Abstract
Cytokines may contribute to the severity of CD4 cell depletion with human immunodeficiency virus (HIV) infection, but quantitative relationships are not well defined. Serum and plasma from 181 HIV-infected individuals were tested with Millipore 30-plex Luminex cytokine assays. Within-individual correlations among cytokines were summarized by two-dimensional hierarchical cluster analysis. Associations with age, sex, race, CD4 count, and HIV viral load were determined with linear regression models. Tests for statistical significance were corrected for multiple comparisons, using a false discovery rate of 0.1. African-Americans had significantly higher levels than whites of six cytokines (IL-2, IL-5, IL-7, IL-15, fractalkine, and IFN-γ), and lower levels of MCP-1. Females had higher fractalkine levels than males. Age was not associated with levels of any cytokine. Six cytokines, including the T-helper (Th) type 1 cytokine IL-15, the Th2 cytokines IL-1ra and IL-10, the chemokines fractalkine and MCP-1, and the growth factor G-CSF were each inversely associated with CD4 count; no cytokine was directly associated with CD4 count. Fractalkine was directly associated with HIV viral load, adjusted for CD4 count. Cytokines clustered by primary function (e.g., Th1, Th2, proinflammatory, chemokines, or growth factors) whereas individuals clustered according to cytokine levels (generally high, intermediate, or low) had significantly different CD4 counts [medians (interquartile range) of 60 (17–162), 131 (62–321), and 155 (44–467), respectively; p<0.0001]. CD4 deficiency is associated with generalized increases in cytokines of various functions. Racial differences in cytokine response to HIV infection could contribute to disparities in disease progression.
Introduction
HIV infection is associated with progressive reduction in CD4 cell count, chronic immune activation, and altered levels of circulating cytokines.1–3 Dysregulation of cytokine signaling is thought to contribute to HIV-associated immune deficiency,4 although effects differ by cell type making systemic relationships hard to predict. For example, macrophage colony-stimulating factor (M-CSF) increases HIV replication in monocyte-derived macrophages (MDM) but not T cells whereas interleukin (IL)-2, IL-7, and IL-15 stimulate HIV replication in T cells but not MDM.2 Cytokine alterations vary over the course of HIV disease progression. Although the T helper (Th) switch does not entirely explain HIV progression, early stage HIV infection has a Th1-predominant profile [characterized by a high production of IL-2 and interferon (IFN)-γ],5 while late HIV infection has a Th2-predominant profile (characterized by increased IL-4 and IL-10 production).4,6 Highly active antiretroviral therapy (HAART) partially reverses the abnormal cytokine profile,7,8 which might contribute to suppression of HIV replication and restoration of CD4 T cell counts. Moreover, the pharmacologic administration of cytokines coupled with antiretroviral treatment has shown clinical benefit in some circumstances, with partial restoration of immune responses.2 We therefore evaluated associations of HIV-related CD4 lymphopenia and HIV viral load with a panel of immunomodulator and inflammatory cytokines.
Materials and Methods
Subjects and samples
We analyzed stored serum (n=23) and plasma (n=158) samples obtained between 1983 and 2003 from 181 HIV-infected individuals. These individuals were originally selected as CD4-matched lymphoma-free controls for a study of AIDS non-Hodgkin lymphoma (NHL) in three National Cancer Institute HIV-infected cohorts.9 Nineteen subjects were from a cohort of 133 HIV-infected male homosexuals from Manhattan and Washington,10 103 were among 2126 HIV-infected hemophilia patients,11 and 59 were in a study of 2803 patients at AIDS treatment and clinical trial sites.12 The study participants were mainly white (83.4%), male (93.4%), and had a median age of 37.0 (IQR 31.4–42.9). Blood samples were transferred at ambient temperature to a central processing laboratory for separation of components within 24 h of phlebotomy. Serum and plasma were then immediately stored at −80°C until testing.
Cytokine assays
Cytokines were measured by Luminex fluorescent bead human cytokine immunoassays (MILLIPLEX MAP, Millipore Corp., Billerica, MA). Thirteen cytokines were part of a high-sensitivity multiplex panel [GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, and TNF-α], 16 were in a standard-sensitivity multiplex panel [EGF, eotaxin, fractalkine, G-CSF, IL-1α, IL-1ra, IL-12 (p40, free form), IL-15, IL-17, IP-10, MCP-1, MIP-1α, MIP-1β, soluble CD40L, TGF-α, and VEGF], and one was measured as a single analyte (RANTES). Measurements on blinded replicate samples included with test samples had coefficients of variation of 10–46%.
Statistical methods
All analyses were conducted using SAS 9.1 (SAS Institute, Cary, NC), BRB Array Tools 3.8.0 beta 3 (developed by the Biometric Research Branch of the U.S. National Cancer Institute http://linus.nci.nih.gov/BRB-ArrayTools.html), and the R statistical package. Pairwise correlations between cytokine levels were assessed by Spearman rank statistics, with p<0.05 considered statistically significant. Cytokine measurements were log-transformed for statistical analyses and undetectable levels were imputed as one-half the limit of detection. We used Chi-square tests to examine the significance of differences between proportions, t-tests for differences between means, and analysis of variance (ANOVA) for differences among more than two means.
Using cytokine centering, Euclidean distance metrics, and complete linkage methods, we conducted hierarchical cluster analysis to construct classifications on two dimensions simultaneously (cytokines and subjects).13 We tested whether the data exhibited more clustering than what is expected by chance from a multivariate normal distribution. As described in more detail by McShane et al., the multivariate normal distribution is unimodal and represents a single cluster.14 To assess the association of individual cytokines with race, sex, age, and CD4 count, we employed class comparison analysis for categorical variables adjusted for material type (i.e., serum or plasma).13 In this analysis, we identified cytokines that were significantly different among the classes by using a multivariate permutation test.13–15 The significance level was set to provide 80% confidence of no more than a 10% false discovery rate, defined as the proportion of cytokines incorrectly identified as significantly differing among the classes (i.e., the number of false positives divided by total positives). Although this technique assumed random variance t-statistics for each cytokine, the permutation test itself is nonparametric and does not require the assumption of Gaussian distribution.16
We used a distance-based regression model to test whether the global pattern of cytokine measurements is influenced by CD4 count. The test for global association is based on a pseudo F statistic with its significance level evaluated through a permutation procedure with 10,000 iterations.17
Antiretroviral therapy could be associated with altered cytokine levels. However, receipt of HAART could indicate severity of disease (i.e., treatment bias) in addition to direct effects on cytokine levels. We therefore examined variation of cytokine associations by year of blood draw as a proxy for treatment availability, rather than by actual treatment received. Accordingly, significant associations from the bivariate associations with CD4 count analysis, class comparison analysis, and quantitative trait analysis were adjusted for individual year of blood draw using linear regression models.
Results
Blood cytokine levels varied widely among the cytokines, with geometric means ranging from 1.0 (for IL-5) to 24,500 (for RANTES) pg/ml. However, interindividual distributions were more similar, with a geometric standard deviation between 2.3 and 5.3 (Table 1). CD4 count significantly declined with year of blood draw (β=−19, p<0001), with medians [interquartile range (IQR)] of 333 (226–459) for subjects enrolled during 1984–1990 (n=36), 65 (29–185) for 1991–1995 (n=70), and 72 (19–140) for 1996–2003 (n=75) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/aid). Adjusted for year of blood draw, median (IQR) CD4 was similar for males and females [169 (143–195) and 122 (21–223), respectively; p=0.4], and for whites and African-Americans [169 (141–197) and 150 (82–218), respectively; p=0.6], and CD4 count was not associated with HIV viral load (β=–15, p=0.2).
Table 1.
Cytokine Levels Among HIV-Infected Subjects
| Cytokine | Percent detectable | Geometric meana(pg/ml) | Geometric standard deviation |
|---|---|---|---|
| RANTES | 100% | 24,500 | 3.1 |
| IL-1ra | 86% | 272 | 3.4 |
| EGF | 78% | 78 | 2.6 |
| TGF-α | 74% | 14 | 2.5 |
| Fractalkine | 56% | 191 | 2.7 |
| IL-15 | 56% | 12 | 2.6 |
| IL-17 | 55% | 23 | 3.2 |
| IL-1a | 70% | 66 | 4.5 |
| G-CSF | 34% | 187 | 3.9 |
| EOTAXIN | 97% | 154 | 2.3 |
| MCP-1 | 100% | 305 | 2.8 |
| Scd40L | 99% | 7,600 | 3.1 |
| IL-12p40 | 72% | 218 | 2.6 |
| MIP-1a | 63% | 38 | 5.5 |
| MIP-1b | 43% | 164 | 5.3 |
| IP-10 | 100% | 1,270 | 2.6 |
| VEGF | 87% | 98 | 2.4 |
| GM-CSF | 44% | 3 | 2.7 |
| IFN-γ | 65% | 8 | 3.3 |
| IL-2 | 75% | 13 | 3.8 |
| IL-4 | 49% | 54 | 4.9 |
| IL-5 | 64% | 1 | 2.3 |
| IL-6 | 95% | 10 | 5.3 |
| IL-7 | 88% | 5 | 2.6 |
| IL-8 | 100% | 15 | 5.3 |
| IL-10 | 98% | 20 | 2.6 |
| IL-13 | 60% | 27 | 3.6 |
| IL-12p70 | 50% | 3 | 2.8 |
| IL-1b | 70% | 2 | 3.1 |
| TNF-α | 100% | 10 | 2.3 |
Excluding nondetectables.
The 30 cytokines were mutually correlated with one another, with the greatest pairwise correlations of 0.80 for sCD40L and RANTES, 0.78 for IFN-γ and interleukin (IL)-2, and 0.75 for IL-13 and IL-4. The cytokine with the lowest maximum correlation was G-CSF, based on its correlation of 0.30 with IL-17. All significant pairwise correlations were positive except for IL-2 with MCP-1, IL-2 with RANTES, IL-2 with Scd40l, and RANTES with IL12p70 (Table 2).
Table 2.
Pairwise Spearman Correlations (pc < 0.05) Among Cytokine Levels
| IL-1a | IL-1b | IL-1ra | IL-2 | IL-4 | IL-5 | IL-6 | IL-7 | IL-8 | IL-10 | IL-12p40 | IL-12p70 | IL-13 | IL-15 | IL-17 | EGF | Eotaxin | Fractalkine | G-CSF | GM-CSF | IFN-γ | IP-10 | MCP-1 | MIP-1a | MIP-1b | RANTES | sCD40L | TGF-α | TNF-α | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-1b | .39 | ||||||||||||||||||||||||||||
| IL-1ra | .34 | .48 | |||||||||||||||||||||||||||
| IL-2 | .45 | .71 | .21 | ||||||||||||||||||||||||||
| IL-4 | .55 | .21 | .23 | ||||||||||||||||||||||||||
| IL-5 | .59 | .41 | .30 | .59 | .37 | ||||||||||||||||||||||||
| IL-6 | .42 | .36 | .36 | .46 | .24 | ||||||||||||||||||||||||
| IL-7 | .39 | .62 | .30 | .56 | .51 | .25 | |||||||||||||||||||||||
| IL-8 | .19 | .27 | .34 | .22 | .66 | .20 | |||||||||||||||||||||||
| IL-10 | .18 | .37 | .36 | .33 | .40 | .23 | .35 | ||||||||||||||||||||||
| IL-12p40 | .35 | .27 | .23 | .37 | .32 | .28 | .25 | ||||||||||||||||||||||
| IL-12p70 | .47 | .58 | .27 | .71 | .21 | .57 | .57 | .37 | .36 | ||||||||||||||||||||
| IL-13 | .71 | .37 | .20 | .40 | .75 | .60 | .48 | .34 | .24 | .20 | .21 | .39 | |||||||||||||||||
| IL-15 | .51 | .39 | .32 | .43 | .31 | .52 | .31 | .38 | .17 | .28 | .37 | .41 | .44 | ||||||||||||||||
| IL-17 | .49 | .43 | .35 | .48 | .45 | .18 | .45 | .38 | .36 | .53 | .20 | .44 | |||||||||||||||||
| EGF | .23 | .18 | .31 | .24 | .48 | .20 | .18 | ||||||||||||||||||||||
| Eotaxin | .16 | .29 | .17 | .18 | .19 | .16 | .19 | ||||||||||||||||||||||
| Fractalkine | .48 | .45 | .39 | .55 | .50 | .50 | .42 | .43 | .67 | .26 | .43 | .68 | .19 | ||||||||||||||||
| G-CSF | .16 | .17 | .19 | .21 | .18 | .28 | .30 | .16 | .20 | ||||||||||||||||||||
| GM-CSF | .30 | .39 | .22 | .37 | .23 | .26 | .23 | .26 | .24 | .33 | .34 | .26 | |||||||||||||||||
| IFN-γ | .42 | .64 | .27 | .78 | .22 | .62 | .16 | .54 | .43 | .33 | .61 | .38 | .52 | .49 | .51 | .19 | .25 | ||||||||||||
| IP-10 | .29 | .17 | .37 | .34 | .20 | .16 | .28 | ||||||||||||||||||||||
| MCP-1 | .28 | .16 | .33 | .42 | .20 | .40 | .38 | .35 | |||||||||||||||||||||
| MIP-1a | .27 | .35 | .42 | .19 | .60 | .19 | .53 | .20 | .15 | .18 | .34 | .29 | .35 | .28 | .22 | .22 | .23 | .27 | .34 | ||||||||||
| MIP-1b | .26 | .46 | .46 | .56 | .24 | .49 | .25 | .16 | .18 | .24 | .27 | .34 | .28 | .17 | .17 | .15 | .27 | .30 | .69 | ||||||||||
| RANTES | .16 | .25 | .21 | .48 | .15 | .62 | .28 | .28 | .19 | ||||||||||||||||||||
| sCD40L | .19 | .21 | .17 | .29 | .50 | .65 | .18 | .18 | .17 | .35 | .36 | .30 | .80 | ||||||||||||||||
| TGF-α | .52 | .35 | .39 | .31 | .19 | .41 | .30 | .41 | .17 | .28 | .30 | .46 | .30 | .41 | .60 | .19 | .17 | .47 | .37 | .35 | .29 | .20 | |||||||
| TNF-α | .34 | .50 | .46 | .29 | .23 | .30 | .48 | .46 | .38 | .39 | .24 | .26 | .29 | .29 | .38 | .18 | .16 | .35 | .17 | .28 | .32 | .37 | .25 | .43 | .40 | .16 | .21 | .35 | |
| VEGF | .26 | .21 | .30 | .17 | .22 | .41 | .26 | .18 | .16 | .24 | .25 | .44 | .52 | .39 | .23 | .29 | .38 | .38 | .32 | .38 | .34 | .33 |
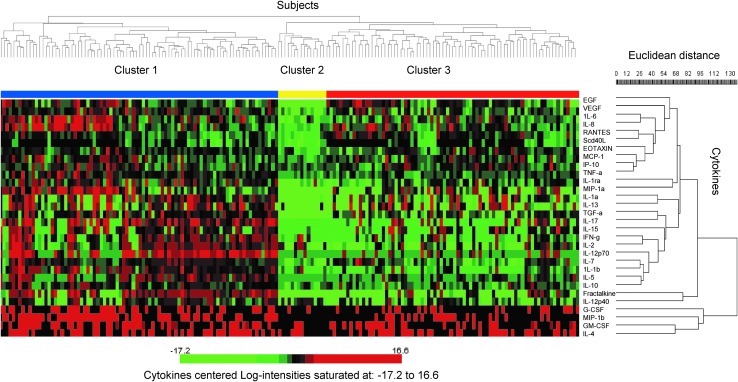
In the hierarchical cluster analysis, the global test of clustering was significant (p=0.001). We identified two main clusters of cytokines. The first cluster included four cytokines (GM-CSF, G-CSF, MIP-1b, and IL-4) that are known for their specific effects on growth, activation, differentiation, or chemotaxis of both neutrophils and B lymphocytes. Within the second cluster, cytokine levels were arranged in groups of similar function, including a predominantly Th1 group (IL-1β, IL-7, IL-12p70, IL-2, INF-γ, IL-15, and TGF-α), a Th2 group (IL-5 and IL-10), a proinflammatory group (IL-6, IL-8, RANTES, EOTAXIN, MCP-1, IP-10, and TNF-α), and a T cell chemokine group (fractalkine and IL-12p40) (Fig. 1, right-hand dendrogram).
FIG. 1.
Two-dimensional cluster analysis of 30 cytokine levels in 181 HIV-infected subjects. The right-hand dendrogram represents similarities among measured cytokines and the top dendrogram represents similarities among cytokine profiles of different subjects. Centered log-2 cytokine concentrations ranging from −17.2 to 16.6 are indicated in gradients of color, where red indicates higher values and green indicates lower values. Each row represents the values for a cytokine, and each column corresponds to a subject. Cluster 1 (green bar) indicates subjects with the highest cytokine concentrations, cluster 2 (orange bar) indicates subjects with the lowest concentrations, and cluster 3 (purple bar) indicates subjects with intermediate concentrations.
In the distance-based regression model, there was a significant global association between CD4 count and the measured cytokines (p<0.0001).
In the class comparison analysis adjusted for sample type, African-Americans had significantly higher (p range 0.0003 to 0.006) levels than whites of six cytokines (IL-2, IL-5, IL-7, IL-15, fractalkine, and IFN-γ) but significantly lower levels of MCP-1 (p=0.004) (Table 3). None of the cytokines significantly differed by age. When we analyzed our results by sex, only fractalkine was significantly higher in females compared to males [medians (IQR) 220 (114–296) versus 24 (0.1–231), respectively, p=0.004].
Table 3.
Cytokines Significantly Associated with Race
| Cytokines | Geometric mean in blacks (n=30) | Geometric mean in whites (n=151) | Fold-difference | Parametric p-value |
|---|---|---|---|---|
| IL-5 | 0.9 | 0.4 | 2.6 | 0.0003 |
| Fractalkine | 84.8 | 4.1 | 20.7 | 0.0004 |
| IL-7 | 7.3 | 3.1 | 2.4 | 0.0008 |
| IL-2 | 15.5 | 2.8 | 5.6 | 0.002 |
| IL-15 | 5.7 | 1.2 | 5.0 | 0.004 |
| IFN-γ | 5.8 | 1.4 | 4.2 | 0.006 |
| MCP-1 | 190 | 334 | 0.6 | 0.004 |
Cytokines were selected to provide 80% confidence that the false discovery rate was less than 10%.
Adjusted for sample type, CD4 count was inversely associated with significantly higher levels of six cytokines: the Th1 cytokine IL-15, the Th2 cytokines IL-1ra and IL-10, the chemokines fractalkine and MCP-1, and the growth factor G-CSF. The strongest association was for IL-1ra (9-fold difference between CD4< 200 and CD4 ≥200, p=0.0001) while the lowest association was for MCP-1 (1.5-fold difference, p=0.009) (Table 4). In the bivariate analysis, 17 of the remaining cytokines also varied inversely with CD4 count and only seven varied directly, but none of these 24 associations was significant with correction for multiple comparisons. Adjusted for blood draw date (as a surrogate for antiretroviral treatment availability), the associations of CD4 with G-CSF, IL-10, IL1-ra, and MCP-1 remained significant although attenuated, but the associations with fractalkine and IL-15 became nonsignificant. Results were qualitatively similar in a sensitivity analysis restricted to samples obtained in the pre-HAART era prior to 1996. Similarly, results did not change in a sensitivity analysis adjusted for draw year and study cohort.
Table 4.
Cytokines Significantly Associated with CD4 Count
| Cytokines | Geometric mean in CD4<200 (n=124) | Geometric mean in CD4≥200 (n=57) | Fold-difference | Parametric p-value |
|---|---|---|---|---|
| IL-1ra | 183.88 | 19.8 | 9.29 | 0.0001 |
| IL-10 | 21.69 | 11.07 | 1.96 | 0.0002 |
| Fractalkine | 15.95 | 1.05 | 15.2 | 0.0009 |
| G-CSF | 2.14 | 0.41 | 5.27 | 0.003 |
| IL-15 | 2.31 | 0.6 | 3.87 | 0.006 |
| MCP-1 | 345.7 | 231.19 | 1.5 | 0.009 |
Cytokines were selected to provide 80% confidence that the false discovery rate was less than 10%.
Of the 181 subjects, 179 fell into three clusters leaving two subjects that did not fit into any cluster (Fig. 1, top dendrogram). Compared to the second and third clusters, the first cluster (n=86) was characterized by higher levels for 28 of the 30 cytokines. The second cluster (n=15) had lower levels than either the first or the third cluster. Finally, the third cluster (n=78) was intermediate, with cytokine levels that were mostly lower than the first cluster and higher than the second cluster (Fig. 1). CD4 counts significantly differed among the three clusters of individuals, with median 60 (IQR, 17–162) in the first cluster with high levels of cytokines, median 155 (IQR, 44–467) in the second cluster with low levels, and median 131 (IQR, 62–321) in the third cluster with intermediate levels (p<0.0001).
HIV viral load did not affect the associations of cytokines with CD4 count, as only fractalkine was correlated (positively) with HIV viral load (r=0.2, p=0.007). Fractalkine remained significantly associated with both HIV viral load and CD4 count (β=0.50, p=0.05, and β=−0.01, p<0.0001, respectively) after mutual adjustment for both variables.
None of the subjects had cancer at the time of blood collection and only four developed cancer thereafter (latency intervals, 0.68 to 6.24 years). Exclusion of those four individuals did not substantially change the results.
Discussion
Cytokines, particularly certain chemokines, affect the risk of HIV acquisition and disease progression.2 However, application of this biology to clinical practice has been hampered by their low levels in circulation, transient effects, and complex relationships. Our current study used new, high sensitivity, multiplex technology to quantify and characterize the relationships of circulating cytokine profiles to CD4 count and HIV viral load in well-characterized HIV-infected individuals.
We found an overall inverse relationship between the levels for 23 of 30 cytokines and CD4 count. These correlations were seen across cytokine functions, including significant associations with Th1, Th2, and proinflammatory cytokines and growth factors. Surprisingly, HIV viral load was associated only with fractalkine.
CD4 count declined significantly with the year of blood draw, reflecting the CD4 distribution of the matched lymphoma cases. Our findings of inverse associations of CD4 count with levels of both Th1 and Th2 cytokines are in contrast with some previous studies, and suggest that the theory of a Th1/Th2 switch is an oversimplification that does not explain the pathogenesis of HIV disease progression.4–6,18,19
African-Americans had higher levels of six cytokines (IL-5, fractalkine, IL-7, IL-2, IL-15, and IFN-γ) and lower levels of MCP-1. Associations with race may be due to genetic variation. Previous studies reported a race-specific distribution of allelic variants in cytokine genes.20–23 Alternatively, variations in cytokine levels by race could reflect differences in socioeconomic status (SES), which might indirectly affect disease severity. Issues associated with lower SES, such as being uninsured, limited access to healthcare, not receiving antiretroviral drugs, and lack of transportation to visit doctors could be related to HIV disease severity and thereby might affect cytokine levels.24
Previous studies of people with HIV have not examined multiple circulating cytokines simultaneously. We evaluated a panel of 30 cytokines, finding many associations that are consistent with previous studies as well as some novel associations, such as lower CD4 with elevated IL-15. This range of findings underscores a strength of our study, namely our ability to begin untangling the cytokine network and its relationship to disease. In accord with our results, previous in vitro studies have demonstrated disease progression with excess production of proinflammatory cytokines such as TNF-α25 and Th2 cytokines such as IL-10.4
We found that fractalkine (CX3CL1), which is the only member of the CX3C chemokine family, was significantly associated not only with lower CD4 count but also with elevated HIV viral load. It had been suggested that fractalkine trafficking of HIV-infected lymphocytes propagates the dissemination of HIV in vivo.26 Of the 30 cytokines that we evaluated, only fractalkine was associated with viral load, and this association remained significant after adjusting for CD4 count. Similarly, Widney et al. found that CXCL13—a B cell stimulatory chemokines—is elevated in HIV infection and correlated with CD4 count and HIV viral load.27 In addition, CXCL13 levels were reported to be decreased with HAART.27,28 However, some studies have found levels of beta-chemokines associated with CD4 changes, although not with HIV viral load,29,30 and studies of other chemokines have yielded inconsistent results.31–36
Our study has the strength of being the first to examine a comprehensive panel of circulating cytokines in HIV-infected individuals, enabling us to examine the individual and joint effects of multiple cytokines in vivo. Limitations of the study should be noted. Serum cytokine levels might be affected by antiretroviral therapy, although cytokine association with CD4 count did not vary by treatment availability, based on year of blood draw as a proxy variable. This adjustment suggests that cytokine associations with CD4 count are not strongly influenced by antiretroviral treatment, but a formal investigation into this assumption would be needed. Another limitation is the varying duration of sample storage prior to testing. However, Hosnijeh et al. previously reported that plasma and serum cytokine levels are stable and reproducible.37
In conclusion, we found HIV-related CD4 deficiency associated with generalized increases in Th1, Th2, and proinflammatory cytokines and growth factors. Racial differences in cytokine response to HIV infection could contribute to disparities in opportunistic infection and malignancy.
Supplementary Material
Acknowledgments
We would like to thank Dr. Eric Engels for his helpful suggestions on a previous version of this manuscript. This study was funded by the Intramural Research Program of the National Cancer Institute.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Rosenberg ES. Billingsley JM. Caliendo AM. Boswell SL. Sax PE. Kalams SA. Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 2.Kedzierska K. Crowe SM. Cytokines and HIV-1: Interactions and clinical implications. Antivir Chem Chemother. 2001;12:133–150. doi: 10.1177/095632020101200301. [DOI] [PubMed] [Google Scholar]
- 3.Mellors JW. Munoz A. Giorgi JV. Margolick JB. Tassoni CJ. Gupta P. Kingsley LA. Todd JA. Saah AJ. Detels R. Phair JP. Rinaldo CR., Jr Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Clerici M. Shearer GM. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 5.Stacey AR. Norris PJ. Qin L. Haygreen EA. Taylor E. Heitman J. Lebedeva M. DeCamp A. Li D. Grove D. Self SG. Borrow P. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barcellini W. Rizzardi GP. Borghi MO. Fain C. Lazzarin A. Meroni PL. TH1 and TH2 cytokine production by peripheral blood mononuclear cells from HIV-infected patients. AIDS. 1994;8:757–762. doi: 10.1097/00002030-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Relucio KI. Beernink HT. Chen D. Israelski DM. Kim R. Holodniy M. Proteomic analysis of serum cytokine levels in response to highly active antiretroviral therapy (HAART) J Proteome Res. 2005;4:227–231. doi: 10.1021/pr049930y. [DOI] [PubMed] [Google Scholar]
- 8.Imami N. Antonopoulos C. Hardy GA. Gazzard B. Gotch FM. Assessment of type 1 and type 2 cytokines in HIV type 1-infected individuals: Impact of highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 1999;15:1499–1508. doi: 10.1089/088922299309784. [DOI] [PubMed] [Google Scholar]
- 9.Rabkin CS. Engels EA. Langren O. Schuurman R. Camargo MC. Pfeiffer R. Goedert JJ. Circulating cytokine levels, Epstein-Barr viremia and risk of AIDS-related non-Hodgkin lymphoma. Am J Hematol. 2011;86:875–878. doi: 10.1002/ajh.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goedert JJ. Sarngadharan MG. Biggar RJ. Weiss SH. Winn DM. Grossman RJ. Greene MH. Bodner AJ. Mann DL. Strong DM, et al. Determinants of retrovirus (HTLV-III) antibody and immunodeficiency conditions in homosexual men. Lancet. 1984;2:711–716. doi: 10.1016/s0140-6736(84)92624-2. [DOI] [PubMed] [Google Scholar]
- 11.Goedert JJ. Kessler CM. Aledort LM. Biggar RJ. Andes WA. White GC., 2nd Drummond JE. Vaidya K. Mann DL. Eyster ME, et al. A prospective study of human immunodeficiency virus type 1 infection and the development of AIDS in subjects with hemophilia. N Engl J Med. 1989;321:1141–1148. doi: 10.1056/NEJM198910263211701. [DOI] [PubMed] [Google Scholar]
- 12.Nawar E. Mbulaiteye SM. Gallant JE. Wohl DA. Ardini M. Hendershot T. Goedert JJ. Rabkin CS. Risk factors for Kaposi's sarcoma among HHV-8 seropositive homosexual men with AIDS. Int J Cancer. 2005;115:296–300. doi: 10.1002/ijc.20887. [DOI] [PubMed] [Google Scholar]
- 13.Simon R. Korn E. McShane LM. Radmacher MD. Wright G. Zhao Y. Design, Analysis of DNA Microarray Investigations. Springer-Verlag; New York: 2003. [Google Scholar]
- 14.McShane LM. Radmacher MD. Freidlin B. Yu R. Li MC. Simon R. Methods for assessing reproducibility of clustering patterns observed in analyses of microarray data. Bioinformatics. 2002;18:1462–1469. doi: 10.1093/bioinformatics/18.11.1462. [DOI] [PubMed] [Google Scholar]
- 15.Korn EL. Troendle JF. McShane LM. Simon R. Controlling the number of false discoveries: Application to high-dimensional genomic data. J Stat Plan Inference. 2004;124:379–398. [Google Scholar]
- 16.Wright GW. Simon RM. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics. 2003;19:2448–2455. doi: 10.1093/bioinformatics/btg345. [DOI] [PubMed] [Google Scholar]
- 17.McArdle BH. Anderson MJ. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. [Google Scholar]
- 18.Suzuki Y. Koyanagi Y. Tanaka Y. Murakami T. Misawa N. Maeda N. Kimura T. Shida H. Hoxie JA. O'Brien WA. Yamamoto N. Determinant in human immunodeficiency virus type 1 for efficient replication under cytokine-induced CD4(+) T-helper 1 (Th1)- and Th2-type conditions. J Virol. 1999;73:316–324. doi: 10.1128/jvi.73.1.316-324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llano A. Esté JA. Chemokines and other cytokines in human immunodeficiency virus type 1 (HIV-1) infection. Inmunología. 2005;24:246–260. [Google Scholar]
- 20.Ness RB. Haggerty CL. Harger G. Ferrell R. Differential distribution of allelic variants in cytokine genes among African Americans and white Americans. Am J Epidemiol. 2004;160:1033–1038. doi: 10.1093/aje/kwh325. [DOI] [PubMed] [Google Scholar]
- 21.Hassan MI. Aschner Y. Manning CH. Xu J. Aschner JL. Racial differences in selected cytokine allelic and genotypic frequencies among healthy, pregnant women in North Carolina. Cytokine. 2003;21:10–16. doi: 10.1016/s1043-4666(02)00489-1. [DOI] [PubMed] [Google Scholar]
- 22.Cox ED. Hoffmann SC. DiMercurio BS. Wesley RA. Harlan DM. Kirk AD. Blair PJ. Cytokine polymorphic analyses indicate ethnic differences in the allelic distribution of interleukin-2 and interleukin-6. Transplantation. 2001;72:720–726. doi: 10.1097/00007890-200108270-00027. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann SC. Stanley EM. Cox ED. DiMercurio BS. Koziol DE. Harlan DM. Kirk AD. Blair PJ. Ethnicity greatly influences cytokine gene polymorphism distribution. Am J Transplant. 2002;2:560–567. doi: 10.1034/j.1600-6143.2002.20611.x. [DOI] [PubMed] [Google Scholar]
- 24.Multiple barriers prevent minorities' early treatment. AIDS stigma, lack of transportation top list. AIDS Alert. 2003;18:137–138. 143. [PubMed] [Google Scholar]
- 25.Esser R. von Briesen H. Brugger M. Ceska M. Glienke W. Muller S. Rehm A. Rubsamen-Waigmann H. Andreesen R. Secretory repertoire of HIV-infected human monocytes/macrophages. Pathobiology. 1991;59:219–222. doi: 10.1159/000163649. [DOI] [PubMed] [Google Scholar]
- 26.Becker Y. The spreading of HIV-1 infection in the human organism is caused by fractalkine trafficking of the infected lymphocytes—a review, hypothesis and implications for treatment. Virus Genes. 2007;34:93–109. doi: 10.1007/s11262-006-0056-x. [DOI] [PubMed] [Google Scholar]
- 27.Widney DP. Breen EC. Boscardin WJ. Kitchen SG. Alcantar JM. Smith JB. Zack JA. Detels R. Martinez-Maza O. Serum levels of the homeostatic B cell chemokine, CXCL13, are elevated during HIV infection. J Interferon Cytokine Res. 2005;25:702–706. doi: 10.1089/jir.2005.25.702. [DOI] [PubMed] [Google Scholar]
- 28.Regidor DL. Detels R. Breen EC. Widney DP. Jacobson LP. Palella F. Rinaldo CR. Bream JH. Martinez-Maza O. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS. 2011;25:303–314. doi: 10.1097/QAD.0b013e32834273ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakkanaiah VN. Ojo-Amaize EA. Peter JB. Concentrations of circulating beta-chemokines do not correlate with viral load in human immunodeficiency virus-infected individuals. Clin Diagn Lab Immunol. 1998;5:499–502. doi: 10.1128/cdli.5.4.499-502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitai R. Zhao ML. Zhang N. Hua LL. Lee SC. Role of MIP-1beta and RANTES in HIV-1 infection of microglia: Inhibition of infection and induction by IFNbeta. J Neuroimmunol. 2000;110:230–239. doi: 10.1016/s0165-5728(00)00315-5. [DOI] [PubMed] [Google Scholar]
- 31.Hosnijeh FS. Krop EJ. Portengen L. Rabkin CS. Linseisen J. Vineis P. Vermeulen R. Stability, reproducibility of simultaneously detected plasma, serum cytokine levels in asymptomatic subjects. Biomarkers. 15:140–148. doi: 10.3109/13547500903340570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.