Abstract
HIV increases risk of non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL). The effect of HIV on presentation, treatment, and outcomes of NHL and HL in routine care in the combination antiretroviral therapy (cART) merits further characterization. We performed a retrospective analysis of HIV-infected patients with NHL and HL receiving care at the University of North Carolina at Chapel Hill from January 1, 2000 until December 31, 2010. Statistical analyses were conducted using SAS, version 9.2 (SAS Institute Inc). Sixty-five HIV-infected patients with NHL and HL were identified. Patients with non-CNS NHL and HL presented with advanced disease (85% stage III or IV) and adverse prognostic features. Patients completed 87% of planned chemotherapy cycles, and 68% of patients completed stage-appropriate therapy. Dose reduction, interruption, and/or delay occurred during more than 25% of administered cycles in 64% of patients. Infectious complications, febrile neutropenia, and myelosuppression accounted for 78% of deviations from planned cumulative dose and dose intensity. Primary CNS lymphoma (PCNSL) was associated with poor prognosis, but 2-year overall survival was 66% for all non-CNS lymphoma. Among patients surviving at least 2 years, 75% had CD4 count >200 cells/μl and 79% had HIV viral load <400 copies/ml at last follow-up. Despite advanced disease and difficulty tolerating chemotherapy with optimal cumulative dose and dose intensity, most patients with non-CNS HIV-associated lymphoma survived more than 2 years after diagnosis, the majority with suppressed HIV RNA.
Introduction
HIV confers a higher risk of non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) than occurs in individuals without HIV.1–3 In the combination antiretroviral therapy (cART) era, NHL incidence declined but has since stabilized, while HL incidence has been stable and possibly even increasing.1,2,4 Additionally, cancer has increased in frequency as a contributor to HIV mortality, with NHL being the most frequent cause of cancer-related death.5–8 Pathogenic mechanisms underlying lymphomagenesis in HIV-infected persons remain poorly understood, but are postulated to include B cell dysregulation, perturbations in intracellular signaling, viral coinfections, and decreased cytotoxic T cell surveillance.9
Risk factors for NHL in HIV-infected individuals include lower CD4 count and cumulative HIV viremia, whereas a consistent association between lower CD4 count and increased HL incidence has not been demonstrated, with there even being some evidence that higher CD4 count is associated with higher HL and Burkitt lymphoma (BL) incidence.10–16
Controversy remains as to the optimal lymphoma and antiretroviral treatment regimens for patients with HIV-associated lymphoma.17 Clinical trials have demonstrated comparable outcomes after HIV-associated NHL to patients without HIV infection.18,19 Extended survival after HIV-associated lymphoma similar to HIV-uninfected individuals has also been reported from European observational cohorts.20,21 However, other studies have found HIV to be an independent risk factor for death among patients with NHL, irrespective of stage and histologic subtype.22
If HIV negatively impacts survival after lymphoma diagnosis, the mechanisms by which this is mediated are unclear, and might include more advanced disease, poorer performance status, difficulty achieving stage-appropriate chemotherapy cumulative dose and dose intensity, reduced effectiveness or greater toxicity of chemotherapy due to interactions with antiretroviral medications, discontinuity or suboptimal concentrations of antiretroviral therapy due to interactions with chemotherapy, diminished anti-lymphoma response of the host immune system, and increased mortality from lymphoma-unrelated causes. Because interactions between HIV and lymphoma remain understudied, we undertook a retrospective analysis of patients with HIV-associated lymphoma at our institution to characterize their initial presentation, receipt of HIV and lymphoma treatment, and clinical outcomes.
Materials and Methods
Patient identification and data collection
We performed a retrospective analysis of HIV-infected patients with lymphoma receiving care at the University of North Carolina at Chapel Hill from January 1, 2000 until December 31, 2010. Patients were identified via comprehensive review of independent, unlinked, institutional HIV and cancer databases. Data were collected via abstraction from the medical record. Social Security Death Index records were reviewed to ascertain final vital status of patients lost to follow-up, with patients matched to Death Index records by name and date of birth. Patients with diffuse large B cell lymphoma (DLBCL) were compared to an existing institutional research database of contemporaneously treated adult DLBCL patients without HIV, without specific matching by calendar year of diagnosis. Morphologic, immunophenotypic, and molecular subtypes of DLBCL were analyzed together as a group rather than separately.
Study definitions
Chemotherapy dose reduction was defined as omission or dose reduction of any medication included in the treating regimen. Chemotherapy interruption was defined as discontinuation of all components of an administered cycle before completion. Chemotherapy delay was defined as failure to administer a chemotherapy dose on the scheduled date.
Stage-appropriate therapy was defined according to National Comprehensive Cancer Center Network (NCCN) clinical practice guidelines for NHL and HL.23,24 For DLBCL, stage-appropriate therapy was defined as completion of three or more chemotherapy cycles with radiotherapy or six or more chemotherapy cycles for Ann Arbor stage I or II disease, and six or more chemotherapy cycles for stage III or IV disease. For BL, stage-appropriate therapy was defined as completion of an NCCN guideline-recommended regimen. For HL, stage-appropriate therapy was defined as completion of four or more chemotherapy cycles for stage I or II favorable disease, four or more chemotherapy cycles with radiotherapy or six or more chemotherapy cycles for stage I or II unfavorable disease, and six or more chemotherapy cycles for stage III or IV disease. If chemotherapy was stopped due to refractory lymphoma, this was also considered stage-appropriate therapy. Complete response was defined according to International Harmonization Project criteria.25
Interruption of antiretroviral therapy was defined as discontinuation of all medications used to treat HIV lasting at least 3 days. Change in antiretroviral therapy was defined as substitution of at least one antiretroviral medication in a regimen.
Statistical analysis
Statistical analyses were conducted using SAS, version 9.2 (SAS Institute Inc.). Bivariate analyses were conducted using Fisher's exact test, two-sample t-test, and Kruskal–Wallis test.
Ethics statement
Our study was approved by the biomedical institutional review board (IRB) of the University of North Carolina at Chapel Hill. The requirement for informed consent was specifically waived by the IRB committee for our study.
Results
Baseline characteristics
Sixty-five patients with HIV-associated lymphoma were identified (47 NHL, 17 HL, 1 nonclassifiable). Baseline characteristics for NHL and HL patients are shown in Table 1. NHL cases were primarily composed of DLBCL (n=17, 41%), primary CNS lymphoma (PCNSL, n=12, 29%), and BL (n=9, 21%). Additionally, there were single cases each of follicular lymphoma, primary effusion lymphoma, primary cutaneous lymphoma, and B cell lymphoma not otherwise specified, and five NHL cases without further histopathologic classification. There were differences between NHL subtypes with respect to CD4 count at the time of lymphoma diagnosis, with BL patients having statistically higher CD4 counts than those with DLBCL or PCNSL. Of 60 patients for whom HIV diagnosis date was known, 22 (36%) were diagnosed with lymphoma within 6 months of HIV diagnosis (45% NHL, 12% HL; p=0.03).
Table 1.
Baseline Characteristics of Patients with HIV-Associated Lymphoma
| All (n=65) | All NHL (n=47) | DLBCL (n=17) | PCNSL (n=12) | BL (n=9) | HL (n=17) | |
|---|---|---|---|---|---|---|
| Age | 42.8 (37.6–48.8) | 42.6 (37.4–49.0) | 42.2 (37.7–49.1) | 43.9 (40.0–48.1) | 38.6 (34.3–48.2) | 43.6 (38.1–47.8) |
| Male | 80% | 81% | 88% | 67% | 89% | 76% |
| Ethnicity | ||||||
| White | 28% | 32% | 47% | 8% | 44% | 18% |
| African-American | 58% | 55% | 47% | 75% | 33% | 65% |
| Latino | 9% | 11% | 6% | 17% | 11% | 6% |
| Other | 5% | 2% | — | — | 11% | 12% |
| Years since HIV diagnosis | 2.6 (0–10.4) | 1.6 (0–8.5) | 0.3 (0–2.6) | 8.9 (0.4–13.2) | 2.0 (0–10.9) | 5.9 (1.9–12.4) |
| Lymphoma diagnosis within 6 months of HIV diagnosis* | 36% | 45% | 53% | 44% | 44% | 12% |
| Median calendar year of lymphoma diagnosis | 2005 | 2006 | 2005 | 2006 | 2007 | 2004 |
| CD4 cells/μl at lymphoma diagnosis** | 107 (46–291) | 128 (36–316) | 112 (46–137) | 18 (9–204) | 314 (166–548) | 73 (55–177) |
| HIV RNA <400 copies/ml at lymphoma diagnosis*** | 39% | 27% | 33% | 18% | 29% | 67% |
p=0.03 for NHL versus HL.
p=0.12 for DLBCL vs. PCNSL, p=0.01 for PCNSL vs. BL, p=0.03 for DLBCL vs. BL.
p=0.03 for NHL vs. HL.
NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B cell lymphoma; PCNSL, primary CNS lymphoma; BL, Burkitt lymphoma; HL, Hodgkin lymphoma.
Median values given with interquartile ranges in parentheses.
Non-CNS lymphoma cases frequently presented with adverse prognostic features, including impaired performance status, Ann Arbor stage III or IV disease, abnormal lactate dehydrogenase (LDH), B symptoms, extranodal and bone marrow involvement, advanced International Prognostic Index (IPI) in NHL, and advanced International Prognostic Score (IPS) in HL (Table 2).
Table 2.
Clinical Stage and Prognostic Features, HIV-Associated Non-CNS Lymphoma Subtypes
| DLBCL (n=17) | BL (n=9) | HL (n=17) | |
|---|---|---|---|
| Performance status | |||
| 0–1 | 50% | 57% | 47% |
| ≥2 | 50% | 43% | 53% |
| Ann Arbor stage | |||
| I | 12% | 11% | 19% |
| II | — | — | — |
| III | 19% | 33% | 37% |
| IV | 69% | 56% | 44% |
| Abnormal lactate dehydrogenase | 86% | 86% | 75% |
| B symptoms | 71% | 56% | 81% |
| Extranodal sites | |||
| 0 | 12% | 33% | 56% |
| 1 | 47% | 33% | 38% |
| ≥2 | 41% | 33% | 6% |
| Bone marrow involved (if assessed) | 29% | 25% | 55% |
| International Prognostic Index | |||
| 0–1 | 7% | 14% | N/A |
| 2 | 29% | 43% | |
| 3 | 43% | 29% | |
| 4–5 | 21% | 14% | |
| International Prognostic Score | |||
| 0 | N/A | N/A | — |
| 1 | 8% | ||
| 2 | 17% | ||
| 3 | 17% | ||
| 4 | 17% | ||
| ≥5 | 42% | ||
CNS, central nervous system; DLBCL, diffuse large B cell lymphoma; BL, Burkitt lymphoma; HL, Hodgkin lymphoma.
Lymphoma treatment
Fifteen of 17 DLBCL patients received chemotherapy. Chemotherapy regimens included CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone; n=10), CDE (cyclophosphamide, doxorubicin, etoposide; n=2), dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; n=2), and hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone, alternating with methotrexate and cytarabine; n=1). Ten of 17 patients (59%) received rituximab. Four patients received intrathecal chemotherapy, and four patients received radiotherapy, one of whom received primary radiotherapy without chemotherapy for stage IA disease based on comorbidities and patient preference. One patient died before initiation of lymphoma therapy.
All BL patients received chemotherapy. Chemotherapy regimens included hyper-CVAD (n=3), CODOX-M/IVAC (cyclophosphamide, doxorubicin, vincristine, methotrexate, alternating with ifosfamide, etoposide, cytarabine; n=2), CHOP (n=1), dose-adjusted EPOCH (n=1), French LMB89 protocol (n=1), and French LMB86 protocol (n=1). Five of nine patients (56%) received rituximab. All patients received intrathecal chemotherapy.
Fifteen of 17 HL patients were treated with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine), 1 patient received gemcitabine and carboplatin, and 1 patient received ChIVPP (chlorambucil, vinblastine, procarbazine, prednisone). Two patients received radiotherapy after completing chemotherapy.
Two of 12 patients with PCNSL received chemotherapy with the R-MPV regimen (rituximab, methotrexate, procarbazine, vincristine). Eight patients received palliative radiotherapy alone, and 2 patients received no lymphoma therapy.
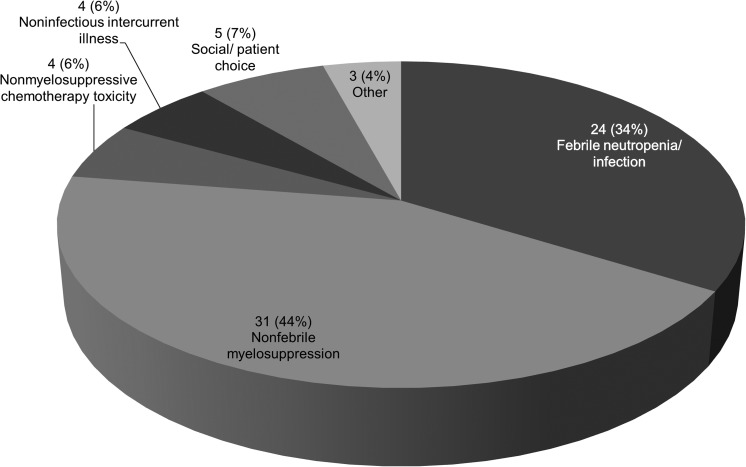
Chemotherapy administration among 39 patients with non-CNS lymphoma, for whom detailed records were available and lymphoma therapy was completed prior to study conclusion, is summarized in Table 3. Overall, patients completed 188 of 217 (87%) planned chemotherapy cycles, and 25 patients (64%) completed stage-appropriate therapy as per NCCN guidelines. Dose reduction, interruption, and/or delay occurred during 71 of 165 cycles (43%) for which data were available, of which 78% were due to infectious complications, febrile neutropenia, and myelosuppression (Fig. 1). Of 35 patients for whom data were available, 23 (66%) experienced chemotherapy dose reduction, interruption, and/or delay during ≥25% of administered cycles.
Table 3.
Chemotherapy Administration for HIV-Associated Non-CNS Lymphoma
| DLBCL (n=15) | BL (n=9) | HL (n=15) | |
|---|---|---|---|
| Cycles planned | |||
| Total | 82 | 55 | 80 |
| Per patient | 5.5 | 6.1 | 5.3 |
| Cycles completed | |||
| Total | 75 | 50 | 63 |
| Per patient | 5.0 | 5.6 | 4.2 |
| % completed | 91% | 91% | 79% |
| Rituximab | |||
| % of cycles | 52% | 56% | — |
| % of patients | 59% | 56% | — |
| Dose reduction | |||
| % of cycles | 31% | 2% | 27% |
| Interruption | |||
| % of cycles | 1% | 5% | 10% |
| Delay | |||
| % of cycles | 19% | 33% | 22% |
| Dose reduction, interruption, or delay | |||
| % of cycles | 43% | 36% | 49% |
| Dose reduction, interruption, or delay in >25% of cycles | |||
| % of patients | 69% | 75% | 57% |
| Completed stage-appropriate therapy | |||
| % of patients | 73% | 44% | 67% |
DLBCL, diffuse large B cell lymphoma; BL, Burkitt lymphoma; HL, Hodgkin lymphoma.
FIG. 1.
Reasons for deviation from planned cumulative dose or dose intensity among 71 chemotherapy cycles requiring dose reduction, interruption, and/or delay.
Of 53 patients with non-CNS lymphoma, 9 (17%) developed refractory or relapsed lymphoma (7 refractory, 2 relapsed), including 4 DLBCL, 1 BL, 3 HL, and 1 nonspecified B cell lymphoma. Both relapses occurred late, 5.6 and 8.8 years after initial diagnosis, in one patient despite continued HIV RNA suppression. Three refractory or relapsed patients were treated with salvage chemotherapy (1 DLBCL, 2 HL), with 2 patients proceeding to high-dose chemotherapy with autologous stem cell rescue (HDT/ASCR).
Interaction of antiretroviral treatment with lymphoma chemotherapy
HIV treatment records were available during receipt of lymphoma chemotherapy for 40 patients overall, with 25 patients either not receiving chemotherapy or receiving HIV treatment at another facility without available records. Of these 40 patients, 26 (65%) were receiving antiretroviral therapy at the time of lymphoma diagnosis. Thirty-one (78%) of 40 patients had HIV RNA results available at lymphoma diagnosis, of whom 13 (42%) had suppressed HIV RNA <400 copies/ml. Twenty of 40 patients (50%) experienced discontinuity of antiretroviral therapy during lymphoma treatment. Ten of 40 patients (25%) had at least one antiretroviral therapy interruption lasting 3 days or more. Patients experiencing antiretroviral therapy interruptions had a mean of 1.2 interruption events per patient (range 1–3), lasting a median of 33 days per interruption event (range 4–111 days). Of 28 antiretroviral interruption and/or change events, stated reasons included gastrointestinal intolerance (n=8, 29%), virologic failure (n=5, 18%), interaction with chemotherapy medications (n=4, 14%), myelosuppresion (n=3, 11%), renal insufficiency (n=2, 7%), and 1 instance each of financial difficulty, inability to swallow, transition to hospice, nonadherence, hyperbilirubinemia, and myalgias. Of 19 antiretroviral interruption and/or change events in which an individual medication was implicated, the most frequently implicated agents were ritonavir (n=9, 47%), non-ritonavir protease inhibitor (n=5, 26%), tenofovir (n=2, 11%), and zidovudine (n=2, 11%).
Clinical outcomes
Complete response rates and overall survival 2 and 5 years after lymphoma diagnosis are summarized in Table 4. Of 58 patients for whom at least 2 years had elapsed from lymphoma diagnosis on December 31, 2010, 56 patients had ascertainable vital status with 2 patients (3%) lost to follow-up. Of 49 patients for whom at least 5 years had elapsed from lymphoma diagnosis on December 31, 2010, 46 patients had ascertainable vital status with 3 patients (6%) lost to follow-up. A complete response was achieved in 36 of 62 patients (58%) overall (52% NHL, 76% HL; p=0.15). Survival 2 years after lymphoma diagnosis was 52% overall (46% NHL, 71% HL; p=0.13). Survival was poorest for PCNSL (median survival 0.2 years), with the longest-surviving patient living 1.5 years after diagnosis. In bivariate analyses, only CNS lymphoma versus non-CNS lymphoma was independently associated with mortality 2 years after lymphoma diagnosis. Patients with non-CNS lymphoma had significantly better complete response rates (72% versus 0%; p<0.0001) and 2-year survival (66% versus 0%; p<0.0001) than those with CNS lymphoma. All three patients with non-CNS lymphoma who received salvage therapy for relapsed or refractory disease were alive at last follow-up, including one HL patient treated with salvage chemotherapy alive 7.0 years after relapse, one HL patient treated with salvage chemotherapy and HDT/ASCR alive 0.2 years after ASCR, and one DLBCL patient treated with salvage chemotherapy and HDT/ASCR alive 0.4 years after ASCR.
Table 4.
Complete Response Rates and Overall Survival After HIV-Associated Lymphoma
| All HIV-associated lymphoma (n=65) | All NHL (n=47) | DLBCL (n=17) | PCNSL (n=12) | BL (n=9) | HL (n=17) | |
|---|---|---|---|---|---|---|
| Complete response | 36/62 (58%) | 23/44 (52%) | 10/16 (62%) | 0/12 (0%) | 8/9 (89%) | 13/17 (76%) |
| 2-year overall survival | 29/56 (52%) | 19/41 (46%) | 8/14 (57%) | 0/12 (0%) | 5/7 (71%) | 10/14 (71%) |
| 5-year overall survival | 16/46 (35%) | 11/35 (31%) | 5/12 (42%) | 0/12 (0%) | 3/5 (60%) | 5/10 (50%) |
NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B cell lymphoma; PCNSL, primary CNS lymphoma; HL, Hodgkin lymphoma.
Twenty-six of 34 patients (76%) who died had an ascertainable cause of death. Of these, 19 (73%) were due to progressive lymphoma, 4 (15%) were due to potentially treatment-related causes (2 deaths from neutropenic sepsis, 1 death from liver failure and disseminated tuberculosis proximate to receipt of chemotherapy, 1 death from liver and kidney failure proximate to receipt of chemotherapy), and 3 deaths (12%) were unrelated to either lymphoma progression or treatment (1 death from pneumonia and metastatic anal squamous cell carcinoma, 1 death from liver failure and hepatocellular carcinoma, 1 death from cardiac arrest and hypoxemia), all occurring more than 2 years after lymphoma diagnosis with no evidence of relapsed lymphoma or recent lymphoma therapy.
Among 34 patients for whom HIV RNA results were available 6 months after lymphoma diagnosis, 26 (76%) had HIV RNA <400 copies/ml (72% NHL, 89% HL, p=0.40). Among 29 patients surviving at least 2 years after lymphoma diagnosis, 15 of 20 (75%) for whom data were available had a CD4 cell count >200 cells/μl, and 15 of 19 (79%) had HIV RNA <400 copies/ml at last follow-up.
Comparison of HIV-associated DLBCL with non-HIV DLBCL
Patients with HIV-associated DLBCL were compared to an existing institutional database of 69 contemporaneously treated DLBCL patients without HIV (Table 5). Patients with HIV were younger and more frequently male and African-American. Patients with HIV presented more frequently with stage III or IV disease, abnormal LDH, B symptoms, extranodal involvement, and advanced IPI score. Despite having less advanced disease, HIV-uninfected patients received more intensive lymphoma treatment with an equivalent mean number of chemotherapy cycles, and significantly greater receipt of rituximab and radiotherapy. Two-year survival after lymphoma diagnosis was 83% for patients without HIV compared with 57% for those with HIV (p=0.07).
Table 5.
Comparison of Patients with Diffuse Large-B Cell Lymphoma by HIV Status
| HIV (n=17) | Non-HIV (n=69) | p value | |
|---|---|---|---|
| Age | 42.9 (8.5) | 58.3 (16.3) | <0.0001 |
| Male | 88% | 55% | 0.01 |
| Ethnicity | |||
| White | 47% | 79% | 0.01 |
| AA | 47% | 14% | |
| Other | 6% | 7% | |
| ECOG performance status ≥2 | 50% | 29% | 0.14 |
| Ann Arbor stage III or IV | 88% | 49% | 0.01 |
| Abnormal lactate dehydrogenase | 86% | 56% | 0.04 |
| B symptoms present | 71% | 36% | 0.01 |
| ≥2 extranodal sites | 41% | 18% | 0.04 |
| International Prognostic Index | |||
| 0–1 | 7% | 43% | 0.04 |
| 2 | 29% | 23% | |
| 3 | 43% | 13% | |
| 4–5 | 21% | 20% | |
| Chemotherapy cycles completed | 5.0 (2.6) | 5.4 (2.0) | 0.6 |
| Received rituximab | 59% | 87% | 0.02 |
| Received radiotherapy | 24% | 58% | 0.03 |
Mean values given with standard deviations in parentheses.
ECOG, Eastern Cooperative Oncology Group.
Discussion
We undertook a contemporary description of patients with HIV-associated lymphoma at a single academic medical center over an 11-year period. Our results support observations made elsewhere, and further characterize the interaction between HIV and lymphoma diagnoses in routine care.
First, our results confirm that presenting CD4 cell count and HIV RNA values vary by lymphoma histopathologic subtype. In our sample, BL occurred at higher CD4 cell counts than other NHL subtypes, similar to recent analysis of the HIV/AIDS Cancer Match registry.16 A similar inverse correlation of HL risk with CD4 cell count has also been observed,15 with HL incidence unchanged or even increasing in the cART era.1,2,4 We found no differences in presenting CD4 cell count between patients with NHL and HL, although this may have been confounded by more frequent bone marrow involvement among those with HL, particularly as patients with HL had a higher rate of HIV RNA suppression at lymphoma diagnosis than those with NHL. However, declining CD4 cell count despite continuous HIV RNA suppression in the year prior to HL diagnosis has also been recently reported from the COHERE study group.4 Lymphocytopenia occurs frequently at HL diagnosis even among HIV-uninfected individuals, correlates with poor survival, and remains poorly understood with postulated mechanisms including cytokine-induced immunosuppression and redistribution of lymphocytes from the periphery into tumor tissue.4,26–31 In addition, the observation that BL and HL incidence in some epidemiologic studies is higher in HIV-infected persons with higher CD4 counts also remains largely unexplained. Proposed mechanisms for HL include chemokine-induced recruitment of CD4 cells to germinal centers inhabited by EBV-transformed lymphocytes, which may promote survival of malignant lymphocytes by protecting them against immune surveillance, and also interactions between CD40 ligand on activated CD4 cells and CD40 receptors on malignant Reed–Sternberg cells, which serve to activate the nuclear factor-κB pathway.4,15,29–32 Alternatively, higher CD4 cell counts may lead to increased B cell activation, thereby increasing the rate at which lymphomagenic molecular events, such as the c-myc immunoglobulin translocation event in BL, are generated.16 These putative mechanisms remain speculative, however, and interactions between HIV infection and the immune system leading ultimately to lymphomagenesis are likely to be complex and variable across lymphoma subtypes.
Second, patients with HIV-associated lymphoma continue to present with advanced stage and adverse prognostic features in the cART era. Eighty-five percent of DLBCL, BL, and HL patients in our study presented with Ann Arbor stage III or IV disease, typically accompanied by abnormal LDH, B symptoms, frequent bone marrow and extranodal involvement, advanced IPI score in NHL, and advanced IPS score in HL. Conversely, 47% of U.S. adults with NHL and 35% with HL diagnosed between 1999 and 2006 in the Surveillance Epidemiology and End Results (SEER) database had distant stage III or IV disease.33,34 Our comparison of DLBCL patients with and without HIV similarly demonstrated more frequent advanced stage and adverse prognostic features among those with HIV.
Third, administering chemotherapy to patients with HIV-associated lymphoma with optimal cumulative dose and dose intensity is challenging, as patients often present with moderate-to-severe immunosuppression, and may be diagnosed with HIV in close proximity to their lymphoma diagnosis, particularly for NHL patients in our study. Sixty-four percent of DLBCL, BL, and HL patients in our study required dose reduction, interruption, and/or delay during ≥25% of administered chemotherapy cycles, principally as a result of infectious complications, febrile neutropenia, and myelosuppression. Additionally, fewer DLBCL patients with HIV received rituximab and radiotherapy compared with HIV-uninfected patients, despite presenting with more advanced stage and adverse prognostic features. Rituximab has been associated with increased risk of neutropenia and infection without clinical benefit in HIV-associated NHL, particularly for patients with a CD4 count <50 cells/μl,35,36 but is also responsible for a 10–15% increase in long-term survival among HIV-uninfected patients with DLBCL, with little additional toxicity even when applied to elderly patients.37,38 The precise role of rituximab in HIV-associated NHL across CD4 cell count strata remains to be clarified.
Fourth, administering continuous antiretroviral therapy to patients with HIV-associated lymphoma is difficult. Patients frequently experience discontinuity in antiretroviral therapy, which may promote the development of resistance and compromise long-term outcomes even among those who are cured of lymphoma. In our study, regimens containing protease inhibitors, especially ritonavir, seemed particularly vulnerable to discontinuity, and preemptive consideration of alternative regimens, perhaps to include newer agents such as HIV integrase inhibitors, may be prudent to enhance gastrointestinal tolerability and to avoid interactions and overlapping toxicities with chemotherapy medications.
We are encouraged that despite advanced initial presentation and significant treatment challenges, 66% of patients with non-CNS lymphoma survived at least 2 years after lymphoma diagnosis, with many patients effectively cured and experiencing long-term survival of more than 5 years. Nevertheless, survival in our study was lower than is reported for HIV-uninfected patients with lymphoma of comparable age and stage, consistent with recent data from the Center for AIDS Research Network of Integrated Clinical Systems (CNICS) cohort.8 Five-year survival for HIV-associated NHL in our population was 31%, compared with 58% for stage III or IV NHL (all ages) and 75% for NHL in adults <45 years of age (all stages) in the SEER database,33 although direct comparison of NHL outcomes is limited by the greater frequency of indolent NHL subtypes among patients without HIV. Similarly, 5-year survival for HIV-associated HL in our population was 50%, compared with 74% for stage III or IV HL (all ages) and 92% for HL in adults <45 years of age (all stages) in the SEER database.34 Our comparison of contemporaneously treated DLBCL patients with and without HIV likewise demonstrated a trend toward inferior 2-year survival among those with HIV, perhaps mediated by more advanced disease and less intensive lymphoma therapy. Of all deaths occurring in our study with ascertainable cause, 73% were due to progressive lymphoma, suggesting that treatment-related mortality and competing causes of death are not the main contributors to reduced overall survival among patients with HIV.
Continued efforts to provide coordinated supportive care to patients with HIV-associated lymphoma, possibly to include growth factor support, antibacterial and antifungal prophylaxis, prompt and continuous antiretroviral therapy, and expert management of drug interactions between antiretroviral and chemotherapy medications, will hopefully result in clinical outcomes that eventually equal results seen in HIV-uninfected patients. Additional research to define optimal first-line and salvage lymphoma treatments, including the role of rituximab and newer agents across CD4 strata, together with efforts to define optimal antiretroviral therapy, will hopefully also result in improved outcomes. Our study also confirms the poor prognosis of HIV-associated PCNSL, consistent with data from the CNICS cohort,8 even among the minority of patients with preserved CD4 cell count and HIV RNA suppression at the time of PCNSL diagnosis. Improved strategies to prevent, treat, and palliate HIV-associated PCNSL are urgently needed.
Limitations of our study include the small sample size and retrospective data collection via abstraction from the medical record. However, given the inclusion of patients at a single center with a fully integrated electronic medical record, we were able to collect accurate, detailed information on the majority of patients, and the Social Security Death Index was used to ascertain final vital status for patients lost to follow-up. In addition, heterogeneity in pathologic descriptions and small sample size limited our ability to consider morphologic, immunophenotypic, and molecular subtypes individually within larger lymphoma groupings. For instance, we were unable to assess the influence of plasmablastic morphology or immunophenotype within the DLBCL group, a subtype that is known to occur more frequently in HIV-infected individuals, and is typically associated with later-stage B cells expressing plasma cell markers rather than pan-B cell markers as in typical DLBCL. Plasmablastic DLBCL may be associated with a poorer response to therapy, and greater frequency of such pathologic features may contribute to more aggressive disease and poorer survival in HIV-infected individuals with DLBCL.
In summary, most patients with non-CNS HIV-associated lymphoma receiving routine care at a single academic medical center survived at least 2 years after diagnosis. Ongoing efforts to optimize coordinated HIV and lymphoma treatment will hopefully result in continued survival gains for this challenging population.
Acknowledgments
We would like to thank Amanda Corbett, Sonia Napravnik, and Ralph Raasch for their manuscript review and comments. This research was supported by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program P30 AI 50410.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Patel P. Hanson DL. Sullivan PS, et al. Adult and adolescent spectrum of disease project and HIV outpatient study investigators. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA. Pfeiffer RM. Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 3.Grulich AE. van Leeuwen MT. Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 4.Bohlius J. Schmidlin K. Boué F, et al. HIV-1-related Hodgkin lymphoma in the era of combination antiretroviral therapy: Incidence and evolution of CD4+ T-cell lymphocytes. Blood. 2011;117:6100–6108. doi: 10.1182/blood-2010-08-301531. [DOI] [PubMed] [Google Scholar]
- 5.Simard EP. Engels EA. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis. 2010;51:957–962. doi: 10.1086/656416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet F. Burty C. Lewden C, et al. Changes in cancer mortality among HIV-infected patients: The Mortalité 2005 Survey. Clin Infect Dis. 2009;48:633–639. doi: 10.1086/596766. [DOI] [PubMed] [Google Scholar]
- 7.Antiretroviral Therapy Cohort Collaboration: Causes of death in HIV-1 infected patients treated with antiretroviral therapy, 1996–2006: Collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achenbach CJ. Cole SR. Kitahata MM, et al. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS. 2011;25:691–700. doi: 10.1097/QAD.0b013e3283437f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gucalp A. Noy A. Spectrum of HIV lymphoma 2009. Curr Opin Hematol. 2010;17:362–367. doi: 10.1097/MOH.0b013e328338f6b6. [DOI] [PubMed] [Google Scholar]
- 10.Zoufaly A. Stellbrink HJ. Heiden MA, et al. HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis. 2009;200:79–87. doi: 10.1086/599313. [DOI] [PubMed] [Google Scholar]
- 11.Engels EA. Pfeiffer RM. Landgren O, et al. Immunologic and virologic predictors of AIDS-related non-Hodgkin lymphoma in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2010;54:78–84. doi: 10.1097/01.qai.0000371677.48743.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group: Incidence and risk factors of HIV-related non-Hodgkin's lymphoma in the era of combination antiretroviral therapy: A European multicohort study. Antivir Ther. 2009;14:1065–1074. doi: 10.3851/IMP1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bower M. Fisher M. Hill T, et al. CD4 counts and the risk of systemic non-Hodgkin's lymphoma in individuals with HIV in the UK. Haematologica. 2009:875–880. doi: 10.3324/haematol.2008.002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guiguet M. Boué F. Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): A prospective cohort study. Lancet Oncol. 2009;10:1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 15.Biggar RJ. Jaffe ES. Goedert JJ, et al. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guech-Ongey M. Simard EP. Anderson WF, et al. AIDS-related Burkitt lymphoma in the United States: What do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. 2010;116:5600–5604. doi: 10.1182/blood-2010-03-275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine AM. Management of AIDS-related lymphoma. Curr Opin Oncol. 2008;20:522–528. doi: 10.1097/CCO.0b013e3283094ec7. [DOI] [PubMed] [Google Scholar]
- 18.Sparano JA. Lee JY. Kaplan LD, et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115:3008–3016. doi: 10.1182/blood-2009-08-231613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montoto S. Wilson J. Shaw K, et al. Excellent immunological recovery following CODOX-M/IVAC, an effective intensive chemotherapy for HIV-associated Burkitt's lymphoma. AIDS. 2010;24:851–856. doi: 10.1097/QAD.0b013e3283301578. [DOI] [PubMed] [Google Scholar]
- 20.Collaboration of Observational HIV Epidemiological Research EUROPE (COHERE) Study Group: Prognosis of HIV-associated non-Hodgkin lymphoma in patients starting combination antiretroviral therapy. AIDS. 2009;23:2029–2037. doi: 10.1097/QAD.0b013e32832e531c. [DOI] [PubMed] [Google Scholar]
- 21.Berenguer J. Miralles P. Ribera JM, et al. Characteristics and outcome of AIDS-related Hodgkin lymphoma before and after the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:422–428. [PubMed] [Google Scholar]
- 22.Chao C. Xu L. Abrams D. Leyden W, et al. Survival of non-Hodgkin lymphoma patients with and without HIV infection in the era of combined antiretroviral therapy. AIDS. 2010;24:1765–1770. doi: 10.1097/QAD.0b013e32833a0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network. NCCN: Clinical practice guidelines in oncology: Non-Hodgkin's lymphomas version 2.2011. National Comprehensive Cancer Network. 2011. http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. [Mar 21;2011 ]. http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf [DOI] [PubMed]
- 24.National Comprehensive Cancer Network. NCCN: Clinical practice guidelines in oncology: Hodgkin lymphomas v2.2010. National Comprehensive Cancer Network. 2010. http://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf. [Mar 21;2011 ]. http://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf [DOI] [PubMed]
- 25.Juweid ME. Stroobants S. Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: Consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 26.Hasenclever D. Diehl V. A prognostic score for advanced Hodgkin's disease: International prognostic factors project on advanced Hodgkin's disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 27.Brauninger A. Schmitz R. Bechtel D, et al. Molecular biology of Hodgkin's and Reed/Sternberg cells in Hodgkin's lymphoma. Int J Cancer. 2006;118:1853–1861. doi: 10.1002/ijc.21716. [DOI] [PubMed] [Google Scholar]
- 28.Romagnani S. Del Prete GF. Maggi E, et al. Displacement of T lymphocytes with the ‘Helper/Inducer’ phenotype from peripheral blood to lymphoid organs in untreated patients with Hodgkin's disease. Scand J Haematol. 1983;31:305–314. doi: 10.1111/j.1600-0609.1983.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 29.Poppema S. Potters M. Visser L. van den Berg AM, et al. Immune escape mechanisms in Hodgkin's disease. Ann Oncol. 1998;9(Suppl 5):S21–S24. doi: 10.1093/annonc/9.suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 30.Van den Berg A. Visser L. Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells: A possible explanation for the characteristic T cell infiltrate in Hodgkin's lymphoma. Am J Pathol. 1999;154:1685–1691. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skinnider BF. Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99:4283–4297. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- 32.Rolf J. Fairfax K. Turner M. Signaling pathways in T follicular helper cells. J Immunol. 2010;184:6563–6568. doi: 10.4049/jimmunol.1000202. [DOI] [PubMed] [Google Scholar]
- 33.National Cancer Institute (2011) Non-Hodgkin lymphoma, 5-year relative, period survival by race, sex, diagnosis year, age. National Cancer Institute surveillance epidemiology and end results 2011. http://seer.cancer.gov/csr/1975_2007/browse_csr.php?section=19&page=sect_19_table.08.html. [Mar 21;2011 ]. http://seer.cancer.gov/csr/1975_2007/browse_csr.php?section=19&page=sect_19_table.08.html
- 34.National Cancer Institute. Hodgkin lymphoma, 5-year relative, period survival by race, sex, diagnosis year, age. National Cancer Institute surveillance epidemiology and end results 2011. http://seer.cancer.gov/csr/1975_2007/browse_csr.php?section=9&page=sect_09_table.08.html. [Mar 21;2011 ]. http://seer.cancer.gov/csr/1975_2007/browse_csr.php?section=9&page=sect_09_table.08.html
- 35.Kaplan LD. Lee JY. Ambinder RF. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. Blood. 2005;106:1538–1543. doi: 10.1182/blood-2005-04-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spina M. Jaeger U. Sparano JA, et al. Rituximab plus infusional cyclophosphamide, doxorubicin, and etoposide in HIV-associated non-Hodgkin lymphoma: pooled results from 3 phase 2 trials. Blood. 2005;105:1891–1897. doi: 10.1182/blood-2004-08-3300. [DOI] [PubMed] [Google Scholar]
- 37.Coiffier B. Thieblemont C. Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfreundschuh M. Trűmper L. Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]