Abstract
Acute HIV-1 infection causes a rapid total body depletion of CD4+ T cells in most individuals and HIV-1-specific CD8+ T cell expansion in response to viral replication. A numerically high CD8 T cell response may indicate limited T cell repertoire against HIV and rapid progression. We present a detailed evaluation of an acutely infected individual with a strong HIV-1-specific CD8 T cell response targeting multiple epitopes demonstrating that the upper limit of CD8 expansion in this setting may be much higher than previously reported and was likely driven by the narrow HIV-specific response.
Introduction
Acute HIV-1 infection (AHI) causes a rapid total body depletion of CD4+ T cells in most individuals.1,2 Prior studies have shown that HIV-1-specific CD8 expansion occurs in the setting of AHI and a substantial proportion of CD8 cells may be HIV-1 specific.3 The specificity and diversity of the response likely influence viral set point and disease progression.4,5 The range of the overall CD8 T cell response (i.e., numbers of CD8+ cells) has not been well described.6 A recent AHI case in North Carolina with markedly elevated total CD8 count prompted us to perform a more detailed analysis of this individual's response and examine CD8 cell numbers in our AHI cohort.
Case Report: In December 2007 a 32-year-old African-American man with a remote history of Chlamydia urethritis was admitted to the hospital after presenting with a 2-week history of fevers to 105°F, chills, decreased appetite, weight loss, sore throat, dysphagia, odynophagia, night sweats, nausea, and vomiting. On physical examination he was found to have thrush and bilateral cervical lymphadenopathy (largest 1.5 cm in diameter). He reported a prior negative HIV test and a new sexual partner. Three days after admission HIV-1 enzyme-linked immunosorbent assay (ELISA) was positive, HIV-1 Western blot was negative, and serum HIV-1 viral load was 5,793,980 copies/ml, consistent with AHI.
Laboratory results included an aspartate aminotransferase of 666 U/liter (normal 19–55 U/liter), alanine aminotransferase of 1174 U/liter (normal 19–72 U/liter), and serum lactate dehydrogenase of 8393 U/liter (normal 338–610 U/liter). An initial CD4 count was 484 cells/mm3 (7%) with elevated CD8 at 5392 cells/μl (78%) 8 days after admission. He had an undetectable serum hepatitis C virus (HCV) and cytomegalovirus (CMV) viral load, and Epstein–Barr virus (EBV) DNA was detected in the serum at less than 500 copies/ml. EBV serologies were VCA IgG positive, EBNA positive, and VCA IgM negative consistent with prior infection. Hepatitis serologies were negative. The patient remained symptomatic for approximately 21 days after his admission with fatigue, dysphagia, nausea, diarrhea, and abdominal pain.
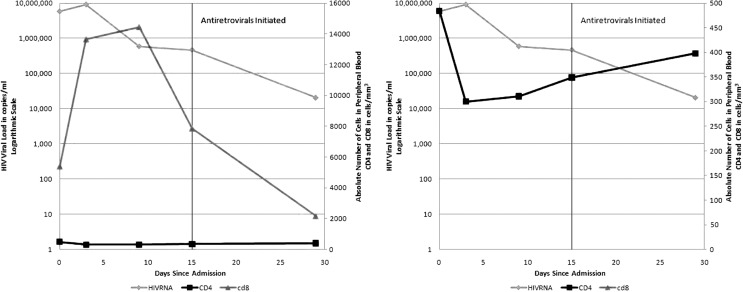
Eleven days after admission his CD4 count was 300 cells/μl (2%) and his absolute CD8 count was 13,654 cells/μl (91%). Seventeen days postadmission the total absolute CD8 count had risen to 14,459 cells/μl (93%). The very high CD8 levels were concerning for hematologic malignancy, therefore diagnostic PCR-based testing (TCR gene rearrangement) on peripheral blood was performed to determine cellular clonality of the expanding population. Analysis of gamma chain rearrangement showed two predominant populations, which is consistent with a clonal population with both alleles rearranged (biallelic gene rearrangement) or with two separate clonal populations. This result was consistent with, but not diagnostic of, a malignancy such as large granular lymphocyte leukemia.7 Antiretroviral medication was started 23 days after admission and a repeat absolute CD8 count was 7861 cells/mm3 prior to the first dose of therapy. Subsequent CD8 counts on therapy were lower, inconsistent with hematologic malignancy (Fig. 1). Antiretroviral therapy was offered because of the severity of the patient's symptoms, the low CD4 nadir, and the initial concern for malignancy. The risks and benefits of starting antiretroviral therapy in the setting of acute infection were discussed with the patient who opted for starting therapy, anticipating lifelong therapy. We therefore examined whether the significant CD8 expansion observed in this patient reflected a very strong adaptive immune response to acute HIV-1 infection.
FIG. 1.
CD8 counts on therapy.
Materials and Methods
A portion of the data was obtained as part of the diagnostic work-up, including the patient's history, routine laboratory data, and TCR gamma rearrangement assay to assess for clonality. The Health Insurance Portability and Accountability Act (HIPAA) confidentiality regulations were followed in compiling this information from the patient's medical records and the patient consented for nonidentifying information to be included in a clinical report. After informed consent was obtained, the patient donated blood for laboratory evaluation as part of a longitudinal study on acutely infected HIV patients.
CD8 activation and HIV-specific responses
The frequency of circulating activated CD8+ T cells was determined by flow cytometric analysis of CD38 and HLA-DR expression. The magnitude of HIV-1-specific CD8+ T cell responses was measured using interferon (IFN)-γ ELISpot assays and by multiparameter flow cytometry for the detection of degranulation (CD107) and production of cytokines (IFN-γ. IL-2, and TNF-α) and the chemokine (MIP-1β) following stimulation with pools of autologous peptides.8 Single genome amplification was performed to determine the patient's transmitted virus sequence.9
Range of absolute CD8 counts among acutely infected individuals
An analysis of the absolute CD8+ response in multiple acutely infected HIV-positive individuals was performed using data collected as part of an acute HIV infection cohort.10 Peak absolute CD8 counts were determined among individuals who had an absolute CD8 count drawn within 6 months of infection and prior to starting antiretroviral therapy.
Results
CD8 activation and HIV-specific responses
At peak, the absolute number of CD3+CD8+ cells was 14,459 cells/μl and 91% of total lymphocytes were CD3+CD8+. Among 65 individuals who had been acutely infected with HIV and had absolute CD8 counts drawn within 6 months of presentation and prior to starting antiretroviral therapy the mean/median peak CD8 count was 1377/1174 (IQR 761:1486, min 132, max 8776 cells/ml). Only 3 out of 65 peak (5%) CD8 counts were greater than 4000 cells/ml. In this subset of acutely infected individuals, 43 of the 65 individuals had a negative enzyme immunoassay (EIA) at the time of diagnosis when the initial CD8 counts were collected (Fiebig stage II or earlier).
In this individual the frequency of circulating activated CD8+ T cells (CD38+HLA-DR+) was 92%, substantially above the normal range observed in healthy individuals (average 8.9%, range 2.69–22.7%) and well above the median observed in our cohort of 100 HIV-1 acutely infected subjects (average 65.6%, range 22.7–95%).
Single genome amplification (SGA) and sequencing techniques demonstrated that this patient was infected with two closely related viruses. Peptides were synthesized to match the major transmitted virus. Different cellular assays were employed to measure the magnitude of HIV-1-specific CD8+ T cell responses at the peak of CD8 expansion and before therapy was initiated. Ex vivo IFN-γ ELISpot assay revealed strong IFN-γ-producing T cell responses to four epitopes (Table 1). In subsequent studies, 9% of all CD3+/CD8+ T cells were responding to a single epitope, binding the YPLTFGWCY B*0702 tetramer. Multiparameter flow cytometry to detect degranulation and production of IFN-γ, IL-2, TNF-α, and MIP-1β following 6 h stimulation with autologous (matching founder virus) and consensus clade B peptide pools revealed that the frequency of HIV-1 Env-, Gag-, and Nef-specific CD8+ T cells represented 18% of the total memory CD8+ T cell subset. The standard method of overnight rest followed by 6 h of antigen-specific stimulation of the T cell population resulted in a loss of 50% of the cells when compared to cell recoveries in chronically infected individuals. The rapid loss of CD8 viability suggests the measured fraction of HIV-1-specific cells underestimates the total HIV-specific response given that only viable cells can be quantified. In addition, HIV-1-specific T cells could not be expanded in vitro, further supporting the HLA-DR/CD38 phenotyping data that cells were highly activated and most likely terminally differentiated.
Table 1.
T Cell Responses Mapped in Acute HIV-1 Infection (Day 18 from Admission)
| Virus | Protein | Sequence | IFN-γ ELISPOT (SFU/106PBMC) | Tetramer frequency (% of CD3/CD8 T cells) | Restriction |
|---|---|---|---|---|---|
| HIV-1 | GP160(585–593)a | RYLKDQQLL | 4220 | ND | CW*0702 |
| HIV | Integrase (263–271) | RKAKIIRDY | 2255 | ND | B*1503 |
| HIV | NEF (135–143) | YPLTFGWCY | 9180 | 9.0% | B*0702 |
| HIV | ENV (637–645) | NYTSLIYNL | 4615 | ND | CW*0702 |
| HCMV | pp65 (415–429)b | TPRVTGGGAM | 160 | ND | B*07 |
Amino acid alignment relative to HXB2 strain.
Alignment relative to HCMV AD169 strain.
IFN-γ, interferon gamma; PBMC, peripheral blood mononuclear cells.
Discussion
This case demonstrates a massive CD8 expansion in the setting of acute HIV infection that was associated with a severe symptomatic infection and a rapid decline in peripheral CD4 count. In the absence of any evidence of malignancy, the strong CD8 cell expansion observed likely reflects the rapid expansion of HIV-1-specific CD8 T cells following infection. We cannot exclude activation and cytokine-driven expansion of non-HIV-1-specific T cells or significant immune system dysfunction, but if non-HIV-1-specific expansion of T cells did occur, it was not reflected in the CMV T cell response to the pp65 TPRVTGGGAM epitope, which was detectable but 50- to 79-fold lower in magnitude than the mapped HIV-1-specific responses (Table 1). In addition, assuming a relationship between specific IFN-γ secretion as measured by ELISpot and tetramer frequency,11 as many as 20% of CD8 T cells in this patient were directed at the four epitopes identified. High frequencies of HIV-1-specific T cells have been reported in other studies, with studies by Wilson et al.3 reporting HIV-1 tetramer-specific frequencies approaching 4% in acute infection and Papagno et al.12 reporting frequencies of 10% tetramer-positive CD8 T cells in chronic HIV-1 infection. More recently, Ferrari et al.,13 employing intracellular cytokine and degranulation (IFN-γ, IL-2, TNF-α, MIP-β, CD107) flow cytometry techniques, reported frequencies of acute HIV-1epitope-specific CD8 T cells in the range of from 0.65 to 7.29% of the total CD8 population within the first 4 weeks of infection. The capacity of the T cell response to expand rapidly in acute viral infections is best demonstrated by studies of acute EBV infection where EBV-specific CD8 T cells comprised up to 44% of the total CD8 T cell response.14
The 40% contraction of absolute CD8 cells within 5 days and results from in vitro assays suggested that cells were functional but highly apoptopic and poorly replicative. These observations are consistent with primary T cell responses and kinetics to live attenuated yellow fever vaccines15 and mouse models of virus infection16 that describe strong activation and expansion of virus-specific primarily effector T cells within weeks of infection followed by a contraction phase limiting the duration of the primary T cell response preventing immunopathology that would no doubt result if T cell expansion remained unchecked.
Recent studies have shown that primary T cell responses make a significant contribution to killing of virus-infected cells but may also select for rapid viral escape during acute viremia.8 Because this patient went on antiretroviral therapy within weeks of admission we have insufficient data on whether these primary HIV-1-specific T cell responses selected for viral escape. It is notable, however, that there was a 1.5 log drop in viremia over a 10-day period coincident to the detection of HIV-1-specific T cell responses. Previous studies on CD8 expansion during AHI have demonstrated that HIV-1-specific expansion may represent a limited T cell repertoire, and may be associated with more rapid progression. Conversely, Ferrari et al.13 described the CD8+ T cell response to acute HIV infection in symptomatic patients. They did not see an association with the magnitude of CD8+ T cell response and the appearance of escape mutants. In this individual, the height of the CD8 T cell response, the systemic symptoms, and elevated LFT in the setting of clonality by TCR gene rearrangement raised concerns that the patient may have presented with a malignant or premalignant syndrome. The rapid decline in CD8 count and the subsequent epitope analysis were not consistent with malignancy.
The question of whether this CD8 cell response was targeted entirely or predominantly against HIV is difficult to answer, but the HIV-specific response was robust, the proportion of activated CD8 cells was remarkably high,and despite the level of immune activation levels of other common viral pathogens (CMV and EBV) were either not detected or below quantification. Certainly the magnitude of the CD8 T cell response contributed to the patient's clinical illness, but whether the response was ineffectual is not certain. We note that the CD4 cell count was rising and the CD8 count and plasma HIV RNA were falling prior to the institution of antiretroviral therapy, which his clinicians believed was indicated based on the symptoms, CD4 decline, and CD8 expansion data at the time.
In conclusion, this case demonstrates that the magnitude of immune activation accompanying AHI can extend beyond what has been described to the point of mimicking malignancy. This massive expansion of CD8 T cells could have contributed to the severe symptoms seen in this individual and may reflect a profound disregulation of the T cell compartment. These results provide insights into the diversity of response to acute HIV infection.
Contributor Information
Collaborators: the Center for HIV/AIDS Vaccine Immunology AI067854
Acknowledgments
This work was funded through NIH Grant 2R01-AI50483-07, 5T32-AI007001-32, the Center for HIV/AIDS Vaccine Immunology AI067854, UNC CFAR-AI50410, and CTSA-RR025747.
Author Disclosure Statement
Dr. Eron is a consultant to Merck, GlaxoSmithKline, BristolMyersSquibb, and Tibotec. He is the principal investigator on research grants to the University of North Carolina from GlaxoSmithKline, Merck, and Taimed. Dr. Hicks has received grant support and/or honoraria or been a consultant for BMS, GSK, Gilead, Tibotec, Merck, Pfizer, and Myriad Pharmaceuticals. Dr. Arnoczy has received grant support from GSK and been on an advisory board for Tibotec.
References
- 1.Guadalupe M. Reay E. Sankaran S. Prindiville T. Flamm J. McNeil A, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao J. McNevin J. Malhotra U. McElrath MJ. Evolution of CD8+ T cell immunity and viral escape following acute HIV-1 infection. J Immunol. 2003;171:3837–3846. doi: 10.4049/jimmunol.171.7.3837. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JD. Ogg GS. Allen RL. Davis C. Shaunak S. Downie J, et al. Direct visualization of HIV-1-specific cytotoxic T lymphocytes during primary infection. AIDS. 2000;14:225–233. doi: 10.1097/00002030-200002180-00003. [DOI] [PubMed] [Google Scholar]
- 4.Streeck H. Jolin JS. Qi Y. Yassine-Diab B. Johnson RC. Kwon DS, et al. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J Virol. 2009;83:7641–7648. doi: 10.1128/JVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pantaleo G. Demarest JF. Soudeyns H. Graziosi C. Denis F. Adelsberger JW, et al. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 6.Tindall B. Barker S. Donovan B. Barnes T. Roberts J. Kronenberg C, et al. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Arch Intern Med. 1988;148:945–949. [PubMed] [Google Scholar]
- 7.Davey MP. Bongiovanni KF. Kaulfersch W. Quertermous T. Seidman JG. Hershfield MS, et al. Immunoglobulin and T-cell receptor gene rearrangement and expression in human lymphoid leukemia cells at different stages of maturation. Proc Natl Acad Sci USA. 1986;83:8759–8763. doi: 10.1073/pnas.83.22.8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goonetilleke N. Liu MK. Salazar-Gonzalez JF. Ferrari G. Giorgi E. Ganusov VV, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keele BF. Giorgi EE. Salazar-Gonzalez JF. Decker JM. Pham KT. Salazar MG, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilcher CD. Fiscus SA. Nguyen TQ. Foust E. Wolf L. Williams D, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med. 2005;352:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 11.Tan LC. Gudgeon N. Annels NE. Hansasuta P. O'Callaghan CA. Rowland-Jones S, et al. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 12.Papagno L. Appay V. Sutton J. Rostron T. Gillespie GM. Ogg GS, et al. Comparison between HIV- and CMV-specific T cell responses in long-term HIV infected donors. Clin Exp Immunol. 2002;130:509–517. doi: 10.1046/j.1365-2249.2002.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari G. Korber B. Goonetilleke N. Liu MKP. Turnbull EL, et al. Relationship between functional profile of HIV-1 specific CD8 T cells, epitope variability with the selection of escape mutants in acute HIV-1 Infection. PLoS Pathog. 2011;7:2. doi: 10.1371/journal.ppat.1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callan MF. Tan L. Annels N. Ogg GS. Wilson JD. O'Callaghan CA, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akondy RS. Monson ND. Miller JD. Edupuganti S. Teuwen D. Wu H, et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol. 2009;183:7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaech SM. Wherry EJ. Ahmed R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]