Abstract
HIV-infected patients have low vitamin D levels as well as an increase in cardiovascular (CVD) risk. We examined the relationship between vitamin D and three markers of arterial dysfunction among HIV-infected individuals on stable antiretroviral (ARV) therapy. Levels of 25-hydroxyvitamin D [25(OH)D] were assessed by chemiluminescent immunoassay (DiaSorin) in 100 enrollees into the Hawaii Aging with HIV-Cardiovascular Cohort Study, a cohort of HIV-infected subjects age ≥40 years on stable (≥6 months) ARV therapy. The relationships between 25(OH)D levels and brachial artery flow-mediated dilation (FMD), right common carotid artery intima-media thickness (cIMT), and coronary artery calcium (CAC) were examined. Analytical methods included Pearson's correlations, Kruskal–Wallis tests, relative risks, and linear regression models. The cohort was 86% male and 60% white with a median age of 52 years and CD4 of 510 cells/mm3. The median (Q1, Q3) level of 25(OH)D was 27.9 ng/ml (21.8, 38.3). There were 72 FMD, 50 cIMT, and 90 CAC measurements available for analyses. A significant correlation was observed between 25(OH)D levels and FMD (r=0.30, p=0.01) but not with cIMT (r=−0.05, p=0.76). In a linear regression model, Framingham risk score attenuated the relationship between FMD and 25(OH)D. Those with lower 25(OH)D levels were at slightly higher risk of having CAC (RR=1.02, p=0.04). Among those with CAC, lower 25(OH)D levels were not associated with higher CAC scores (p=0.36). Lower vitamin D levels are associated with evidence of subclinical arterial dysfunction in HIV-infected individuals. The significance of these findings warrants further investigation.
Introduction
Observational studies in the general population have identified an association between low vitamin D levels and cardiovascular disease (CVD) risk.1 Although the exact relationship remains controversial, this issue is of potential concern in the HIV-infected population as both low vitamin D levels2,3 and increased CVD risk4 have been reported. We examined the associations between 25-hydroxyvitamin D [25(OH)D] levels and three markers of arterial dysfunction [brachial artery flow-mediated dilatation (FMD), carotid artery intima-media thickness (cIMT), and coronary artery calcium (CAC)] measured in the same individuals to determine if correlations between 25(OH)D levels and markers of arterial dysfunction were present in a cohort of HIV-infected subjects.
Materials and Methods
Subjects and study design
Analyses utilized baseline (entry) data from the Hawaii Aging with HIV-Cardiovascular [HAHC-CVD] study, a 5-year longitudinal cohort study of HIV-infected individuals designed to investigate the pathogenesis of CVD. The study is linked to a HIV-CVD consortium funded by the National Heart, Lung and Blood Institute (NHLBI), which provided centralized services for reading of FMD, cIMT, and CAC. The HAHC-CVD study was approved by the University of Hawaii Committee on Human Subjects and informed consents were obtained from all subjects. At the time of the initial consent, subjects also provided informed consent to have their blood and urine specimens banked and utilized for future research related to HIV and/or cardiovascular health.
Recruitment of subjects targeted the general HIV-infected population in Hawaii, which is predominantly male with the major HIV risk factor being male-to-male sex. This study analyzed the first 100 subjects entered into the cohort over the first 2 years of the study. By ethnicity, the population is approximately 60% white, 25% Asian/Pacific Islander, and 15% other. Entry criteria for the HAHC-CVD study required subjects to have documentation of HIV infection, be ≥40 years, and to be on stable highly active antiretroviral therapy (HAART) for ≥6 months. Routine HIV and CVD assessments were performed at baseline including fasting (nothing by mouth for 12 h) blood tests for total, high-density lipoprotein (HDL), and directly measured low-density lipoprotein (LDL) cholesterol and for triglycerides by enzymatic, colorimetric assay, and for fasting levels of glucose by glucose oxidase assay and insulin by electrochemiluminescence immunoassay.
25-Hydroxy [25(OH)] vitamin D levels
Stored EDTA plasma specimens frozen at −140°C from the first 100 enrollees into the HAHC-CVD Study were forwarded to LipoScience Inc. (Raleigh, NC) and 25(OH)D levels were assayed by chemiluminescent immunoassay (DiaSorin) per the manufacturer's guidelines.
Subclinical markers of arterial dysfunction
Three different markers of arterial dysfunction were obtained on the same set of participants. All were obtained locally in Honolulu, Hawaii with FMD and cIMT performed at Queen's Medical Center and CAC at Accuimaging. Technical quality assurance and centralized analyses of FMD, cIMT, and CAC were provided, respectively, by the University of Wisconsin Atherosclerosis Imaging Research Program (J. Stein), University of Southern California Atherosclerosis Research Unit Core Imaging and Reading Center (ARU CIRC) (H. Hodis), and Los Angeles Biomedical Research Institute (M. Budoff).
FMD testing was performed in the morning following a 12-h fast utilizing previously published methodology.5–7 Those who regularly used tobacco products refrained for at least 8 h prior to the test. The test was conducted by forearm occlusion using a high-resolution linear array vascular ultrasound transducer (Siemens ACUSON Cypress) after a 10-min rest in a temperature-controlled room (70–76°F). Each study was recorded digitally and measured in triplicate by a single blinded reader at the core FMD laboratory.
High-resolution B-mode ultrasound images of the right carotid common artery (CCA) were obtained from each patient after the FMD procedure using previously described techniques.8–11 A single reader measured the intima-media thickness of the far wall of the distal common carotid artery along a 1-cm length just distal to the carotid artery bulb with automated computerized edge detection (Prowin, Patents 2005, 2006) at the University of Southern California ARU CIRC.
Computerized tomography examinations for CAC were performed on a dual source CT (DSCT) scanner (Siemens 64-slice Somatom) following previously published methods.12 Images were forwarded to the Los Angeles Biomedical Research Institute where a radiologist or cardiologist blinded to clinical data quantified CAC score, using an interactive scoring system to calculate Agatston score, with any Agatston score >0 defining the presence of CAC.13
Statistical methods
Pearson product-moment correlation coefficient was used to measure the strength of the correlation between 25(OH)D levels and continuous variables; Kruskal–Wallis methods were used to test for the association between 25(OH)D levels and categorical variables. The relationship between CAC and 25(OH)D was analyzed in two ways: (1) by relative risk regression14 where the response was a dichotomous CAC outcome: (CAC >0) or (CAC=0), followed by linear regression, with the measured value of CAC among subjects with CAC >0 as the response; and (2) by cumulative logit models, where the response was ordinal CAC score (0, 1–99, 100–400, >400). This model was fit assuming proportional odds, e.g., the effect of 25(OH)D is identical across each cumulative logit. More specifically, odds of CAC being greater than 400 is the same as odds of being greater than 100 or odds of CAC being greater than 0. This assumption was investigated using formal hypothesis testing.
Results
Cohort characteristics
The general demographic as well as the metabolic and cardiovascular risk characteristics of the 100 subjects in the cohort are shown in Table 1. In general, the cohort was representative of the HIV-infected community in Hawaii. Of the 100 subjects, the median (Q1, Q3) level of 25(OH)D was 27.9 ng/ml (21.8, 38.3) with a minimum of 10.3 and maximum of 70.5 ng/ml. Utilizing manufacturer-suggested reference ranges, 47% had adequate 25(OH)D levels (30–100 ng/ml), 53% had insufficient levels (10–30 ng/ml), and none was 25(OH)D deficient (<10 ng/ml).
Table 1.
Patient Characteristics
| Age [years; median (IQR)] | 52 (47, 57) |
| Male gender (%) | 86% |
| Ethnicity (%) | |
| White | 60% |
| Non white or mixed | 40% |
| CD4 count [cells/mm3; median (IQR)] | 510 (361, 660) |
| HIV RNA (% undetectable) | 85% |
| Nadir CD4 count [cells/mm3; median (IQR)] | 150 (30, 260) |
| BMI (mean±SD) | 26.4 (4.5) |
| Waist-to-hip ratio [median (IQR)] | 0.92 (0.89, 0.98) |
| Blood pressure (mm Hg) | |
| SBP | 121.0 (112.5, 130.0) |
| DBP | 75.5 (68.0, 81.0) |
| Smoking (%) | |
| Never | 34% |
| Past | 38% |
| Current | 28% |
| Diabetes mellitusa (%) | 10% |
| Impaired fasting glucose and/or glucose intoleranceb (%) | 19% |
| HOMA-IR [median (IQR)] | 1.19 (0.79, 2.04) |
| Triglyceridesc [mg/dl; median (IQR)] | 122.5 (86.0, 167.5) |
| Total cholesterolc [mg/dl; median (IQR)] | 176.5 (155.0, 197.5) |
| HDL cholesterolc [mg/dl; median (IQR)] | 39.0 (32.0, 50.5) |
| Directly measured LDL cholesterolc [mg/dl; median (IQR)] | 108.5 (83.5, 127.5) |
| Framingham score % [median (IQR)] | 7% (4, 10) |
| Brachial artery flow-mediated dilatation [%, median (IQR)] | 3.74 (2.09, 6.14) |
| Carotid intima media thickness [mm; median (IQR)] | 0.73 (0.67, 0.84) |
| Coronary artery calcium | |
| Undetectable (%) | 47% |
| Detectable [%, median (IQR)] | 53% |
Diabetes defined as fasting glucose >125 mg/dl or an OGGT 2-h glucose >200 mg/dl.
Impaired fasting glucose: fasting glucose between 100 and 125 mg/dl; impaired glucose tolerance: OGGT 2-h glucose between 140 and 199 mg/dl.
Performed fasting ≥12 h.
IQR, interquartile range; BMI, body mass index; LDL, low-density lipoprotein.
Seasonal variation in 25-OH vitamin D levels
The National Weather Service Forecast Office (http://www.prh.noaa.gov/hnl/pages/climate_summary.php) considers Hawaii to have only two seasons: “summer” between May and October and “winter” between October and April. Analysis of seasonal difference in 25(OH)D levels using Wilcoxon rank sum test [based on the month that the study visit occurred during which blood for 25(OH)D was drawn] revealed a statistically significant difference in summer vs. winter: 35 ng/ml vs. 25 ng/ml, p<0.01.
Association of 25-OH vitamin D levels with various cohort characteristics
Lower 25(OH)D levels were seen in nonwhites (median, white=33.9, nonwhite=24.6, p<0.01 by Kruskal–Wallis test). Significant correlations were seen with current CD4 (r=0.21, p=0.03) and CD4% (r=0.31, p<0.01) levels with a trend for nadir CD4 count (r=0.19, p=0.06). The association between 25(OH)D levels and plasma HIV RNA levels could not be assessed as HIV RNA levels were detectable in only 15 of the 100 individuals, mostly at very low levels.
An inverse correlation was found between 25(OH)D levels and waist-to-hip ratio (WHR) (r=−0.26, p=0.02), but not with body mass index (BMI). A significant correlation was observed with 25(OH)D levels and total cholesterol (−0.23, p=0.02) but not separately with LDL cholesterol (r=−0.18, p=0.08) or with HDL cholesterol (r=−0.15, p=0.14); nor was a correlation found with triglyceride levels (−0.14, p=0.15). No significant correlation was observed between 25(OH)D levels and fasting glucose levels and no association was found between 25(OH)D levels and glucose homeostasis categories (normal, elevated fasting glucose, diabetes); however, a marginal correlation was found with insulin resistance as assessed by HOMA-IR (r=−0.20, p=0.06).
Association of vitamin D with arterial dysfunction
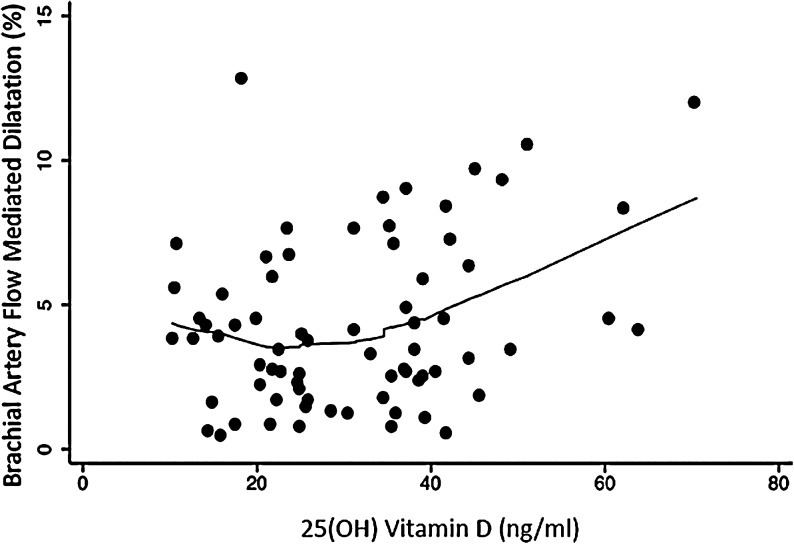
A total of 72 FMD, 50 cIMT, and 90 CAC measurements were available for analyses. A significant correlation was found between 25(OH)D levels and FMD (r=0.30, p=0.01) (Fig. 1) but not with cIMT (r=−0.05, p=0.76). Kruskal–Wallis tests did not reveal significant difference in FMD by gender and ethnicity groups.
FIG. 1.
Correlation between flow-mediated dilation and 25-hydroxyvitamin D levels.
We examined the impact of various potential factors (age, ethnicity, smoking status, hypertension, LDL cholesterol, HDL cholesterol, diabetes, and Framingham score) using multiple linear regression models where log-transformed FMD was the response variable. By simple linear regression, we found associations between log-transformed FMD and 25(OH)D levels (p=0.03), Framingham score (p<0.01), LDL cholesterol (p=0.03), and hypertension (p<0.01) but not with HDL cholesterol, age, smoking status, diabetes, and ethnicity. The Framingham score incorporates the impact of hypertension and cholesterol (as total cholesterol) in addition to age and smoking; therefore we elected to construct a multiple linear regression model utilizing 25(OH)D levels and only the Framingham score in the model. This model showed the Framingham score to be a significant predictor of log-transformed FMD, β=−1.99, p<0.01) but not 25(OH)D (β=0.003, p=0.30). Inclusion of seasonal variation in 25(OH)D levels in the model did not alter the results.
We investigated the association between 25(OH)D and CAC score; CAC was present in 53% of subjects. Considering CAC response as positive (CAC > 0) or not (CAC=0), those with lower 25(OH)D were at greater risk of being positive for CAC (RR=1.02, p=0.04). Among those with CAC, there was no evidence of association of lower 25(OH)D with higher CAC scores (p=0.36) by linear regression. The alternative analytic approach investigated the association between 25(OH)D and categorical CAC score (0, 1–99, 100–400, >400) using cumulative logit models. This analysis showed that lower levels were associated with a modestly higher likelihood of classification into a higher CAC category (OR=1.03, p=0.028). The likelihood ratio test of the model after inclusion of gender and ethnicity failed to reject the null hypothesis. Formal testing did not reject the assumption of proportional odds. As in the linear regression analysis described above, when applying the cumulative logit model only to those with CAC > 0, the association between 25(OH)D and classification of CAC category was not significant (OR=1.03, p=0.23); however, the point estimate was the same as that observed when the model was fit to all data on all patients. Thus we can say with assurance that lower 25(OH)D is associated with CAC positivity, but we are not certain whether such levels imply higher CAC levels among those with CAC > 0.
We extended the cumulative logit models involving the entire cohort to determine the relationship between categorical CAC score and vitamin D level after adjustment for age, ethnicity, smoking status, hypertension, LDL cholesterol, HDL cholesterol, diabetes, and Framingham score. Diabetes and age were the only factors that showed significant univariate correlations with the CAC categories. When age and diabetes were included in the model together with 25(OH)D, age remained significant (OR=0.89, p<0.01) and a significant interaction was present for 25(OH)D and diabetes (p=0.01). For nondiabetic patients, higher 25(OH)D levels were associated with lower CAC categories, while among diabetic patients, higher 25(OH)D was associated with higher CAC categories. Inclusion of seasonal variation in the cumulative logit model did not change the results.
Finally, we dichotomized our cohort into subjects with adequate vs. insufficient 25(OH)D levels, and using Wilcoxon rank sum test assessed for differences between the groups in FMD, cIMT, and CAC values. With median values of adequate vs. insufficient for FMD of 4.13% vs. 3.44%, for cIMT of 0.72 mm vs. 0.75 mm, and for CAC of 0% vs. 11.2%, no significance differences were found for any neuroimaging modality.
Discussion
Among HIV-infected patients on HAART, we found associations between 25(OH)D levels and two measures of arterial dysfunction. Lower 25(OH)D levels were associated by FMD with endothelial dysfunction, an early stage of arterial dysfunction. Lower 25(OH)D levels were also associated with CAC presence. The small sample size may have precluded the ability to see a relationship between 25(OH)D levels and cIMT, or to confirm a relationship between 25(OH)D and CAC levels among those who were CAC positive. While controversy exists as to what level of 25(OH)D represents a deficiency of vitamin D, 53% of subjects in this study had levels below 30 ng/ml, which most experts agree suggests some degree of vitamin D insufficiency.1 Consistent with other published data, lower levels of 25(OH)D were seen in nonwhite subjects compared to white subjects in this study.15
In the general population, lower vitamin D levels are associated with prevalent myocardial dysfunction, incident CAC,16 increased cIMT,17 and lower FMD18 as well as multiple CVD risk factors including diabetes, hypertension, and lipid abnormalities.19–24 Despite a substantial degree of association in cross-sectional studies, the association between vitamin D status and cardiometabolic outcomes in longitudinal studies done in the general population remains uncertain. Furthermore, no clear consensus has been reached in the literature regarding the efficacy of vitamin D supplementation in ameliorating CVD.25
The effects of vitamin D on CVD may involve direct effects on the vasculature as well as indirect effects mediated through modification of CVD risk factors. In our study of the association between vitamin D and endothelial dysfunction by FMD, adjustment for Framingham score substantially weakened this association, suggesting that indirect mechanisms of action may at least partially explain the association. Our findings of an interaction between 25(OH)D and diabetes deserve further investigation.
Vitamin D deficiency is frequent in ambulatory HIV-positive patients2,3 and may be associated with disease progression.26 We found an association between current CD4 count and 25(OH)D levels. Vitamin D receptors are ubiquitously distributed and found not only on heart and blood vessels but also on T and B lymphocytes and monocytes. It is interesting to speculate that the association between vitamin D and CVD risk in the HIV population may be mediated by immune response. Indeed, vitamin D deficiency has been linked to higher C-reactive protein27,28 and vitamin D treatment has been reported to reduce tumor necrosis factor (TNF)-α and increase interleukin (IL)-10 in a small study of patients with heart failure.29 However, it is also possible that the association may simply reflect chronic, nonspecific illness.
This study did not collect data on vitamin D supplementation or on hours of sun exposure for each subject. While 25(OH)D levels obtained in this study varied by whether the assays were conducted in summer or winter, this seasonal variation did not alter any analyses of the association between vitamin D and arterial dysfunction conducted in this study.
The current study was limited by its observational nature as well as its small sample size and the lack of non-HIV-infected controls. The number of individuals with positive CAC was particularly small. Future research will examine the relationship between vitamin D and markers of arterial dysfunction with an increased sample size and will attempt to elucidate the importance of HIV-specific as well as non-HIV-related factors in this relationship. Nevertheless, the markers of arterial dysfunction in this current study were performed using standardized methodology and careful quality assurance measures. The identified association of 25(OH)D levels with FMD and CAC, two validated markers of arterial dysfunction, as well as the association of 25(OH)D levels with current CD4 count are intriguing and warrant further investigation.
Acknowledgments
The authors wish to thank the patients for their participation in this study and the staff at the Hawaii Center for AIDS for their contribution to this study. Funding support was provided through the NIH National Heart, Lung, and Blood Institute (5R01HL095135). Partial funding for laboratory and imaging work as well as assistance with general study coordination was provided by the University of Washington's CVD and Metabolic Complications of HIV/AIDS Data Coordinating Center (5R01HL095126). Local infrastructure structure support at the University of Hawaii was also provided by National Centers for Research Resources grant U54RR026136.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Reddy Vanga S. Good M. Howard PA. Vacek JL. Role of vitamin D in cardiovascular health. Am J Cardiol. 2010;106:798–805. doi: 10.1016/j.amjcard.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez M. Daniels B. Gunawardene S. Robbins GK. High frequency of vitamin D deficiency in ambulatory HIV-positive patients. AIDS Res Hum Retroviruses. 2009;25:9–14. doi: 10.1089/aid.2008.0183. [DOI] [PubMed] [Google Scholar]
- 3.Van Den Bout-Van Den Beukel CJ. Fievez L. Michels M, et al. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: Effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 4.Triant VA. Lee H. Hadigan C. Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein JH. Klein MA. Bellehumeur JL, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 6.Stein JH. Merwood MA. Bellehumeur JL, et al. Effects of pravastatin on lipoproteins and endothelial function in patients receiving human immunodeficiency virus protease inhibitors. Am Heart J. 2004;147:E18. doi: 10.1016/j.ahj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Torriani FJ. Komarow L. Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selzer RH. Hodis HN. Kwong-Fu H, et al. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 9.Selzer RH. Mack WJ. Lee PL. Kwong-Fu H. Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 10.Hodis HN. Mack WJ. LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hodis HN. Mack WJ. Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 12.Carr JJ. Nelson JC. Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: Standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS. Janowitz WR. Hildner FJ. Zusmer NR. Viamonte M., Jr Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 15.Ginde AA. Liu MC. Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Boer IH. Kestenbaum B. Shoben AB. Michos ED. Sarnak MJ. Siscovick DS. 25-Hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–1812. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Targher G. Bertolini L. Padovani R, et al. Serum 25-Hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 2006;65:593–597. doi: 10.1111/j.1365-2265.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 18.Tarcin O. Yavuz DG. Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 19.Martins D. Wolf M. Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 20.Melamed ML. Muntner P. Michos ED, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: Results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:1179–1185. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilz S. Marz W. Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93:3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 22.Dobnig H. Pilz S. Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 23.Kendrick J. Targher G. Smits G. Chonchol M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205:255–260. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Wang TJ. Pencina MJ. Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittas AG. Chung M. Trikalinos T, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta S. Giovannucci E. Mugusi FM, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS ONE. 2010;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timms PM. Mannan N. Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: Mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 28.Van den Berghe G. Van Roosbroeck D. Vanhove P. Wouters PJ. De Pourcq L. Bouillon R. Bone turnover in prolonged critical illness: Effect of vitamin D. J Clin Endocrinol Metab. 2003;88:4623–4632. doi: 10.1210/jc.2003-030358. [DOI] [PubMed] [Google Scholar]
- 29.Schleithoff SS. Zittermann A. Tenderich G. Berthold HK. Stehle P. Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]