AC, Ritchie MA, Hunt LG, Steane SE, Davies KG, Bocking SP, et al. Post-translational hydroxylation at the N-terminus of the prion protein reveals presence of PPII structure in vivo. EMBO J. 2000;19:5324–31. doi: 10.1093/emboj/19.20.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen JP, Maddison BC, Whitelam GC, Gough KC. Use of thermolysin in the diagnosis of prion diseases. Mol Biotechnol. 2007;35:161–70. doi: 10.1007/BF02686111. [DOI] [PubMed] [Google Scholar]
- 34.Féraudet C, Morel N, Simon S, Volland H, Frobert Y, Créminon C, et al. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J Biol Chem. 2005;280:11247–58. doi: 10.1074/jbc.M407006200. [DOI] [PubMed] [Google Scholar]