Abstract
Characterization of HIV-1 strains is important for surveillance of the HIV-1 epidemic. In Vietnam HIV-1-infected pregnant women often fail to receive the care they are entitled to. Here, we analyzed phylogenetically HIV-1 env sequences from 37 HIV-1-infected pregnant women from Ha Noi (n=22) and Hai Phong (n=15), where they delivered in 2005–2007. All carried CRF01_AE in the gp120 V3 region. In 21 women CRF01_AE was also found in the reverse transcriptase gene. We compared their env gp120 V3 sequences phylogenetically in a maximum likelihood tree to those of 198 other CRF01_AE sequences in Vietnam and 229 from neighboring countries, predominantly Thailand, from the HIV-1 database. Altogether 464 sequences were analyzed. All but one of the maternal sequences colocalized with sequences from northern Vietnam. The maternal sequences had evolved the least when compared to sequences collected in Ha Noi in 2002, as shown by analysis of synonymous and nonsynonymous changes, than to other Vietnamese sequences collected earlier and/or elsewhere. Since the HIV-1 epidemic in women in Vietnam may still be underestimated, characterization of HIV-1 in pregnant women is important to observe how HIV-1 has evolved and follow its molecular epidemiology.
Mother-to-child transmission of HIV-1 has decreased to a minimum in developed countries primarily by antiretroviral therapy during pregnancy, at delivery and to the newborn child, nonbreastfeeding, and often elective Cesarean section. A decrease has also been achieved in many developing countries, although it is often not possible to abstain from breastfeeding due to its protection against lethal respiratory and/or gastrointestinal infection. Also there may be economic and other constraints to proper antiretroviral prevention.
In Vietnam HIV-1 infection is now endemic in some of the largest cities, such as Ho Chi Minh City, with a prevalence of 1.2%.1,2 The most profound prevalence of the HIV-1 epidemic in northern Vietnam is in the coastal province of Quang Ninh, which borders southern China, in the port city Hai Phong (1.1%), and in the capital Ha Noi (0.9%).2 In pregnant women the prevalence in these cities was 0.6% and 0.1%, respectively, in 2006.3
The genetic epidemiology of HIV-1 has become increasingly important in understanding how, wherefrom, and whereto the HIV-1 epidemic is changing. The env gene is the most variable and is commonly used for the definition and characterization of genotypes.4 The major (M) group of HIV-1 genotypes is divided into subtypes A–D, F–H, J–K, and 49 circulating recombinant forms (CRF). In Vietnam, CRF01_AE has been reported to be the dominant HIV-1 genotype, even more common than in the neighboring countries.5–8
Due to the widespread discrimination against HIV-1-infected people in Vietnam,9–11 HIV-1-infected pregnant women fear being revealed.12–15 The number of pregnant women having an HIV test in antenatal care decreased from 22% in 2004 to about 16% in 2006,16 but has since increased.17 Still, only 25% received the test in 2009.17 HIV-1-infected pregnant women choose elective abortion in close to 70%,18 which may be a reflection of their fear.
Thus HIV-1 in pregnant women in Vietnam has not been studied, most likely due to the difficulty of obtaining samples from them. It is not known to what extent HIV-1 in pregnant women is a reflection of HIV-1 in different risk groups, such as intravenous drug users or commercial sex workers, or has spread beyond this group. Therefore we wanted to explore the phylogenetic relation of HIV-1 in pregnant women to other strains of HIV-1 from northern Vietnam and elsewhere.
The 37 HIV-infected mothers studied here were part of a prospective study in 2004–2007 concerning follow-up of children to 135 HIV-1-infected mothers, the first in Vietnam.19 They were followed prospectively after a gynecological check up and delivery in 2005–2007 at the Obstetrics and Gynecology hospitals in Ha Noi (n=22) and Hai Phong (n=15), respectively.19 All pregnant women were offered an HIV test and counseling, if found to be HIV infected. All consenting women (182/243) were offered participation for a thorough follow-up of their children up to a year of age; 135 mothers participated. Most women did not provide a sample from themselves. We have had access to 37 samples for the present study. The small number may restrict the conclusions that could be drawn, but still represents the first report from this important group. They included most (7/11) mothers of infected children and a selection of mothers of uninfected children, collected during the same time and from the same cities. Their age ranged from 17 to 36 with a median of 25, which was similar to other groups of pregnant women. The CD4 counts were less than 200×106/liter in 5/7 mothers of infected children and over 200×106/liter in the others tested. Six women were treated with combination therapy within 3 weeks before delivery. None of those women had a child who contracted HIV-1. The others were treated with nevirapine at delivery (n=25), or not at all (n=6). None of the children was breastfed.
The National Institute of Hygiene and Epidemiology and the Obstetric and Gynecology Hospitals in Ha Noi and Hai Phong had given permission for the study. All subjects were treated respectfully and signed consents were obtained, according to the Helsinki declaration of human rights from 2000. Also the regional board of ethical vetting in Stockholm had approved of the study on February 26, 2008: 2207/1496-31/3. The study was registered at the Clinical trials.gov with ID: NCT 00669604 at (https://register.clinicaltrials.gov./prs/app/action/ FilterOrSelectProtocol/selectaction/View/ts/6/uid/U0000MHB).
The HIV status of the women was discovered when they went to the hospital for an antenatal check up late in gestation or in most cases at delivery. Three milliliters of defibrinated venous blood was taken 1 or 2 days after delivery from the mothers and processed within 8 h. Plasma was separated from the cellular fraction. Peripheral blood mononuclear cells were isolated by Lymphoprep (Axis-Shield PoC AS, Oslo, Norway). Two million cells were lysed in 0.2 ml buffer containing protease K and detergent as a source of HIV-1 DNA. RNA was extracted from 140 μl plasma (nine samples) by QIAamp (QIAGEN Gmbh, Hilden Germany). Viral RNA was used as a template to run cDNA synthesis by Omniscript kit (QIAGEN, Hilden, Germany). To avoid contamination of samples tubes were not opened at the same time. A positive and a negative control of HIV-1-infected cells and water, respectively, were included in each run. The sequences gave no indication that contamination had occurred.
The V3 loop region of the HIV-1 env gene was amplified in a nested PCR, using the outer primers JA167 and JA170 and inner primers JA168 and JA169.4 In samples with remaining material the reverse transcriptase of the pol gene was also amplified for different HIV-1 subtypes in a nested PCR, using the outer primers GAG2, PR1, RT137, and RT3303. The inner PCR primers RT1 and RT4 spanned the reverse transcriptase gene, amino acids 30 to 227 of the pol gene.20 Sequencing was performed using Big Dye Terminator 3.1 (AB Applied Biosystems, Foster City, CA). Sequencing reactions were purified by the Dye Ex 2.0 spin kit (QIAGEN). The product was run and sequenced on the ABI PRISM 3700 DNA sequencer (Applied Biosystems, Foster City, CA) or submitted to MWG Operon Eurofins Company, Erbesberg, Germany. The length of the V3 sequences was about 350 bp for the env gene and 646 bp for the reverse transcriptase gene. The chromatograms were evaluated using the program Sequencher 4.1 (Gene Codes, Ann Arbor, MI).
To determine the phylogenetic relation between the new and present CRF01_AEenvV3 sequences from the pregnant women from northern Vietnam to sequences characterized in the past in Vietnam and neighboring countries, 14 sequences, obtained from intravenous drug users in Ha Noi from 2002,21 were added, as well as all other (n=413) available sequences of CRF01_AE from the Los Alamos HIV database, retrieved in June 2008. The criteria of selection were that they should include the C2V3C3 region of the env protein. One sequence per individual was selected at random to avoid bias, reducing the number of sequences used. The number of sequences from each region was over or around 100 and could be expected to represent the epidemic in that area. The numbers of maternal sequences and of intravenous drug users were less, but may still be a reflection of their risk group. Sequences from pregnant women are the first reported from this group in Vietnam. The papers, from which the Vietnamese and Chinese sequences were obtained, were reviewed.5–7,21–25
A maximum likelihood phylogenetic tree was inferred using PhyML v3.0 (PHYlogenetic inferences using maximum likelihood), using a GTR+G+I model with BioNJ program initial tree and an NNI search. A tree diagram was plotted with FigTree v1.1.2 by Andrew Rambaut, available at [http:/mac.softpedia.com/get/Graphics/FigTree.shtml]. It was rooted to the midpoint. For details of the individual sequences see the supplementary material. Nonsynonymous and synonymous nucleotide substitutions were calculated for all pairwise sequence comparisons within each geographically determined group using the SNAP program (Los Alamos HIV database), http://www.hiv.lanl.gov26 according to the Nei and Gojobori method.27 Student's t-test was used for the statistical analysis. A significance level of p<0.001 was chosen.
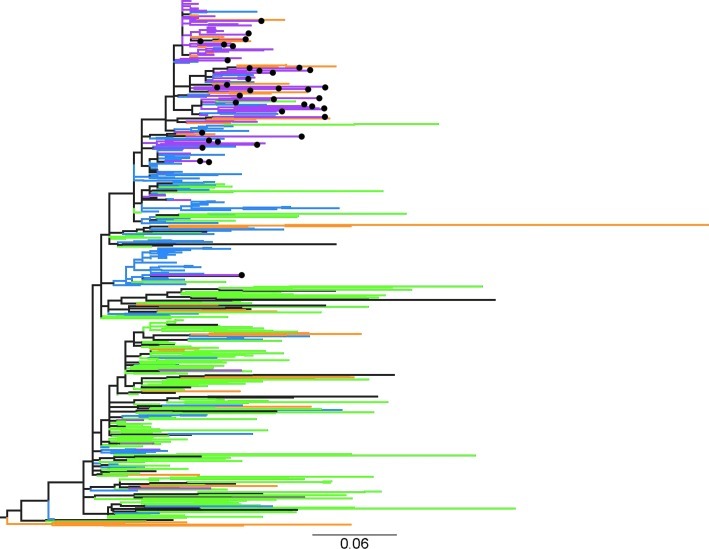
A total of 464 sequences were analyzed; 159 came from Thailand, 43 from China, and 198 from Vietnam. The remaining came from other countries such as Japan (n=8), Indonesia (n=7), Singapore (n=2), and Korea (n=9). The sequences were presented in a phylogenetic tree (Fig. 1), which displayed the nodes in ascending order. Each sequence can be identified in the supplementary file (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/aid). The bottom part of the tree was dominated by sequences from Thailand (in green). It also contained sequences from China (orange), Vietnam (blue for northern or purple for southern Vietnam), and from other countries (black). The upper part was dominated by sequences from northern Vietnam, including sequences from the pregnant women, and sequences from southern China, across the Vietnamese border. The bootstrap values indicated that the phylogenetic relations were complex and without clear phylogenetic paths. The bootstrap value support of the branching was not sufficiently high to allow the tree to be a basis for statistical analysis. However, the topology of the tree was similar to a recently reported phylogenetic analysis of the spread of HIV-1 CRF01_AE from Thailand to Southern Vietnam and then to the North.28
FIG. 1.
Phylogenetic tree of the CRF01_AE envV3 molecular epidemic in Vietnam and related countries in Southeast Asia. The phylogenetic tree shows the sequences from Thailand in green, from China in orange, from the south of Vietnam in blue, and from the north of Vietnam in purple. Sequences from other countries are shown in black. The indicated scale bar is in units of substitutions/site according to a GTR+I+G model. The maternal sequences are indicated by filled black circles.
The closest relationships between the sequences from northern Vietnam and a foreign source was found with the HIV-1 Pingxiang strains of HIV-1-infected individuals from the Guangxi province5,6 in southern China, which borders with the Lang Son province in northern Vietnam.
There was an interesting separation of the Chinese sequences between the upper and lower part of the tree, as all 22 of the Chinese sequences in the upper part came from areas at the border between China and Vietnam and colocalized with sequences from northern Vietnam, while 22/23 (95.7%) of the Chinese sequences in the lower part of the tree, dominated by sequences from Thailand, came from Liaonging and Fuijan25 in the east of China. These latter ones were, similar to the samples from the pregnant women, of relatively recent date (from 2000 to 2006), indicating that there may be two (ongoing) paths of entry of CRF01_AE into China.
The visual impression of the maternal sequences (black circles) was that they were scattered throughout the other sequences, collected from northern Vietnam and southern China, apart from one sequence, which appeared together with sequences from southern Vietnam.
Another way to study the relation to other groups of HIV-infected people would be to describe the synonymous (ds) and nonsynonymous (dn) amino acid changes, where the synonymous changes would represent a background of mutations and nonsynonymous would increase in relation to different pressures. Both would accumulate over time. Studying the individual nucleotide changes according to whether they were ds or dn showed that the maternal sequences had significantly larger numbers of synonymous changes than those of the intravenous drug users in Ha Noi in 2002 and others from northern Vietnam, respectively (p<0.001) See Table 1. The nonsynonymous changes were not significantly different from the intravenous drug users, p>0.001, but were significantly higher than the older ones from northern Vietnam (p<0.001). Altogether, the comparisons of the respective dn and ds means may reflect the time-related evolution of the maternal sequences versus the older ones from Vietnam.
Table 1.
Comparison of Synonymous (ds) and Nonsynonymous (dn) Nucleotide Changes in Sequences from Different Groups/Areas
| Maternal sequences | IVDU in Ha Noi | Northern Vietnam | Southern Vietnam | Thailand | |
|---|---|---|---|---|---|
| Number of sequences | 37 | 14 | 53 | 128 | 157 |
| Number of comparisons | 652 | 79 | 1967 | 6295 | 12217 |
| ds (mean) | 0.076 | 0.035469 | 0.042911 | 0.082226 | 0.09325 |
| Standard deviation | 0.0146 | 0.0117 | 0.011764 | 0.017775 | 0.008841 |
| Confidence interval 99.9% | 0.0741–0.0778 | 0.031–0.039 | 0.042–0.044 | 0.0815–0.083 | 0.093–0.0935 |
| dn (mean) | 0.056 | 0.052667 | 0.035386 | 0.047654 | 0.098924 |
| Standard deviation | 0.01 | 0.00891 | 0.008015 | 0.008554 | 0.012591 |
| Confidence interval 99.9% | 0.0547–0.0572 | 0.049–0.056 | 0.035–0.036 | 0.0473–0.048 | 0.098–0.0992 |
Extending the analysis to geographically more distant groups, the maternal sequences had less synonymous changes than those from southern Vietnam, but more with regard to nonsynonymous changes. With regard to sequences from Thailand, these were significantly more evolved than the maternal sequences and all others with regard to both synonymous and nonsynonymous changes (p<0.001). This may be a sign of the longstanding epidemic in Thailand, broader range of sources, and a wider range in time of collection.
In Lang Son in northern Vietnam the HIV-1 prevalence rates among intravenous drug users increased dramatically between 1995 and 1998 from 0% to 12%29 and in Pingxiang, Guangxi, from 8% to 42% in a year.30 This was interpreted to be related to the major heroin transshipment routes from the Golden Triangle [Myanmar (Burma), Thailand, and Laos] to Hong Kong, by separate routes for different subtypes.23,30,31 For CRF01_AE this route goes from Laos into Ha Noi and then turns north.30,31 Since there were no sequences from Myanmar and Laos, a relation of HIV-1 to those countries may be unrevealed.
Recently the epidemic of HIV CRF01A_AE in Vietnam was analyzed phylogenetically, involving near full length sequences, collected in 1997–1998.28 The epidemic first seemed to come from Thailand by heterosexual spread into the south of Vietnam and spread to intravenous drug users in the late 1980s and subsequently to intravenous drug users in the north during the mid-1990s. It is of interest that the sequences in our tree displayed a similar pattern. However, also their study was hampered by a lack of sequences from Myanmar and Laos.
It would be important to know if the epidemic now may be moving into the general population, here represented by pregnant women. This would be expected to be associated with a greater degree of evolution than the rapid spread associated with intravenous drug use.32,33 Thus it has been shown that HIV-1 evolves less among intravenous drug users than among heterosexuals.32 This has also been observed in the context of CRF01_AE in northern Vietnam and Southern China since the 1990s to more recently.5,6,21,30
The analysis of synonymous and nonsynonymous nucleotide changes indicated that the maternal sequences had evolved when comparing them to the older sequences from northern Vietnam. It was not possible to discern whether HIV-1 in pregnant women has evolved as a result of spreading into the general population, or is still closely related to the epidemic among intravenous drug users, a group that dominates as a source of HIV sequences in this part of the country. In a recent study on the pol gene phylogeny of sequences from Northern Vietnam, there were signs of HIV spread in different groups, such as among intravenous drug users and from sexual spread.34
The samples here come from an important and sensitive group, from which it is difficult to receive samples and information. Since the HIV epidemic in women in Vietnam may still be underestimated,9 characterization of HIV-1 in pregnant women is important to assist in the surveillance of mother-to-child transmission of HIV-1.
Accession Numbers
The following accession numbers of previously reported sequences from intravenous drug users in Ha Noi were used: AY283413–AY283427. The following accession numbers were given to the new HIV-1 env sequences created for this publication: FJ009330–FJ009366.
Supplementary Material
Acknowledgments
Economic support was given by SIDA (Swedish International Development Cooperation Agency) for costs in northern Vietnam and to A.E., SWE 2006-467. Tran Ha was given a grant from the Karolinska Institutet Research Training Program (KIRT) and the Swedish Nutrition Foundation. Simani Gaseitsiwe was awarded a Ph.D. training grant and Tran Ha and Rozina Caridha received a 6 month training grant each, all from EU Marie Curie Early Training program, and support from the Karolinska Institutet. P.V.H. received travel grants from the Maud and Birger Gustavsson's Foundation. Tran Ha held an NIH-DOE interagency agreement grant: Y1-AI-1500-07. We would like to thank Thomas Leitner for scientific support in generating the phylogenetic tree, Ina Maljkovic Berry for similar support and for critical evaluations and discussions, and her and Joakim Coster for reviewing the manuscript. R. Caridha and T.T.T. Ha contributed equally to this work and shared first authorship.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Nguyen TH. Nguyen TL. Trinh QH. HIV/AIDS epidemics in Vietnam: Evolution and responses. AIDS Educ Prev. 2004;16(Suppl A):137–154. doi: 10.1521/aeap.16.3.5.137.35527. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health. Vietnam: Summary of the HIV epidemic in Vietnam. http://www.unaids.org.vn/facts/docs/key_messages_sep_2006_e.pdf http://www.unaids.org.vn/facts/docs/key_messages_sep_2006_e.pdf
- 3.WHO reports. http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_VN.pdf http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_VN.pdf
- 4.Leitner T. Korovina G. Marquina S. Smolskaya T. Albert J. Molecular epidemiology and MT-2 cell tropism of Russian HIV type 1 variant. AIDS Res Hum Retroviruses. 1996;12:1595–1603. doi: 10.1089/aid.1996.12.1595. [DOI] [PubMed] [Google Scholar]
- 5.Kato K. Shiino T. Kusagawa S, et al. Genetic similarity of HIV type 1 subtype E in a recent outbreak among injecting drug users in northern Vietnam to strains in Guangxi Province of southern China. AIDS Res Hum Retroviruses. 1999;15:1157–1168. doi: 10.1089/088922299310250. [DOI] [PubMed] [Google Scholar]
- 6.Kato K. Kusagawa S. Motomura K, et al. Closely related HIV-1 CRF01_AE variant among injecting drug users in northern Vietnam: Evidence of HIV spread across the Vietnam-China border. AIDS Res Hum Retroviruses. 2001;17:113–123. doi: 10.1089/08892220150217201. [DOI] [PubMed] [Google Scholar]
- 7.Lan NT. Recordon-Pinson P. Hung PV, et al. HIV type 1 isolates from 200 untreated individuals in Ho Chi Minh City (Vietnam): ANRS 1257 Study. Large predominance of CRF01_AE and presence of major resistance mutations to antiretroviral drugs. AIDS Res Hum Retroviruses. 2003;19:925–928. doi: 10.1089/088922203322493111. [DOI] [PubMed] [Google Scholar]
- 8.Nerurkar VR. Nguyen HT. Woodward CL, et al. Sequence and phylogenetic analyses of HIV-1 infection in Vietnam: Subtype E in commercial sex workers (CSW) and injection drug users (IDU) Cell Mol Biol. 1997;43:959–968. [PubMed] [Google Scholar]
- 9.Khoat DV. Hong LD. An CQ. Ngu D. Reidpath DD. A situational analysis of HIV/AIDS-related discrimination in Hanoi, Vietnam. AIDS Care. 2005;17(Suppl 2):S181–S193. doi: 10.1080/09540120500119940. [DOI] [PubMed] [Google Scholar]
- 10.Thi MD. Brickley DB. Vinh DT, et al. A qualitative study of stigma and discrimination against people living with HIV in Ho Chi Minh City, Vietnam. AIDS Behav. 2008;12(Suppl 4):S63–S70. doi: 10.1007/s10461-008-9374-4. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen TA. Oosterhoff PA. Hardon HN. Tran RA. Coutinho RA. Wright P. A hidden HIV epidemic among women in Vietnam. BMC Public Health. 2008;8:37–47. doi: 10.1186/1471-2458-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinh TH. Detels R. Nguyen MA. Factors associated with declining HIV testing and failure to return for results among pregnant women in Vietnam. AIDS. 2005;19:1234–1236. doi: 10.1097/01.aids.0000176228.09474.59. [DOI] [PubMed] [Google Scholar]
- 13.Oosterhoff P. Hardon AP. Nguyen TA. Pham NY. Wright P. Dealing with a positive result: Routine HIV testing of pregnant women in Vietnam. AIDS Care. 2008;20:654–659. doi: 10.1080/09540120701687026. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TA. Oosterhoff P. Ngoc YP. Wright P. Hardon A. Barriers to access prevention of mother-to-child transmission for HIV positive women in a well-resourced setting in Vietnam. AIDS Res Ther. 2008;5:7. doi: 10.1186/1742-6405-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brickley DB. Le Dung Hanh D. Nguyet LT. Mandel JS. Giang le T. Sohn AH. Community, family, and partner-related stigma experienced by pregnant and postpartum women with HIV in Ho Chi Minh City, Vietnam. AIDS Behav. 2009;13:1197–1204. doi: 10.1007/s10461-008-9501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UNAIDS reports: The Socialist Republic of Viet Nam. The third country report on following up the implementation to the declaration of commitment on HIV and AIDS. Reporting period: Janaury 2006–December 2007. http://unaids.org.vn/sitee/index.php? http://unaids.org.vn/sitee/index.php?
- 17.UNAIDS reports: The Socialist Republic of Viet Nam. Reporting period: January 2008–December 2009; Update on the HIV epidemic in Viet Nam. http://www.unaids.org.vn/sitee/index.php?option=com_content&task=view&id=28&Itemid=72 http://www.unaids.org.vn/sitee/index.php?option=com_content&task=view&id=28&Itemid=72
- 18.Bùi KC. Gammeltoft T. Nguyen TT. Rasch V. Induced abortion among HIV-positive women in Quang Ninh and Hai Phong, Vietnam. Trop Med Int Health. 2010;15(10):1172–1178. doi: 10.1111/j.1365-3156.2010.02604.x. [DOI] [PubMed] [Google Scholar]
- 19.Ha TTT. Tuan PL. Bao NH. Anh NM. Cam PD. Chiodi F. Ehrnst A. Successful reduction of mother-to-child transmission of HIV-1 by nevirapin and non-breastfeeding in Hanoi and Haiphong. Retrovirology. 2008;5(S1):26–27. [Google Scholar]
- 20.Steegen K. Demecheleer E. De Cabooter N, et al. A sensitive in-house RT-PCR genotyping system for combined detection of plasma HIV-1 and assessment of drug resistance. J Virol Methods. 2006;133:137–145. doi: 10.1016/j.jviromet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Tran TTH. Maljkovic I. Swartling S. Phung DC. Chiodi F. Leitner T. HIV-1 CRF01_AE in intravenous drug users in Ha Noi, Vietnam. AIDS Res Hum Retroviruses. 2004;20:341–345. doi: 10.1089/088922204322996581. [DOI] [PubMed] [Google Scholar]
- 22.Menu E. Truong TX. Lafon ME, et al. HIV type 1 Thai subtype E is predominant in south Vietnam. AIDS Res Hum Retroviruses. 1996;12:629–633. doi: 10.1089/aid.1996.12.629. [DOI] [PubMed] [Google Scholar]
- 23.Yu XF. Chen J. Shao Y, et al. Emerging HIV infections with distinct subtypes of HIV-1 infection among injection drug users from geographically separate locations in Guangxi Province, China. J Acquir Immune Defic Syndr. 1999;22:180–188. doi: 10.1097/00126334-199910010-00011. [DOI] [PubMed] [Google Scholar]
- 24.Caumont A. Lan NT. Uyen NT, et al. Sequence analysis of env C2/V3, gag p17/p24, and pol protease regions of 25 HIV type 1 isolates from Ho Chi Minh City, Vietnam. AIDS Res Hum Retroviruses. 2001;17:1285–1291. doi: 10.1089/088922201750461357. [DOI] [PubMed] [Google Scholar]
- 25.Hai-Long H. Jian Z. Ping-Ping Y, et al. Genetic characterization of CRF01_AE full length HIV type 1 sequences from Fujian, China. AIDS Research Human Retroviruses. 2007;23:569–574. doi: 10.1089/aid.2006.0257. [DOI] [PubMed] [Google Scholar]
- 26.Korber B. HIV signature, sequence variation analysis. In: Rodrigo AG, editor; Learn GH, editor. Computational Analysis of HIV Molecular Sequences. Kluwer Academic Publishers; Dordrecht, Netherlands: 2000. pp. 55–72. [Google Scholar]
- 27.Nei M. Gojobori T. Simple methods for estimating the numbers of synonymous, nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 28.Liao H. Tee KK. Hase S, et al. Phylodynamic analysis of the dissemination of HIV-1 CRF01_AE in Vietnam. Virology. 2009;391:51–56. doi: 10.1016/j.virol.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Hammett TM. Patrick J. Kling JR, et al. Correlates of HIV status among injection drug users in a border region of southern China and northern Vietnam. J Acquired Immune Defic Syndr Hum Retrovirol. 2005;38:228–235. doi: 10.1097/00126334-200502010-00016. [DOI] [PubMed] [Google Scholar]
- 30.Yu XF. Liu W. Chen J, et al. Maintaining low HIV type 1 env genetic diversity among injection drug users infected with a B/C recombinant and CRF01_AE HIV type 1 in southern China. AIDS Res Hum Retroviruses. 2002;18:167–170. doi: 10.1089/08892220252779719. [DOI] [PubMed] [Google Scholar]
- 31.Beyrer C. Razak MH. Lisam K. Chen J. Lui W. Yu XF. Overland heroin trafficking routes and HIV-1 spread in south and south-east Asia. AIDS. 2000;14:75–83. doi: 10.1097/00002030-200001070-00009. [DOI] [PubMed] [Google Scholar]
- 32.Bobkov A. Cheingsong-Popov R. Selimova L, et al. An HIV type 1 epidemic among injecting drug users in the former Soviet Union caused by a homogeneous subtype A strain. AIDS Res Hum Retroviruses. 1997;13:1195–1201. doi: 10.1089/aid.1997.13.1195. [DOI] [PubMed] [Google Scholar]
- 33.Maljkovic Berry I. Ribeiro R. Kothari M, et al. Unequal evolutionary rates in the human immunodeficiency virus type 1 (HIV-1) pandemic: The evolutionary rate of HIV-1 slows down when the epidemic rate increases. J Virol. 2007;81:10625–10635. doi: 10.1128/JVI.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bontell I. Cuong DD. Agneskog E. Diwan V. Larsson M. Sönnerborg A. Transmitted drug resistance, phylogenetic analysis of HIV CRF01_AE in Northern Vietnam. Infect Genet Evol. 2011 May 19; doi: 10.1016/j.meegid.2011.04.034. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.