Abstract
The BED capture enzyme immunoassay (BED-CEIA) was developed for estimating HIV incidence from cross-sectional data. This assay misclassifies some individuals with nonrecent HIV infection as recently infected, leading to overestimation of HIV incidence. We analyzed factors associated with misclassification by the BED-CEIA. We analyzed samples from 383 men who were diagnosed with HIV infection less than 1 year after a negative HIV test (Multicenter AIDS Cohort Study). Samples were collected 2–8 years after HIV seroconversion, which was defined as the midpoint between the last negative and first positive HIV test. Samples were analyzed using the BED-CEIA with a cutoff of OD-n ≤0.8 for recent infection. Logistic regression was used to identify factors associated with misclassification. Ninety-one (15.1%) of 603 samples were misclassified. In multivariate models, misclassification was independently associated with highly active antiretroviral treatment (HAART) for >2 years, HIV RNA <400 copies/ml, and CD4 cell count <50 or <200 cells/mm3; adjusted odds ratios (OR) and 95% confidence intervals (CI) were 4.72 (1.35–16.5), 3.96 (1.53–10.3), 6.85 (2.71–17.4), and 11.5 (3.64–36.0), respectively. Among 220 men with paired samples, misclassification 2–4 years after seroconversion was significantly associated with misclassification 6–8 years after seroconversion [adjusted OR: 25.8 (95% CI: 8.17–81.5), p<0.001] after adjusting for race, CD4 cell count, HIV viral load, and HAART use. Low HIV viral load, low CD4 cell count, and >2 years of HAART were significantly associated with misclassification using the BED-CEIA. Some men were persistently misclassified as recently infected up to 8 years after HIV seroconversion.
Introduction
Accurate methods for determining the HIV incidence using samples from cross-sectional surveys are needed to monitor the HIV/AIDS epidemic and to evaluate the effectiveness of interventions for HIV prevention.1 Most laboratory tests that are currently used to estimate HIV incidence are based on analysis of anti-HIV antibodies.2,3 The BED capture enzyme immunoassay (BED-CEIA)4 is currently used for surveillance purposes in the United States5,6 and around the world7 to estimate incidence and identify populations with high levels of new infections. This assay measures the proportion of antibodies that binds to an HIV peptide; results are reported as normalized optical density units (OD-n). In a recent study of 756 adults,8 individuals who were classified by the BED-CEIA as recently infected (based on a BED-CEIA result ≤0.8 OD-n) had a mean estimated duration of infection of 176 days [95% confidence interval (CI): 164–188 days]. However, some individuals with nonrecent HIV infection are misclassified by the BED-CEIA as recently infected. This type of misclassification can lead to a significant overestimation of HIV incidence rates.9 For this reason, the Joint United Nations Programme on HIV/AIDS (UNAIDS) has discouraged the use of the BED-CEIA for estimating HIV incidence.10 The CDC responded by issuing a document outlining possible causes of false recent BED-CEIA results; these included testing samples from uninfected individuals, poor specimen handling, chronic infection or hyper-gamma-globulinemia, HIV subtype heterogeneity, AIDS, and antiretroviral use.11 The UNAIDS followed in 2010 with a recommendation that if the BED-CEIA is used, that the frequency of misclassification should be determined in the population being surveyed.12
Factors that have been associated with misclassification by the BED-CEIA in African populations include low HIV viral load, low CD4 cell count, and long-term use of highly active antiretroviral therapy (HAART).13–17 Longitudinal testing of samples collected up to 2 years after HIV seroconversion has demonstrated that some individuals have low, stable BED-CEIA results consistent with recent infection8,14,18; however, it is not known whether individuals can remain misclassified over longer periods of time after HIV seroconversion. Few studies have evaluated the frequency of misclassification by the BED-CEIA in Western populations apart from an investigation in women from Atlanta.19 Recent reports have demonstrated differences in the immune response among men and women.20 Because the majority of new HIV infections in the United States occur in men who have sex with men (MSM),6 it is important to know the misclassification frequency in that risk group. In this report, we evaluated the frequency of misclassification by the BED-CEIA in MSM from the United States who were followed in a cohort study for at least 2 years after documentation of HIV seroconversion. We also evaluated factors associated with misclassification by the BED-CEIA in this cohort.
Materials and Methods
Samples used for analysis
We analyzed archived samples from 383 men enrolled in the Multicenter AIDS Cohort Study (MACS), a longitudinal study of the natural and treated history of HIV infection in MSM that has followed men semiannually since 1984.21 The samples analyzed in this study were collected between 1987 and 2009 from men whose last negative HIV test and first positive HIV test were obtained less than 1 year apart. The date of HIV seroconversion was defined as the midpoint between the dates of these two study visits. Two sets of samples were collected. Samples from 2–4 years after seroconversion (N=339) were collected a median of 3.7 years after the last negative HIV test (range: 2.4–4.0 years); samples from 6–8 years after HIV seroconversion (N=264) were collected a median of 7.7 years after the last negative HIV test (range: 6.0–8.0 years). The time between the first positive HIV test and sample collection ranged from 2.3 to 7.8 years. Epidemiologic and laboratory data, including HIV viral load, CD4 cell count, and serologic test results for herpes simplex virus type 2 (HSV-2),22 human herpesvirus 8 (HHV-8),23 and hepatitis C virus (HCV),24 were included in the analysis.
Laboratory testing
The BED-CEIA was performed according to the manufacturer's instructions (Calypte Biomedical Corporation, Lake Oswego, OR), and OD-n values were calculated. The BED-CEIA measures the proportion of total IgG that binds to a branched synthetic tripeptide that contains three 18-amino acid components derived from an immunodominant region of gp41 (regions corresponding to positions 590 to 607 of HXB2 gp160 in HIV subtypes B and D and CRF01_AE).25 For the purposes of this study, we defined recent infection as <1 year after HIV seroconversion. Because the men in this study were known to have been HIV infected for at least 2.3 years, we considered them to be misclassified if they had an OD-n value ≤0.8.
Statistical analysis
Samples were stratified by time after HIV seroconversion (2–4 years versus 6–8 years). Age, HIV viral load, CD4 cell count, duration of HAART at the time of sample collection, and year of sample collection were treated as categorical variables (Table 1). Serologic status for HSV-2, HHV-8, and HCV infection were treated as dichotomous variables (positive or negative; data for HSV-2 were missing for two men; data for HHV-8 were missing for 104 men); the number of positive results for these pathogens was treated as a continuous variable. The association of categorical factors with misclassification was examined using Fisher's exact test or the Chi square test. Logistic regression was performed using data stratified by duration of infection (2–4 years versus 6–8 years) to determine the odds of misclassification for all factors analyzed.
Table 1.
Factors Associated with Misclassification by the BED Capture Enzyme Immunoassay (OD-n ≤0.8) in Men Who Have Sex with Men with Nonrecent HIV Infection (Multicenter AIDS Cohort Study: 1987–2009)
| |
|
2 to 4 years after seroconversion |
6 to 8 years after seroconversion |
||
|---|---|---|---|---|---|
| Factor | All samples % Misclassified | % Misclassified | OR (95% CI) | % Misclassified | OR (95% CI) |
| All | 15.1% (91/603) | 14.5% (49/339) | — | 15.9% (42/264) | — |
| Age (years)a | |||||
| <35 | 7.1% (11/155) | 8.6% (10/117) | 1 | 2.6% (1/38) | 1 |
| 35–39 | 14.9% (21/141) | 15.1% (11/73) | 1.90 (0.76–4.72) | 14.7% (10/68) | 6.38 (0.78–51.9) |
| 40–44 | 14.9% (18/121) | 11.5% (7/61) | 1.39 (0.50–3.85) | 18.3% (11/60) | 8.31 (1.03–67.2)b |
| ≥45 | 22.0% (41/186) | 23.9% (21/88) | 3.35 (1.49–7.56)c | 22.4% (20/98) | 9.49 (1.22–73.4)b |
| Sample yeara | |||||
| <1990 | 5.7% (7/123) | 5.7% (7/123) | 1 | — | |
| 1990–1994 | 11.4% (22/193) | 16.7% (17/102) | 3.31 (1.31–8.34)b | 5.5% (5/91) | 1 |
| 1994–1998 | 11.0% (15/136) | 5.0% (3/60) | 0.87 (0.27–3.50) | 15.8% (12/76) | 3.22 (1.08–9.61)b |
| ≥1998 | 24.1% (19/79) | 40.7% (22/54) | 11.4 (4.47–29.6)c | 25.8% (25/97) | 5.97 (2.18–16.4)c |
| Racea | |||||
| White | 13.6% (74/530) | 12.9% (38/295) | 1 | 14.5% (34/235) | 1 |
| Not white | 26.0% (19/73) | 25.0% (11/44) | 2.25 (1.05–4.83)b | 27.6% (8/29) | 2.25 (0.92–5.49) |
| HIV viral load (copies/ml)a | |||||
| >10,000 | 11.8% (40/339) | 10.8% (23/214) | 1 | 13.6% (17/125) | 1 |
| 10,000 to 400 | 6.1% (9/147) | 6.0% (5/84) | 0.53 (0.19–1.43) | 6.4% (4/63) | 0.43 (0.14–1.34) |
| <400 | 35.9% (42/117) | 51.2% (21/41) | 8.72 (4.1–18.5)c | 27.6% (21/76) | 2.43 (1.18–4.97)b |
| CD4 cell count (cells/mm3)a | |||||
| >500 | 14.1% (38/269) | 15.0% (25/167) | 1 | 12.8% (13/102) | 1 |
| 200–500 | 12.3% (29/236) | 11.5% (15/131) | 0.73 (0.37–1.46) | 13.3% (14/105) | 1.05 (0.47–2.37) |
| 50–199 | 20.9% (14/67) | 21.2% (7/33) | 1.53 (0.60–3.90) | 20.6% (7/34) | 1.77 (0.64–4.90) |
| <50 | 32.3% (10/31) | 25.0% (2/8) | 1.89 (0.36–9.92) | 34.8% (8/23) | 3.65 (1.29–10.3)b |
| HAARTa | |||||
| No | 9.7% (48/485) | 9.9% (30/302) | 1 | 9.3% (17/183) | 1 |
| Yes, <2 years | 26.0% (13/50) | 33.3% (8/24) | 4.53 (1.79–11.5)c | 19.2% (5/26) | 2.32 (0.78–6.95) |
| Yes, ≥2 years | 45.6% (32/68) | 84.6% (11/13) | 49.9 (10.6–236)c | 36.7% (20/55) | 5.58 (2.66–11.72)c |
| First BED-CEIA result ≤0.8d | |||||
| No | — | — | — | 4.3% (8/188) | 1 |
| Yes | — | — | — | 59.4% (19/32) | 32.9 (12.1–89.4)c |
p value <0.05 by chi square.
p value <0.05.
p value <0.01.
Samples were available from both 2–4 years after seroconversion and 6–8 years after HIV seroconversion for 220 men; first BED-CEIA result refers to the test result obtained for the sample collected 2–4 years after seroconversion.
OR, odds ratio; CI, confidence intervals; HAART, highly active antiretroviral therapy; CEIA, capture enzyme immunoassay; OD-n, normalized optical density. Statistically significant values are shown in bold text.
All factors associated with misclassification in the univariate analysis with p<0.1 were included in the multivariate logistic regression analysis. To account for individuals who had samples from two time points (2–4 years and 6–8 years after seroconversion), we included a variable with the 2–4 year result as a predictor of the 6–8 year result. This allowed us to determine whether men who were misclassified at 2–4 years were likely to be misclassified at 6–8 years. We also investigated two composite variables based on CD4 cell count, HIV viral load, and HAART use. The first composite variable was positive if an individual had a CD4 cell count <50 cells/mm3 or an HIV viral load <400 copies/ml. The second composite variable was positive if either of these conditions was present, or if the individual was receiving HAART. All analyses were performed using STATA v11 (StataCorp, College Station, TX).
Human subjects
All work was conducted in accordance with the Declaration of Helsinki, with informed consent from each participant and approval by appropriate internal review boards.
Results
We analyzed 603 samples from 383 men (see Materials and Methods). Paired samples from 2–4 and 6–8 years after HIV seroconversion were available for 220 (57.4%) of the 383 men; 119 men had a single sample from 2–4 years after seroconversion, and 44 men had a single sample from 6–8 years after seroconversion. Most of the men (86.5%, 332/383) were white. At the time of sample collection, the median age was 40.3 years (range: 23.2 to 73.5 years), the median CD4 cell count was 480 cells/mm3 (range: 1 to 1711 cells/mm3), and the median HIV viral load was 73,400 copies/ml (range: <50 to 2,967,000 copies/ml); 117 (19.4%) of the 603 samples had HIV viral loads <400 copies/ml. Samples were collected from 1987 to 2009; of the 603 samples analyzed, 424 (70.3%) were collected before 1997 (prior to widespread availability of HAART) and 118 (19.6%) were from men who were receiving HAART at the time of sample collection. The prevalences of a seropositive status for coinfection with other viruses were 6.5% (39/603) for HCV, 67.5% (407/601) for HSV-2 (two men were missing data), and 60.7% (303/499) for HHV-8 (104 men were missing data).
Ninety-one (15.1%) of the 603 samples tested were misclassified as recently infected, and 66 (17.2%) of the 383 men tested were misclassified at one or both of the time points tested. In univariate analyses, the following factors were significantly associated with misclassification: age, year of sample collection, race, HIV viral load, CD4 cell count, and duration of HAART use (Table 1). Of note, 77% (10/13) of individuals who initiated HAART within 1 year of seroconversion were misclassified as recently infected when they were tested years later. Coinfection with HCV, HSV-2, or HHV-8 was not associated with misclassification. The frequency of misclassification from samples that were serologically reactive for the following coinfections was 12.8% (5/39) for HCV positive vs. 15.3% (86/564) for HCV negative, 14.0% (57/407) for HSV-2 positive vs. 17.0% (33/194) for HSV-2 negative, and 11.9% (36/303) for HHV-8 positive vs. 12.4% (24/196) for HHV-8 negative. Additionally, no association was seen as a total number of coinfection with misclassification by the BED-CEIA (data not shown). The frequency of misclassification was higher in samples from African-American and Hispanic men (12/44=27.3% and 6/22=27.3%, respectively) than in white men (72/530=13.6%, p=0.04). The frequency of misclassification was similar for samples collected 2–4 years versus 6–8 years after HIV seroconversion (14.5% versus 15.9%). Among men who were misclassified 2–4 years after seroconversion, 59.4% (19/32, 95% CI: 41–76%) were also misclassified 6–8 years after seroconversion. The misclassification frequency was reduced from 15.1% to 13.4% when men with CD4 cell counts <200 cells/mm3 were excluded from analysis, to 8.1% when men with HIV viral loads <400 copies/ml were excluded from analysis, and to 6.5% when men with either of these conditions were excluded from analysis.
In a standard multivariate model that did not include composite variables (Table 2, Model 1), the following factors were independently associated with misclassification: HIV viral load <400 copies/ml, CD4 cell count <50 cells/mm3, and duration of HAART >2 years. Furthermore, compared to men who were not misclassified 2–4 years after seroconversion, men who were misclassified 2–4 years after seroconversion were significantly more likely to be misclassified 6–8 years after seroconversion [adjusted odds ratio (aOR)=25.8, p<0.01]. In multivariate logistic regression models using composite variables to account for CD4 cell count, HIV viral load and HAART use (Table 2, Models 2 and 3), misclassification 2–4 years after HIV seroconversion predicted misclassification 6–8 years after seroconversion. In Model 2, the effect of HAART use for 2 or more years on misclassification was marginally statistically significant (p=0.048), even after adjusting for low CD4 cell count and low HIV viral load. The effects of age and year of sample collection were attenuated when the model was adjusted for CD4 cell count, HIV viral load, and HAART use (Models 2 and 3).
Table 2.
Adjusted Odds of Misclassification by the BED Capture Enzyme Immunoassay (OD-n ≤0.8) in Men Who Have Sex with Men with Nonrecent HIV Infection (Multicenter AIDS Cohort Study: 1987–2009)
| Factor | Model 1 aOR (95% CI) | Model 2 aOR (95% CI) | Model 3 aOR (95% CI) |
|---|---|---|---|
| All | — | — | — |
| Age (years) | |||
| 23–35 | 1 | 1 | 1 |
| 35–39 | 2.57 (1.08–6.13)a | 2.36 (1.01–5.52)a | 2.20 (0.95–5.10) |
| 40–44 | 1.63 (0.65–4.05) | 1.71 (0.70–4.17) | 1.74 (0.72–4.17 |
| 45–74 | 2.05 (0.88–4.78) | 2.08 (0.90–4.76) | 2.13 (0.94–4.80) |
| Sample year | |||
| 1987–1990 | 1 | 1 | 1 |
| 1990–1994 | 3.19 (1.21–8.34)a | 2.69 (1.06–6.82)a | 2.60 (1.03–6.57)a |
| 1994–1998 | 2.35 (0.81–6.86) | 1.95 (0.71–5.42) | 1.80 (0.66–4.95) |
| 1998–2009 | 2.70 (0.72–10.2) | 1.98 (0.57–6.90) | 3.26 (1.15–9.18)a |
| Race | |||
| White | 1 | 1 | 1 |
| Not white | 1.71 (0.80–3.66) | 2.07 (1.02–4.21)a | 1.83 (0.91–3.67) |
| HIV viral load (copies/ml) | |||
| >10,001 | 1 | ||
| 401 to 10,000 | 0.86 (0.36–2.05) | ||
| <400 | 3.96 (1.53–10.3)b | ||
| CD4 cell count (cells/mm3) | |||
| >500 | 1 | ||
| 200–500 | 1.76 (0.90–3.43) | ||
| 51–199 | 6.85 (2.71–17.4)b | ||
| <50 | 11.5 (3.64–36.0)b | ||
| HAART | |||
| No | 1 | 1 | |
| Yes, <2 years | 1.82 (0.56–5.91) | 1.54 (0.52–4.58) | |
| Yes, ≥2 years | 4.72 (1.35–16.5)a | 3.16 (1.01–9.84)a | |
| Visit-dependent variable | |||
| 2–4 years after SC | 1 | 1 | 1 |
| 6–8 years after SC, no 2–4 year sample | 0.48 (0.18–1.27) | 0.71 (0.29–1.72) | 0.79 (0.34–1.88) |
| 6–8 years after SC, not misclassified at 2–4 years | 0.14 (0.06–0.30)b | 0.18 (0.08–0.37)b | 0.20 (0.10–0.40)b |
| 6–8 years after SC, misclassified at 2–4 years | 3.56 (1.19–10.6)a | 4.01 (1.42–11.4)b | 4.56 (1.69–12.3)b |
| Composite variable 1 | |||
| HIV VL >400 and CD4 >50 | 1 | ||
| HIV VL <400 or CD4 <50 | 3.88 (1.95–7.73)b | ||
| Composite variable 2 | |||
| HIV VL >400 and CD4 >50 and no HAART | 1 | ||
| HIV VL <400 or CD4 <50 or HAART | 3.78 (1.94–7.36)b | ||
p value <0.05.
p value <0.01.
aOR, adjusted odds ratio; CI, confidence interval; HAART, highly active antiretroviral therapy; SC, seroconversion. Statistically significant values are shown in bold text.
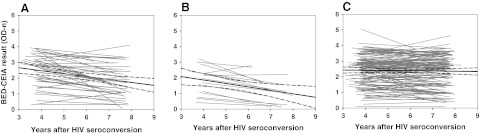
We next compared BED-CEIA values obtained for paired samples collected 2–4 and 6–8 years after HIV seroconversion to evaluate trends in misclassification in individual study participants. Different patterns of longitudinal BED-CEIA results were obtained for different subgroups of participants (Fig. 1). Among men who initiated HAART between the two study visits, BED-CEIA results decreased by a median of 0.54 OD-n units [interquartile range (IQR): 0.22 to 0.70, Fig. 1A]. In men who experienced significant immune decline between the two study visits (those with a CD4 cell count >50 cells/mm3 2–4 years after HIV seroconversion and <50 cells/mm3 6–8 years after HIV seroconversion, Fig. 1B), BED-CEIA results decreased by a median of 0.70 OD-n units (IQR: 0.44 to 0.83). For these men, the average decrease in CD4 cell count was 342 cells/mm3 (IQR: 165 to 482, range 53 to 770). In contrast, BED-CEIA values were stable in the subgroup of 137 men who did not initiate HAART and who had CD4 cell counts >50 cells/mm3 both 2–4 and 6–8 years after seroconversion [median change: 0.00 OD-n units (IQR: −0.28 to 0.34, Fig. 1C)]. Seven of the 137 men (5.0%) who were not on HAART and had CD4 cell counts >50 cells/mm3 and viral loads >400 copies/ml at both study visits had an OD-n ≤0.8 at both time points. These data show that misclassification by the BED-CEIA is not a transient phenomenon.
FIG. 1.
Comparison of paired BED capture enzyme immunoassay (CEIA) results within an individual 2–4 and 6–8 years after HIV seroconversion (Multicenter AIDS Cohort Study: 1987–2009). The x-axis shows the number of years between HIV seroconversion and sample collection. The y-axis shows the BED-CEIA result in normalized optical density (OD-n) units. Two samples from each individual were tested with the BED-CEIA: one collected 2–4 years after HIV seroconversion and one collected 6–8 years after HIV seroconversion; results from the two samples from each individual are connected with a gray line. (A) Data for men who had a CD4 cell count >50 cells/mm3 and an HIV viral load >400 copies/ml at 2–4 years who initiated HAART between the two time points and were receiving HAART 6–8 years after seroconversion. (B) Data for men who had a CD4 cell count >50 copies/mm3 2–4 years after seroconversion and a CD4 cell count <50 copies/mm3 6–8 years after seroconversion. (C) Data for men who had a CD4 cell count >50 cells/mm3 and an HIV viral load >400 copies/ml at both time points, and who were not receiving HAART. In each panel of the figure, a solid black line shows the regression line for all paired data; dashed black lines show the 95% confidence intervals for the regression line.
Discussion
In this study, 15.1% of men who were followed for more than 2 years after HIV seroconversion were identified as recently infected using the BED-CEIA. In multivariate models, low CD4 cell count, low viral load, and time on HAART were significantly associated with misclassification. Since production of anti-HIV antibodies is diminished when viral replication is suppressed by the immune system or by HAART, and since the BED-CEIA measures the proportion of IgG that is specific for HIV, it is not surprising that some men with low HIV viral loads had low BED-CEIA results. The proportion of antibodies specific for HIV is also likely to decrease when the immune system collapses. Therefore, it is also not surprising that some men with low CD4 cell counts had low BED-CEIA results. It is notable, however, that when individuals with low CD4 cell counts and low viral loads were excluded from our analyses, the misclassification frequency of the BED-CEIA was 6.5%; this is higher than the misclassification frequency noted in a previous report.26
We previously demonstrated the stability of BED-CEIA results over time in a cohort of HIV-infected Ugandan women.27 Interestingly, most men in the MACS also had stable BED-CEIA results over a period of several years (Fig. 1C) if they did not become virally suppressed (Fig. 1A) or severely immune compromised (Fig. 1B) at the later time point. In addition, we identified a subgroup of men in the MACS who had stable, low BED-CEIA values (≤0.8 OD-n) that were not explained by low HIV viral load, low CD4 cell count, or HAART use. The factor most strongly associated with misclassification 6–8 years after HIV seroconversion was previous misclassification by the BED-CEIA. We recently demonstrated that repeated freeze thaws or prolonged incubation at various temperatures did not impact results obtained with the BED-CEIA; therefore, use of archived samples that may have been thawed previously was not likely to have led to artificially low BED-CEIA results in this study.27 Studies are underway to determine whether persistent misclassification is explained by a viral factor (e.g., if the gp41 sequence in HIV from participants with persistently low BED-CEIA values differs significantly from the sequence of the peptide target in the BED-CEIA assay).
The misclassification by race can be explained by the differences in IgG concentrations in African-Americans and Hispanics as compared to whites. A study by McGowan et al.28 demonstrated higher levels of total IgG in African-Americans and Hispanics compared to whites for both HIV-infected and HIV-uninfected individuals. An additional study found that HIV-infected African-Americans had significantly higher levels of total IgG compared to HIV-infected whites across all levels of CD4 counts.29 Since the BED-CEIA measures the proportion of IgG specific for HIV of the total IgG, African-Americans and Hispanics may be more likely to be misclassified than whites as the proportion of IgG specific for HIV of total IgG would be lower. However, data are not available comparing the level of antibodies specific to the gp41 target antigen used in the BED-CEIA in different racial groups.
The specificity of the BED-CEIA can be increased by lowering the cut-off value that is used to define recent HIV infection. In one study, the BED-CEIA cut-off value was reduced from 0.8 to 0.4 OD-n to reduce the frequency of misclassification.7 However, this also reduces the sensitivity for detection of recently infected individuals.4 In this study, factors that were associated with misclassification at the higher cut-off of 0.8 OD-n (e.g., low HIV viral load, low CD4 cell count, and duration of HAART use) were still associated with misclassification at a cut-off of 0.4 OD-n (data not shown). The frequency of misclassification was reduced when men with low CD4 cell counts and/or low viral loads were classified as not recently infected (without changing the assay cut-off from 0.8 OD-n).
This report provides information on the nature and frequency of misclassification of the BED-CEIA in MSM from the United States who are likely to be infected with subtype B HIV. The observed misclassification rate of 15.1% is much higher than the misclassification rate of 3% that is stated in the BED-CEIA package insert. Consideration of CD4 cell count and HIV viral load data in the evaluation of HIV incidence using the BED-CEIA can reduce the misclassification rate by identifying individuals who are unlikely to have been infected within the past year; in our study, this approach reduced the misclassification frequency from 15.1% to 6.5%. Inclusion of criteria such as a CD4 cell count above 200 cells/mm3 and an HIV viral load greater than 400 copies/ml in a cross-sectional HIV incidence testing algorithm should not significantly impact the sensitivity of detection of recently infected individuals (sensitivity of the algorithm). In the MACS in the absence of HAART, only 1.7% (7/406) of seroconverters had a CD4 cell count below 200 cells/mm3 and only 5.4% (22/406) had a viral load below 400 copies/ml within the first year after seroconversion. Further studies are needed to determine the window period for recent HIV infection using different cut-offs for the BED-CEIA, and to determine whether consideration of CD4 cell count, HIV viral load, or other biomarkers increases the precision of HIV incidence estimates obtained using the BED-CEIA. Studies are also needed to determine whether findings from this report can be generalized to women, since gender-related differences in the immunologic response to HIV infection have been noted,20 and to individuals infected with non-subtype B HIV.
Acknowledgments
The authors thank Stacey Meyerer for her assistance with sample analysis and the MACS study team and MACS participants for providing the samples and data used in this study. This work was supported by (1) the HIV Prevention Trials Network (HPTN) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the NIH, DHHS (U01-AI46745, U01-AI48054, U01-AI068613, and UM1-AI068613), (2) 1R01-AI095068, (3) the International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) Network (U01-AI068632), (4) the Multicenter AIDS Cohort Study (MACS) sponsored by the NIAID, with additional supplemental funding from the National Cancer Institute and the National Heart, Lung and Blood Institute (UO1-AI35042, UL1-RR025005, UO1-AI35043, UO1-AI35039, UO1-AI35040, and UO1-AI35041), and (5) the Division of Intramural Research, NIAID, NIH. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Institutes of Health. Use of trade names is for identification purposes only and does not constitute endorsement by the National Institutes of Health and Prevention or the Department of Health and Human Services.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Brookmeyer R. Measuring the HIV/AIDS epidemic: Approaches and challenges. Epidemiol Rev. 2010;32:26–37. doi: 10.1093/epirev/mxq002. [DOI] [PubMed] [Google Scholar]
- 2.Murphy G. Parry JV. Assays for the detection of recent infections with human immunodeficiency virus type 1. Euro Surveill. 2008;13:pii18966. [PubMed] [Google Scholar]
- 3.Busch MP. Pilcher CD. Mastro TD. Kaldor J. Vercauteren G. Rodriguez W, et al. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS. 2010;24:2763–2771. doi: 10.1097/QAD.0b013e32833f1142. [DOI] [PubMed] [Google Scholar]
- 4.Dobbs T. Kennedy S. Pau CP. McDougal JS. Parekh BS. Performance characteristics of the immunoglobulin G-capture BED-enzyme immunoassay, an assay to detect recent human immunodeficiency virus type 1 seroconversion. J Clin Microbiol. 2004;42:2623–2628. doi: 10.1128/JCM.42.6.2623-2628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall HI. Song R. Rhodes P. Prejean J. An Q. Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prejean J. Song R. Hernandez A. Ziebell R. Green T. Walker F, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mermin J. Musinguzi J. Opio A. Kirungi W. Ekwaru JP. Hladik W, et al. Risk factors for recent HIV infection in Uganda. JAMA. 2008;300:540–549. doi: 10.1001/jama.300.5.540. [DOI] [PubMed] [Google Scholar]
- 8.Parekh BS. Hanson DL. Hargrove J. Branson B. Green T. Dobbs T, et al. Determination of mean recency period for estimation of HIV type 1 incidence with the BED-Capture EIA in persons infected with diverse subtypes. AIDS Res Hum Retroviruses. 2011;27:265–273. doi: 10.1089/aid.2010.0159. [DOI] [PubMed] [Google Scholar]
- 9.Barnighausen T. McWalter TA. Rosner Z. Newell ML. Welte A. HIV incidence estimation using the BED capture enzyme immunoassay: Systematic review and sensitivity analysis. Epidemiology. 2010;21:685–697. doi: 10.1097/EDE.0b013e3181e9e978. [DOI] [PubMed] [Google Scholar]
- 10.UNAIDS Reference Group on Estimates Modeling and Projections. Statement on the use of the BED assay for estimation of HIV-1 incidence or epidemic monitoriing. Weekly Epidemiol Rec. 2006;81:33–40. [PubMed] [Google Scholar]
- 11.United States Centers for Disease Control. Using the BED HIV-1 capture EIA assay to estimate incidence using STARHS in the context of surveillance in the United States. 2007. http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/BED.htm http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/BED.htm
- 12.Gouws E. Methods for estimating HIV incidence. UNAIDS quarterly update on HIV epidemiology/1Q 2010.
- 13.Barnighausen T. Wallrauch C. Welte A. McWalter TA. Mbizana N. Viljoen J, et al. HIV incidence in rural South Africa: Comparison of estimates from longitudinal surveillance and cross-sectional cBED assay testing. PLoS One. 2008;3:e3640. doi: 10.1371/journal.pone.0003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karita E. Price M. Hunter E. Chomba E. Allen S. Fei L, et al. Investigating the utility of the HIV-1 BED capture enzyme immunoassay using cross-sectional and longitudinal seroconverter specimens from Africa. AIDS. 2007;21:403–408. doi: 10.1097/QAD.0b013e32801481b7. [DOI] [PubMed] [Google Scholar]
- 15.Marinda ET. Hargrove J. Preiser W. Slabbert H. van Zyl G. Levin J, et al. Significantly diminished long-term specificity of the BED capture enzyme immunoassay among patients with HIV-1 with very low CD4 counts and those on antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;53:496–499. doi: 10.1097/qai.0b013e3181b61938. [DOI] [PubMed] [Google Scholar]
- 16.Hladik W. Olara D. Mermin J. Moore D. Were W. Alexander L. Downing R. Effect of CD4+ T-cell count and antiretroviral treatment on two serological HIV incidence assays. AIDS Res Hum Retroviruses. 2011 Mar 11; doi: 10.1089/aid.2010.0347. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Hayashida T. Gatanaga H. Tanuma J. Oka S. Effects of low HIV type 1 load, antiretroviral treatment on IgG-capture BED-enzyme immunoassay. AIDS Res Hum Retroviruses. 2008;24:495–498. doi: 10.1089/aid.2007.0150. [DOI] [PubMed] [Google Scholar]
- 18.McDougal JS. Parekh BS. Peterson ML. Branson BM. Dobbs T. Ackers M. Gurwith M. Comparison of HIV type 1 incidence observed during longitudinal follow-up with incidence estimated by cross-sectional analysis using the BED capture enzyme immunoassay. AIDS Res Hum Retroviruses. 2006;22:945–952. doi: 10.1089/aid.2006.22.945. [DOI] [PubMed] [Google Scholar]
- 19.Nesheim S. Parekh B. Sullivan K. Bulterys M. Dobbs T. Lindsay M, et al. Temporal trends in HIV Type 1 incidence among inner-city childbearing women in Atlanta: Use of the IgG-capture BED-enzyme immunoassay. AIDS Res Hum Retroviruses. 2005;21:537–544. doi: 10.1089/aid.2005.21.537. [DOI] [PubMed] [Google Scholar]
- 20.Klein SL. Jedlicka A. Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaslow RA. Ostrow DG. Detels R. Phair JP. Polk BF. Rinaldo CR., Jr The Multicenter AIDS Cohort Study: Rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 22.Summerton J. Riedesel M. Laeyendecker O. Gaydos C. Maldeis NE. Hardick A, et al. Effect of sexually transmitted disease (STD) coinfections on performance of three commercially available immunosorbent assays used for detection of herpes simplex virus type 2-specific antibody in men attending Baltimore, Maryland, STD clinics. Clin Vaccine Immunol. 2007;14:1545–1549. doi: 10.1128/CVI.00120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson LP. Jenkins FJ. Springer G. Munoz A. Shah KV. Phair J, et al. Interaction of human immunodeficiency virus type 1 and human herpesvirus type 8 infections on the incidence of Kaposi's sarcoma. J Infect Dis. 2000;181:1940–1949. doi: 10.1086/315503. [DOI] [PubMed] [Google Scholar]
- 24.Tobler LH. Stramer SL. Lee SR. Masecar BL. Peterson JE. Davis EA, et al. Impact of HCV 3.0 EIA relative to HCV 2.0 EIA on blood-donor screening. Transfusion. 2003;43:1452–1459. doi: 10.1046/j.1537-2995.2003.00521.x. [DOI] [PubMed] [Google Scholar]
- 25.Parekh BS. Kennedy MS. Dobbs T. Pau CP. Byers R. Green T, et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: A simple assay for detecting recent HIV infection and estimating incidence. AIDS Res Hum Retroviruses. 2002;18:295–307. doi: 10.1089/088922202753472874. [DOI] [PubMed] [Google Scholar]
- 26.Hargrove JW. Humphrey JH. Mutasa K. Parekh BS. McDougal JS. Ntozini R, et al. Improved HIV-1 incidence estimates using the BED capture enzyme immunoassay. AIDS. 2008;22:511–518. doi: 10.1097/QAD.0b013e3282f2a960. [DOI] [PubMed] [Google Scholar]
- 27.Laeyendecker O. Latimore A. Eshleman SH. Summerton S. Oliver AE. Gamiel J, et al. The effect of sample handling on cross sectional HIV incidence testing results. PLoS One. 2011;6(10):e25899. doi: 10.1371/journal.pone.0025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGowan JP. Shah SS. Small CB. Klein RS. Schnipper SM. Chang CJ. Rosenstreich DL. Relationship of serum immunoglobulin, IgG subclass levels to race, ethnicity, behavioral characteristics in HIV infection. Med Sci Monit. 2006;12:CR11–CR16. [PubMed] [Google Scholar]
- 29.Lucey DR. Hendrix CW. Andrzejewski C. Melcher GP. Butzin CA. Henry R, et al. Comparison by race of total serum IgG, IgA, and IgM with CD4+ T-cell counts in North American persons infected with the human immunodeficiency virus type 1. J Acquir Immune Defic Syndr. 1992;5:325–332. [PubMed] [Google Scholar]