Abstract
Determining arterial mechanical properties is important for understanding the work done by the heart and how it changes with cardiovascular disease. Ex vivo tests are necessary to apply various loads to the artery and obtain data to model and predict the behavior under any load. Most ex vivo tests are performed within 24 h of dissection, so the tissue is still “alive.” For large elastic arteries; however, the passive mechanical behavior is attributed mostly to the very stable proteins, elastin, and collagen. If the testing equipment fails, is in use, or is located at another facility, it would be useful to store the vessels and postpone the tests until the equipment is available. The goal of this study is to determine the effects of storage time on the mechanical behavior of the common carotid artery from adult mice. Each artery was tested after storage for 1–28 days in physiologic saline at 4°C. storage time on the arterial diameter or force at each pressure, but there were significant effects on the stretch ratio and stress at each pressure. The significant effects on the stretch ratio and stress were due to decreases in the unloaded dimensions with storage time, when measured from cut arterial rings. When the unloaded dimensions were measured instead from histology sections, there were no significant changes with storage time. We conclude that histology sections yield a more consistent measurement of the unloaded dimensions and that there are no significant changes in the mechanical behavior of mouse carotid artery with storage up to 28 days.
Keywords: blood vessel storage, mechanical testing, stress, stretch, elastin, collagen, carotid artery
Introduction
Segments of blood vessels are useful for a variety of physiological studies. For instance, scientists and surgeons are working toward using transplanted vessel segments as grafts for their patients. Other scientists are investigating remodeling in development and disease using data from mechanical tests on vessel segments. In either application, the blood vessels may not be used promptly after dissection. This could be due to lack of immediate access to the patient receiving the graft in the first example or unpredicted failure or unavailability of the experimental equipment in the second example. The vessels may have to be stored for hours to days and it is essential to understand how the storage time and conditions affect different properties.
For vascular grafts, properties of the endothelial cells (ECs) and smooth muscle cells (SMCs) are critical for vessel function and patency. The effects of storage duration and storing agent on the preservation of the ECs [1,2], SMCs [1,3], and vascular reactivity [2,4] have been previously investigated. Additional work has focused on cryopreservation of blood vessels [5,6], but cryopreservation may not always be a promising method for blood vessel storage [7]. Some of the studies concluded that transplanted vessels must be used within 3–7 days due to possible biochemical and molecular changes that occur after this point [7], and extending the storage time beyond 7 days can significantly lower the long-term patency rates [8].
For mechanical studies of vessel segments, properties of the extracellular matrix may be more important than cellular properties. In the large elastic arteries, the passive mechanical properties are determined mostly by elastin and collagen, which are very stable proteins [9]. Therefore, to determine changes in the passive mechanical properties of vessel segments with development, aging or disease, it may be reasonable to store the vessels for longer than 7 days. Most previous work on vessel storage has studied the changes in the passive mechanical properties in limited detail and has focused on uniaxial testing of tissue strips [10,11]. Our goal is to perform a systematic examination of the changes in mechanical behavior of common carotid arteries from adult mice after storage at 4 °C in physiologic saline. Our hypothesis is that the passive mechanical behavior will not change significantly up to 28 days of storage.
Materials and Methods
Animals.
87 C57BL/6J male mice were used for this study (age = 60 ± 3 days, weight = 25 ± 2.5 g). Small carbon particles were used to mark the in vivo length of the left and right common carotid arteries (LCC and RCC). The arteries were removed, the ex vivo lengths were measured, and the arteries were stored in a 4°C refrigerator in physiological saline solution (PSS) [12]. After storage for 1, 3, 7, 14, or 28 days, LCCs were used for mechanical tests and RCCs were used for histology. Each vessel was tested at only one time point.
Mechanical Testing.
Mechanical tests were performed on a pressure myograph (Danish Myotechnology), as described previously [12]. Briefly, the LCCs were mounted on custom-made stainless steel cannulae in PSS at 37°C. The arteries were stretched to their in vivo length and preconditioned for three pressure cycles. The pressure, outer diameter and longitudinal force were then recorded for three additional cycles from 0–175 mmHg in steps of 25 mmHg and 12 s/step. The LCCs were removed and several thin rings (∼500 μm thick) were cut and imaged. Unloaded dimensions were measured with Image J software (NIH).
Data Analysis.
The loaded inner diameter was calculated assuming incompressibility of the arterial wall:
| (1) |
where d and D are the loaded and unloaded diameters, with subscripts i for inner and o for outer diameters, and λz = l/L is the longitudinal stretch ratio, where l and L are the loaded and unloaded reference lengths of the artery. The average stresses in the longitudinal and circumferential directions, assuming a thin-walled cylinder with no shear stress, are respectively [13]:
| (2) |
| (3) |
where F is the longitudinal force and P is the gauge luminal static pressure. Also, the stretch ratio of the artery in the circumferential direction is defined as [14]:
| (4) |
Compliance, which is the tendency of the artery to distend in response to the change in luminal pressure, is defined in this work as the percent change in outer diameter for each pressure step:
| (5) |
where j denotes the index of the pressure step.
Histology.
RCCs were fixed in the unloaded state in 10% formalin, dehydrated in a graded series of ethanol, embedded in paraffin and sectioned into 5 μm thick slices. The sections were stained with Verhoeff−Van Gieson (VVG) to visualize elastic fibers. Images were recorded of each section and the outer diameter of the adventitia, media and the inner diameter of the vessel were measured using Image J software. The images were then manually thresholded to determine the percent area of the media occupied by elastic fibers.
Statistics.
ANOVA or two-tailed t-tests, with unequal variance, were used to determine statistical differences. P ≤ 0.05 was considered significant.
Results
Unloaded Geometry From Arterial Rings.
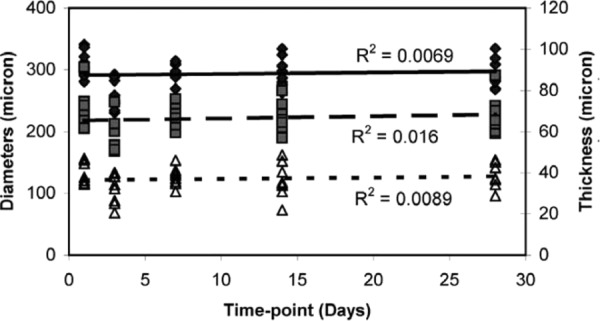
The unloaded outer diameter and thickness of the arterial rings decrease with storage time (Fig. 1). Compared to day 1, the outer diameter is significantly different at day 28 and the thickness is significantly different at days 7, 14, and 28 (Table 1). The inner diameter does not change with storage time and there are no significant differences between day 1 and any other time points.
Fig. 1.
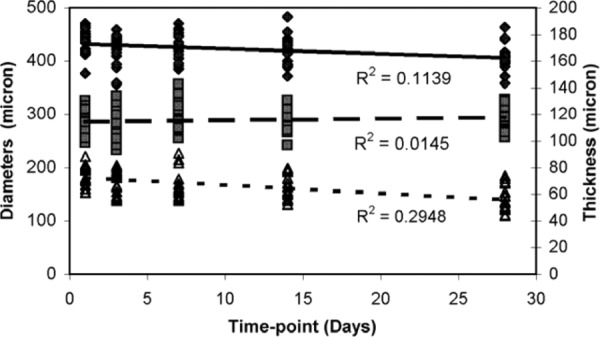
Unloaded dimensions from cut ring measurements. Outer diameter and thickness decrease with time, while the inner diameter does not change with time. N1 = 12, N3 = 11, N7 = N14 = N28 = 9 (N and i are the number of samples and the time-point, respectively). ♦ outer diameter, ▪ inner diameter, thickness.
Table 1.
Storage time points showing statistically significant differences (P < 0.05) for the unloaded dimensions measured from cut rings
| Storage timepoint (day) | |||||
|---|---|---|---|---|---|
| 1 | 3 | 7 | 14 | 28 | |
| Unloaded thickness (THK) | 7,14,28 | 28 | 1,28 | 1,28 | 1,3,7,14 |
| Unloaded outer diameter (OD) | 28 | - | - | - | 1 |
| Unloaded inner diameter (ID) | - | - | - | - | - |
Mechanical Testing.
The loaded outer diameter-pressure data (Fig. 2(a)) demonstrate that storage time does not affect the behavior, despite variations between different storage times. Compared to day 1, there are no significant differences between the diameters at each pressure for any of the storage times. There are significant differences between days 3 and 7 at 100 mmHg (Table 2). It has been shown previously for ex vivo tests on mouse carotid arteries that the longitudinal force decreases with pressure at the measured in vivo length [12]. We observe similar behavior in this study (Fig. 2(b)) and find no significant differences in the longitudinal force at each pressure between day 1 and any other storage times (Table 2). There are significant differences at the low to medium pressure range between days 3 and 7. The storage time does not significantly affect the compliance-pressure behavior (Fig. 2(c) and Table 2), although there is a trend toward shifting the compliance peak to lower pressures with longer storage.
Fig. 2.
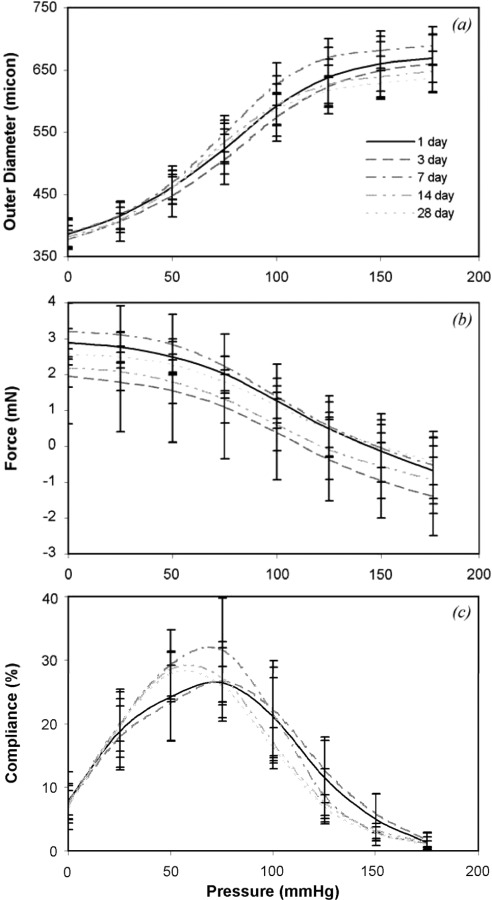
Effects of pressure and storage time on (a) outer diameter, (b) longitudinal force, and (c) compliance. There is no consistent trend for changes in the curves with storage time. N1 = 12, N3 = 11, N7 = N14 = N28 = 9.
Table 2.
Storage time points showing statistically significant differences (P < 0.05) at each applied pressure for the ex vivo mechanical tests
| Outer Diameter | Longitudinal Force | Compliance | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure (mmHg) | 1 | 3 | 7 | 14 | 28 | 1 | 3 | 7 | 14 | 28 | 1 | 3 | 7 | 14 | 28 |
| 0 | - | - | - | - | - | - | 7 | 3 | - | - | - | - | - | - | - |
| 25 | - | - | - | - | - | - | 7 | 3 | - | - | - | - | - | - | - |
| 50 | - | - | - | - | - | - | 7 | 3 | - | - | - | - | - | - | - |
| 75 | - | - | - | - | - | - | 7 | 3 | - | - | - | - | - | - | - |
| 100 | - | 7 | 3 | - | - | - | - | - | - | - | - | - | - | - | - |
| 125 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 150 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 175 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Circumferential Stretch | Circumferential Stress | Longitudinal Stress | |||||||||||||
| Pressure (mmHg) | 1 | 3 | 7 | 14 | 28 | 1 | 3 | 7 | 14 | 28 | 1 | 3 | 7 | 14 | 28 |
| 0 | - | - | - | - | - | - | 14,28 | - | - | - | - | 7 | 3 | - | - |
| 25 | - | - | - | - | - | - | 14,28 | - | 3 | 1,3 | - | 7 | 3 | - | - |
| 50 | - | 14 | - | 3 | - | 28 | 7,14,28 | - | 3 | 1,3 | - | 7,28 | 3 | - | 3 |
| 75 | - | 14,28 | - | 3 | 3 | 14,28 | 7,14,28 | 3 | 1,3 | 1,3 | - | 7,28 | 3 | - | 3 |
| 100 | - | 7 | 3 | - | - | 28 | 7,14,28 | 3 | 3 | 3 | - | 7,28 | 3 | - | 3 |
| 125 | - | - | - | - | - | 28 | 7,14,28 | 3 | 3 | 3 | - | 7,28 | 3 | - | 3 |
| 150 | - | - | - | - | - | - | 7,14,28 | 3 | 3 | 3 | - | 7,28 | 3 | - | 3 |
| 175 | - | - | - | - | - | - | 7,14,28 | 3 | 3 | 3 | - | 7,14,28 | 3 | 3 | 3 |
The unloaded dimensions from the arterial rings were used to calculate circumferential stretch ratio, circumferential stress and longitudinal stress. The circumferential stretch-pressure relationship is similar for most storage times (Fig. 3(a)). There are no significant differences between day 1 and any other days, but there are significant differences between day 3 and some of the later time points (Table 2). Figure 3(b) shows that there are relatively large variations in the mean circumferential stress-pressure relationship for different storage times, but only days 14 and 28 show significant differences from day 1 (Table 2). There are also significant differences between day 3 and days 7, 14, and 28, making circumferential stress the variable with the most significant differences between storage times. The mean longitudinal stress-pressure is similar for most storage time points (Fig. 3(c)). There are no significant differences between day 1 and any other time points (Table 2). There are significant differences between day 3 and all other time points. Circumferential stress-stretch curves (Fig. 4), which demonstrate the nonlinear behavior and the incremental elastic modulus of the arterial wall, show similar behavior for all storage times.
Fig. 3.
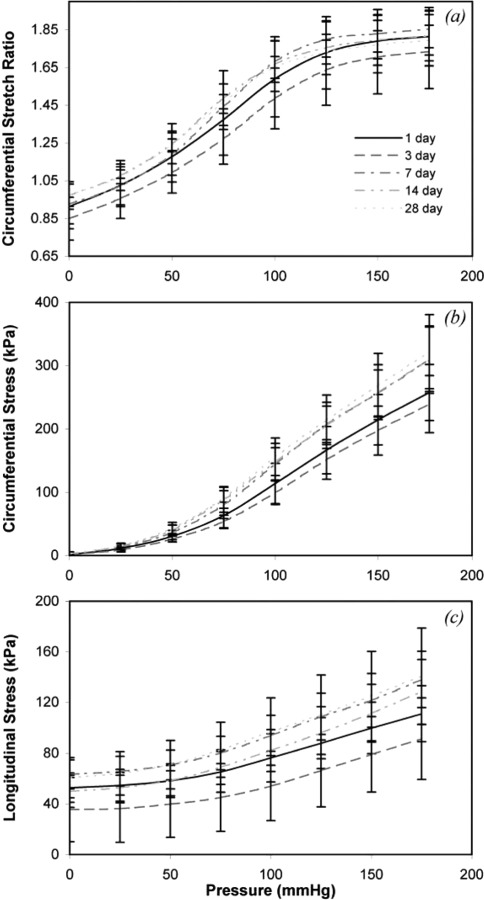
Effects of pressure and storage time on (a) circumferential stretch ratio, (b) circumferential stress, and (c) longitudinal stress. There is no consistent trend for changes in the curves with storage time. N1 = 12, N3 = 11, N7 = N14 = N28 = 9.
Fig. 4.
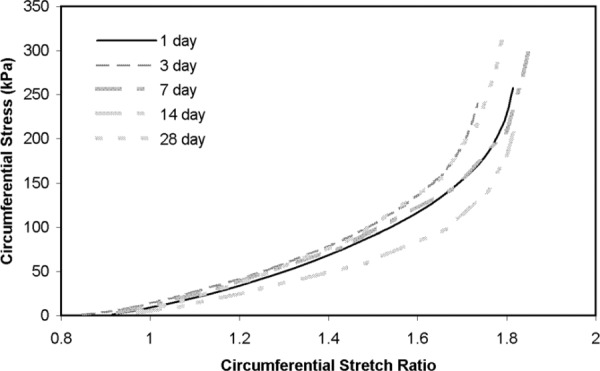
Circumferential stress-stretch relationship at different storage times. The local slope of the curves gives an incremental measure of the elastic modulus of the arterial wall. Although there are some variations from one time point to the other, there is no consistent trend for changes in the curves with storage time. N1 = 12, N3 = 11, N7 = N14 = N28 = 9.
Histology.
The geometric and mechanical data indicated that there may be changes in the composition or structure of the arterial wall with storage time. We performed histology to validate the geometry measurements and investigate changes in the amount or organization of the elastic fibers. Figure 5 shows a cut arterial ring, a VVG stained histology section, and the same section after the image was thresholded to measure the elastic fiber area fraction. Note that determining the outer diameter of the cut ring requires some interpretation due to loose connective tissue and difficulty in focusing the entire boundary. Determining the outer diameter on the histology section is also subject to variation due to loose adventitia that is sometimes lost in tissue processing. Determining the outer boundary of the media on the histology section is relatively straight forward.
Fig. 5.
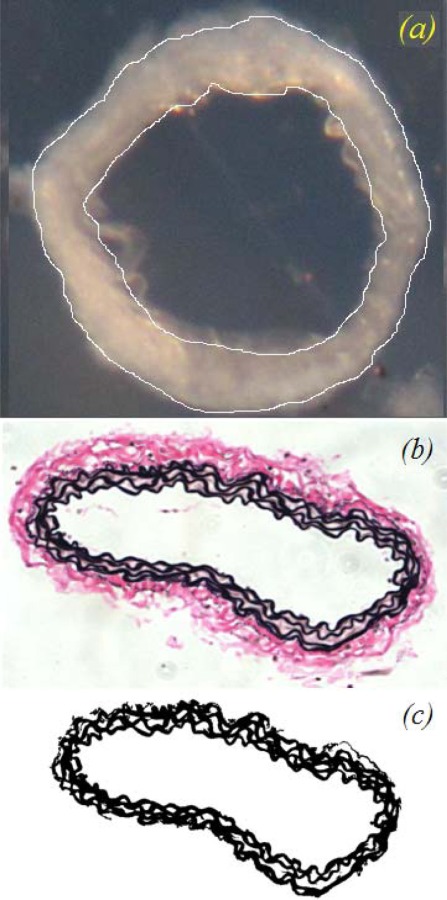
Images used for dimension and component measurements: (a) representative cut arterial ring with the inner and outer diameter outlined, (b) VVG stained histology section which clearly shows the boundaries of the media and adventitia in the artery wall, and (c) VVG stained histology image thresholded to highlight the elastic lamellae for area fraction measurements
Figure 6 shows the outer and inner diameters and thickness of the arteries as measured from the histology sections. Unlike the arterial ring measurements (Fig. 1), there is no consistent relationship between changes in the unloaded dimensions and the storage time. We address the conflicting results of these two measurements in the discussion. There are significant differences in outer and inner diameter between day 1 and day 3, and significant differences in the thickness between day 1 and day 14. To understand the relationship between the ring and histology measurements for the unloaded arterial dimensions, we plotted the unloaded diameter for the cut rings versus the unloaded diameter for the histology sections at each storage time. This relationship indicates that the tissue shrinks 31–58% with histology processing, with an average shrinkage of 44% for all storage times.
Fig. 6.
Unloaded dimensions obtained from histology sections. Unlike the measurements from cut arterial rings in Fig. 1, there are no consistent trends for changes in the dimensions with storage time. N1 = N3 = 7, N7 = N14 = N28 = 6.
We also quantified the area fraction of elastic fibers in the artery wall from the histology images to ensure that this was not changing over time and causing the observed changes in stress. Because of the loss of some adventitial tissue during histology processing, we calculated the ratio of dark staining elastic fibers (Ae) to the total medial area (Am). The area fraction is shown in Fig. 7. There are no significant differences in elastic fiber area fraction between day 1 and any other storage time points.
Fig. 7.
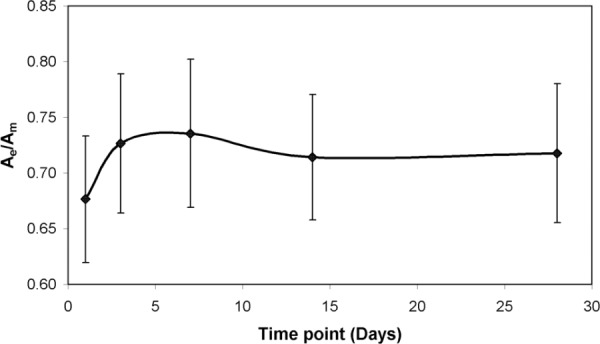
Fractional area of the elastic lamellae (Ae) [shown in Fig. 5(c)] compared to the total in medial area (Am) in the carotid artery at different storage time points. There are no significant differences between any time points.
Discussion
Storage of vessel segments may be necessary before implantation as grafts or before ex vivo mechanical testing. For vascular grafts, the maintenance of cellular activity is critical and has been studied in previous work [1,2]. For mechanical testing, the passive mechanical properties are often of primary interest and previous work has been limited to uniaxial tissue strips [10,11]. Passive mechanical properties are important to gain insight on arterial remodeling in development, aging and disease. Subsequent changes in mechanical properties due to this remodeling will affect the work done by the heart and overall cardiovascular and cardiac function. We hypothesize that the passive mechanical properties of mouse carotid arteries will not change significantly up to 28 days of storage at 4°C in physiologic saline. The ability to store arteries for days or weeks before testing would be convenient for collaborations across different facilities and would ensure the accuracy of mechanical data when the test equipment is not immediately available.
Pressure-Diameter and -Force Relationships do not Change With Storage Time.
In our mechanical test system, the outer diameter and longitudinal force are recorded for each pressure step. Although there is some variation in the data with each time point, there are no significant differences in these directly measured variables between day 1 and any other time point up to 28 days. At one pressure, there are significant differences between days 3 and 7. The 3 day time point shows the most differences for all variables between the other time points. Short term changes in the mechanical behavior could be caused by changes in the EC or SMC function or vessel reactivity that occur after the first few days [4], but we did not investigate this in the current study. If cellular changes were responsible; for example if the changes were due to cell death within the first few days, one would expect the later time points to more closely match the 3 day time point, not the 1 day time point. The differences for the 3 day time point may be a statistical anomaly caused by using different mice over the nine month study that had natural variations in arterial size and mechanical properties. We tried to avoid this by using several groups of mice over the study period for each storage time point.
Arterial Ring Dimensions Change With Storage Time, but Histology Section Dimensions do not.
The different trends between dimensions measured from the cut rings and the histology sections may be due to the fact that fewer samples were included in the histology measurements. However, we believe it is an artifact of the ring measurements that diminishes with time. As shown in Fig. 5(a), the arterial rings have loose connective tissue on the outside of the wall. This may be disturbed during dissection so that it contributes significantly to the overall outer diameter. When the arteries are stored in PSS, this connective tissue may become compacted and contribute less to the outer diameter over time. When considering the parts of the wall that are important for the passive mechanical behavior of the artery, this loose connective tissue is probably negligible and should not be included in the overall dimensions. Another alternative is that there is fluid movement out of the tissue wall during the storage time so that the dimensions decrease with time. The inner diameter does not change with storage time, which may be due to the lack of loose connective tissue at this surface or the EC barrier that prevents fluid movement at this surface. The histology dimensions are measured from dehydrated tissue and would not capture these differences. The PSS was made to closely mimic extracellular fluid and the pH did not change significantly with storage time (maximum standard deviation of pH among all time points was 0.10), but we did not specifically investigate fluid movement in the wall.
The histology sections and analysis of the elastic fiber area fraction show that there are no significant changes in dimensions of these solid, load bearing constituents with storage time. However, histology processing also shrinks the tissue and so a scaling factor must be used to determine the dimensions of the unprocessed tissue if these dimensions are used to calculate stretch ratios and stresses. An alternative approach to determine the unloaded dimensions is to measure the inner and outer diameters when the artery is loaded on the test system in the unloaded state [15], but we have found that determining the inner diameter of the thick carotid artery is difficult with the imaging capabilities of the pressure myograph system. We conclude that histology measurements are the most consistent and accurate determination of the unloaded arterial geometry, but must be scaled by the shrinkage factor to obtain the unloaded dimensions in the hydrated arterial wall.
Stresses and Stretch Ratios Change With Some Storage Time Increments.
The stresses and stretch ratios are calculated in this study from the measured pressure, loaded outer diameter, and force from the mechanical tests and the unloaded outer diameter, thickness and in vivo stretch ratio of the excised arteries and cut rings. Because the loaded outer diameter and force were not significantly different between day 1 and any of the other storage times, but the unloaded dimensions were, it is reasonable to deduce that most of the differences in stresses and stretch ratios with storage time were caused by the changes in unloaded dimensions of the cut rings. For example, the loaded outer diameter-pressure relationships of the 1 and 28 day time points are very similar, but the unloaded outer diameter and thickness are significantly different, leading to significant differences for the circumferential stress at some pressures. There is also some interplay between nonsignificant differences in multiple independent variables that can lead to significant differences in the calculated variables. For example, there are no significant differences for the outer diameter- or force-pressure relationship, or the unloaded dimensions between day 3 and 14, but there are significant differences for the stresses and stretch ratios.
Using the histology measurements that do not change with storage time and the diameter- or force-pressure relationships that generally do not change with storage time would reduce the number of significant differences in the stresses and stretch ratios between the time points. Unfortunately, the histology sections were cut from arteries that were not used for the mechanical tests; therefore the stresses and stretch ratios cannot be calculated directly with those dimensions in the current study. We conclude that the mechanical behavior of the adult mouse carotid artery does not change significantly between 1 day of storage in PSS at 4°C and up to 28 days of storage. Significant differences between some of the other storage time points would be eliminated by measuring the unloaded dimensions from histology sections and avoiding artifacts from loose connective tissue on the outer wall.
Our results are in agreement with previous findings on the passive mechanical properties of human or pig aorta demonstrating that storage at 4°C does not affect the subfailure stress, ultimate strength, and the Young's modulus (defined in the linear region of stress-strain curve) [10,11]. However, these studies were limited to uniaxial tests of tissue strips and focused primarily on the difference between refrigerated tissue and frozen tissue, not on the effects of days to weeks of refrigerated storage.
Conclusion
We performed mechanical tests on mouse carotid arteries stored at 4°C in physiologic saline for 1–28 days. The diameter- and force-pressure behaviors are not significantly different between day 1 and any of the storage time points. The circumferential stress-pressure behavior is significantly different only between 1 and 28 days, but this is most likely due to changes in the unloaded dimensions as calculated from cut arterial rings with loose connective tissue that became compacted over time. When the unloaded dimensions are determined from histologic sections, there are no significant changes with storage time. Therefore, the passive mechanical properties of large elastic arteries are stable over weeks of storage, but care must be taken in measuring the unloaded dimensions for stress and stretch ratio calculations.
Acknowledgment
This work was supported in part by the National Institutes of Health (HL087563, HL105314) and by the National Science Foundation (REU0849621).
Contributor Information
Mazyar Amin, Department of Biomedical Engineering, , St. Louis University, , 3507 Lindell Blvd., , St. Louis, MO 63103 , e-mail: mamin2@slu.edu.
Amber G. Kunkel, Department of Computational and Applied Math , Rice University, , 6350 Main St., , Houston, TX 77005 , e-mail: amber.g.kunkel@rice.edu
Victoria P. Le, e-mail: vle2@slu.edu
Jessica E. Wagenseil, e-mail: jwagense@slu.edu; Department of Biomedical Engineering, , St. Louis University, , 3507 Lindell Blvd., , St. Louis, MO 63103 .
References
- [1]. Kristek, F. , Torok, J. , and Sikulova, J. , 1993, “Morphological and Functional Alterations in Endothelium, Smooth Muscle, and Nerve Fibers in Rabbit Aorta After Storage at 4 Degrees C,” Cryobiology, 30(4), pp. 376–385. 10.1006/cryo.1993.1037 [DOI] [PubMed] [Google Scholar]
- [2]. Török, J. , Kristek, F. , and Mokrásová, M. , 1993, “Endothelium-Dependent Relaxation in Rabbit Aorta After Cold Storage,” Eur. J. Pharmacol.: Environ. Toxicol. Pharmacol., 228(5-6), pp. 313–319. 10.1016/0926-6917(93)90066-Y [DOI] [PubMed] [Google Scholar]
- [3]. Shibata, S. , 1969, “Effect of Prolonged Cold Storage on the Contractile Response of Strips of Rabbit Aorta to Various Agents,” Circ. Res., 24, pp. 179– 187. 10.1161/01.RES.24.2.179 [DOI] [PubMed] [Google Scholar]
- [4]. McIntyre, C. A. , Williams, B. C. , Lindsay, R. M. , McKnight, J. A. , and Hadoke, P. W. , 1998, “Preservation of Vascular Function in Rat Mesenteric Resistance Arteries Following Cold Storage, Studied by Small Vessel Myography,” Br. J. Pharmacol., 123(8), pp. 1555–1560. 10.1038/sj.bjp.0701768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Jeremy, J. Y. , Stansby, G. , Fuller, B. , Rolles, K. , and Hamilton, G. , 1992, “The Effect of Cold Storage of Rat Thoracic Aortic Rings in Organ Preservation Solutions–A Study of Receptor-Linked Vascular Prostacyclin Synthesis,” Transplantation, 53(5), pp. 999–1002. 10.1097/00007890-199205000-00007 [DOI] [PubMed] [Google Scholar]
- [6]. Ku, D. D. , Willis, W. L. , and Caulfield, J. B. , 1990, “Retention of Endothelium-Dependent Vasodilatory Responses in Canine Coronary Arteries Following Cryopreservation,” Cryobiology, 27(5), pp. 511–520. 10.1016/0011-2240(90)90039-7 [DOI] [PubMed] [Google Scholar]
- [7]. Peirce, E. C. , Gross, R. E. , Bill, A. H. , and Merrill, K. , 1949, “Tissue–Culture Evaluation of the Viability of Blood Vessels Stored by Refrigeration,” Ann. Surg., 129(3), pp. 333–348. 10.1097/00000658-194903000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Crowe, D. M. , Hurley, J. V. , Mitchell, G. M. , Niazi, Z. , and Morrison, W. A. , 1998, “Long-Term Studies of Cold-Stored Rabbit Femoral Artery and Vein Autografts,” Br. J. Plast. Surg., 51(4), pp. 291–299. 10.1054/bjps.1998.0020 [DOI] [PubMed] [Google Scholar]
- [9]. Dobrin, P. B. , 1997, “Physiology and Pathophysiology of Blood Vessels,” The Basic Science of Vascular Disease, Sidawy A. N., DePalma R. G., and Sumpio B. E., eds., Futura, New York, pp. 69–105. [Google Scholar]
- [10]. Adham, M. , Gournier, J.-P. , Favre, J.-P. , De La Roche, E. Ducerf, C. , Baulieux, J. , Barral, X. , and Pouyet, M. , 1996, “Mechanical Characteristics of Fresh and Frozen Human Descending Thoracic Aorta,” J. Surg. Res., 64(1), pp. 32–34. 10.1006/jsre.1996.0302 [DOI] [PubMed] [Google Scholar]
- [11]. Stemper, B. D. , Yoganandan, N. , Stineman, M. R. , Gennarelli, T. A. , Baisden, J. L. , and Pintar, F. A. , 2007, “Mechanics of Fresh, Refrigerated, and Frozen Arterial Tissue,” J. Surg. Res., 139(2), pp. 236–242. 10.1016/j.jss.2006.09.001 [DOI] [PubMed] [Google Scholar]
- [12]. Wagenseil, J. E. , Nerurkar, N. L. , Knutsen, R. H. , Okamoto, R. J. , Li, D. Y. , and Mecham, R. P. , 2005, “Effects of Elastin Haploinsufficiency on the Mechanical Behavior of Mouse Arteries,” Am. J. Physiol. Heart Circ. Physiol., 289(3), pp. H1209–1217. 10.1152/ajpheart.00046.2005 [DOI] [PubMed] [Google Scholar]
- [13]. Takamizawa, K. , and Hayashi, K. , 1987, “Strain Energy Density Function and Uniform Strain Hypothesis for Arterial Mechanics,” J. Biomech., 20(1), pp. 7–17. 10.1016/0021-9290(87)90262-4 [DOI] [PubMed] [Google Scholar]
- [14]. Malvern, L. E. , 1969, Introduction to the Mechanics of a Continuous Medium, Prentice-Hall, Englewood Cliffs, NJ. [Google Scholar]
- [15]. Dye, W. W. , Gleason, R. L. , Wilson, E. , and Humphrey, J. D. , 2007, “Altered Biomechanical Properties of Carotid Arteries in Two Mouse Models of Muscular Dystrophy,” J. Appl. Physiol., 103(2), pp. 664–672. 10.1152/japplphysiol.00118.2007 [DOI] [PubMed] [Google Scholar]


