The initial report from the PACS01 trial demonstrated a benefit at 5 years for disease-free survival and overall survival rates with the sequential administration of docetaxel after fluorouracil, epirubicin, and cyclophosphamide for patients with node-positive, operable breast cancer. This article evaluates the impact of this regimen at 8 years.
Keywords: Breast cancer, Adjuvant treatment, Epirubicin, Docetaxel, Sequential treatment, Randomized trial
Abstract
Purpose.
The initial report from the Programme Action Concertée Sein (PACS) PACS01 trial demonstrated a benefit at 5 years for disease-free survival (DFS) and overall survival (OS) rates with the sequential administration of docetaxel after FEC100 (fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2) for patients with node-positive, operable breast cancer. We evaluate here the impact of this regimen at 8 years.
Patients and Methods.
Between June 1997 and March 2000, a total of 1,999 patients (age <65) with localized, resectable, non-pretreated, unilateral breast cancer were randomly assigned to receive either standard FEC100 for 6 cycles or 3 cycles of FEC100 followed by 3 cycles of 100 mg/m2 docetaxel (FEC-D), both given every 21 days. Radiotherapy was mandatory after conservative surgery and tamoxifen was given for 5 years to hormone receptor (HR)-positive patients. Five-year DFS was the trial's main endpoint. Updated 8-year survival data are presented.
Results.
With a median follow-up of 92.8 months, 639 patients experienced at least one event. A total number of 383 deaths were registered. Eight-year DFS rates were 65.8% with FEC alone and 70.2% with FEC-D. OS rates at 8 years were 78% with FEC alone and 83.2% with FEC-D. Cox regression analysis adjusted for age and number of positive nodes showed a 15% reduction in the relative risk of relapse and a 25% reduction in the relative risk of death in favor of FEC-D. Significant relative risk reductions were observed in the HR-positive, HER2-positive, and Ki67 ≥20% subpopulations.
Conclusion.
Benefits for DFS and OS rates with the sequential FEC-D regimen are fully confirmed at 8 years.
Introduction
Multidrug chemotherapy based on the association of anthracyclines (epirubicin, doxorubicin) and taxanes (docetaxel, paclitaxel) for the adjuvant treatment of women with operable node-positive early breast cancer allowed unprecedented high survival rates. The 5-year disease-free survival (DFS) and overall survival (OS) rates near 80% and 90%, respectively, have been repeatedly obtained in large randomized phase III trials [1–3]. These results were recently corroborated by a vast meta-analysis on 44,000 individual patient data, confirming that the addition of a taxane in anthracycline regimens reduced breast cancer mortality by an average of ∼33% [4].
In the PACS01 phase III study, 1,999 patients were randomly assigned to receive either standard FEC100 (fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2) or sequential FEC100 and docetaxel (FEC-D). Significant 4%–5% absolute increases in 5-year DFS and OS rates were recorded, corresponding to relative reductions of 18% and 27% in the risks of relapse and death, respectively [1]. Sequential or concomitant anthracycline-taxane combinations are now widely used as standard adjuvant treatment for high-risk breast cancer. Overall, taxane administration results in an absolute 5-year risk reduction of 5% for DFS and 3% for OS rates [5].
Safety and long-term toxicity are other important concerns when dealing with anthracycline-taxane combinations. Despite lower doses of each drug administered, combination therapy must be monitored for severe acute toxicities and potentially detrimental long-term side effects. In the PACS01 5-year follow-up, sequential FEC-D emerged as potentially safer than 6 cycles of FEC100 with significantly fewer cardiac events and secondary leukemia compared with FEC100 alone.
Identifying particular subgroups of patients who respond differently to the anthracycline-taxane combination deserves further examination. It would allow the physician to fit chemotherapies to the biological characteristics of the patient's tumor. In the 5-year analysis of PACS01, exploratory subgroup analysis has pointed out that FEC-D benefited more postmenopausal women and women with limited axillary node involvement (pN 1–3) than premenopausal women and women with >3 pN [1]. Moreover, it was shown that expression of the biomolecular marker Ki67 could be relevant to identify estrogen receptor (ER)-positive tumors with higher sensitivity to docetaxel in the adjuvant setting [6, 7].
We present here the 8-year follow-up analysis of PACS01, investigating the experimental arm of 3 cycles of FEC100 plus 3 cycles of docetaxel versus the control arm of 6 cycles of FEC100 as adjuvant therapy for patients with node-positive breast cancer.
Patients and Methods
Study Population
Women between 18 and 64 years old with node-positive unilateral operable breast cancer who had undergone primary surgery with clear margins (modified mastectomy or tumorectomy) plus axillary dissection were eligible for the study independent of their menopausal status. Main eligibility criteria included World Health Organization performance status <2 and adequate renal, hepatic, and cardiac function. Women with a documented history of cardiac disease contraindicating anthracyclines were excluded. Chemotherapy was to be administered within 42 days after surgery. Written informed consent was obtained before randomization. Baseline assessments included bone scan, chest radiograph, abdominal ultrasound, and contralateral mammography. The protocol was reviewed and approved by the ethics committee/institutional review board and the study was conducted according to the Declaration of Helsinki.
Study Design and Treatment
Patients were randomly assigned to receive either 6 cycles of FEC100 (fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2) on day 1 every 21 days, or 3 cycles of FEC100 followed by 3 cycles of docetaxel (100 mg/m2) on day 1 every 21 days (FEC-D). Randomization was stratified by age (<50 and ≥50 years), positive nodes (1–3 and >3), and treatment center. Dose adaptation, delays, and the use of granulocyte-colony stimulating factor have been described previously [1].
After chemotherapy completion, tamoxifen (20 mg/day) was started and continued for 5 years. For hormone receptor (HR)-negative patients, the administration of tamoxifen was at the investigator's discretion for postmenopausal patients but prohibited for premenopausal patients.
Radiotherapy was initiated within 4 weeks after the last chemotherapy cycle and was mandatory for all patients who had undergone breast-conserving surgery. Other indications (chest wall, supraclavicular area, and internal mammary chain) were recommended but left to the investigator's discretion. Irradiation of the axilla was prohibited. Of note, the PACS01 trial design is given as supplemental online data.
Evaluations
Physical examinations were performed every 4 months for the first 2 years, every 6 months for the following 3 years, and thereafter annually for the next 5 years. Mammography, chest radiograph, liver ultrasound, and bone scan were performed 1 year after the initial surgery and yearly thereafter for 5 years. Beyond this period, a mammography was performed annually.
Biological Marker Status Evaluation (HER2 and Ki67)
HER2 oncogene expression was evaluated by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). HER2 positivity was defined as IHC scores >3 or IHC scores >2 and amplified FISH, whereas IHC scores of 0 or >1 or >2 scores and nonamplified FISH were ascribed to HER2 negativity. Ki67 expression was scored according to a procedure previously reported [7]. Scores ≥20% were ascribed to Ki67 positivity and <20% to Ki67 negativity.
Statistical Analysis
The PACS01 study's primary endpoint was 5-year DFS. DFS was defined as the time from randomization until first relapse (local, regional, or distant), contralateral breast cancer, or death from any cause, whichever occurred first. The secondary endpoints were OS and safety. In the present article, an 8-year update of the data on survival (DFS and OS) and safety was performed using the same intent-to-treat (ITT) population of patients, criteria, and methods as previously published [1].
The DFS and OS rates for the ITT population and patient subgroups were calculated by the Kaplan-Meier method. Subgroup analysis was unplanned. HER2 and Ki67 status was centrally assessed for 1,190 (60%) patients. Treatment arms were compared using a log-rank test stratified by the number of positive axillary lymph nodes positive nodes (1–3 vs. >3) and age (<50 years vs. ≥50 years). A supportive multivariate analysis (Cox regression model) was adjusted for age (<50 years vs. ≥50 years), positive nodes (1–3 vs. >3), histological tumor size (<20 mm vs. ≥20 mm), estrogen receptor (ER) and progesterone receptor (PR) status, HR status, Scarff-Bloom Richardson grade (I, II, III, not gradable), and tamoxifen use. Each interaction between these variables and DFS has been explored.
Results
Patient Baseline Characteristics
Between June 1997 and March 2000, a total of 85 centers in France and Belgium enrolled 1,999 women (996 on the FEC arm and 1,003 on the FEC-D arm). Baseline characteristics were well balanced between treatment arms, median age, conservative surgery, Scarff-Bloom Richardson grade, 1–3 involved nodes, and the other items, except for combined HR status and ER status (p = .02) [1].
Treatment Characteristics
Six treatment cycles were completed by 97% of patients in the FEC group and by 96.1% of the patients in the FEC-D group. There were 36 (3.6%) dose reductions in the FEC arm and 61 (6.1%) in the FEC-D arm, 41 (4.1%) of which occurred specifically for docetaxel. Almost all treated patients received radiotherapy. Tamoxifen use was well balanced between arms (65.5% in the FEC arm and 68.4% in the FEC-D arm; p = .16), including both premenopausal and postmenopausal women.
Efficacy at 8 Years
The cutoff date for the present analysis was April 2009, and the median follow-up time was 92.8 months from randomization. In the ITT population, 639 patients have experienced at least one event versus 482 in the previous analysis (Table 1): 301 in the FEC-D arm and 338 in the FEC arm, which represents an increase of respectively 38.1% in FEC-D arm and 28% events in FEC arm as compared to the results at 5 years [1]. The 8-year DFS and OS rates were respectively 70.2% and 83.2% for the FEC-D arm versus 78.4% and 90.7% in the previous 5-year analysis. Of the 383 deaths, 214 occurred in the FEC group and 169 in the FEC-D group (Table 1).
Table 1.
Analysis of events in the intent-to-treat population
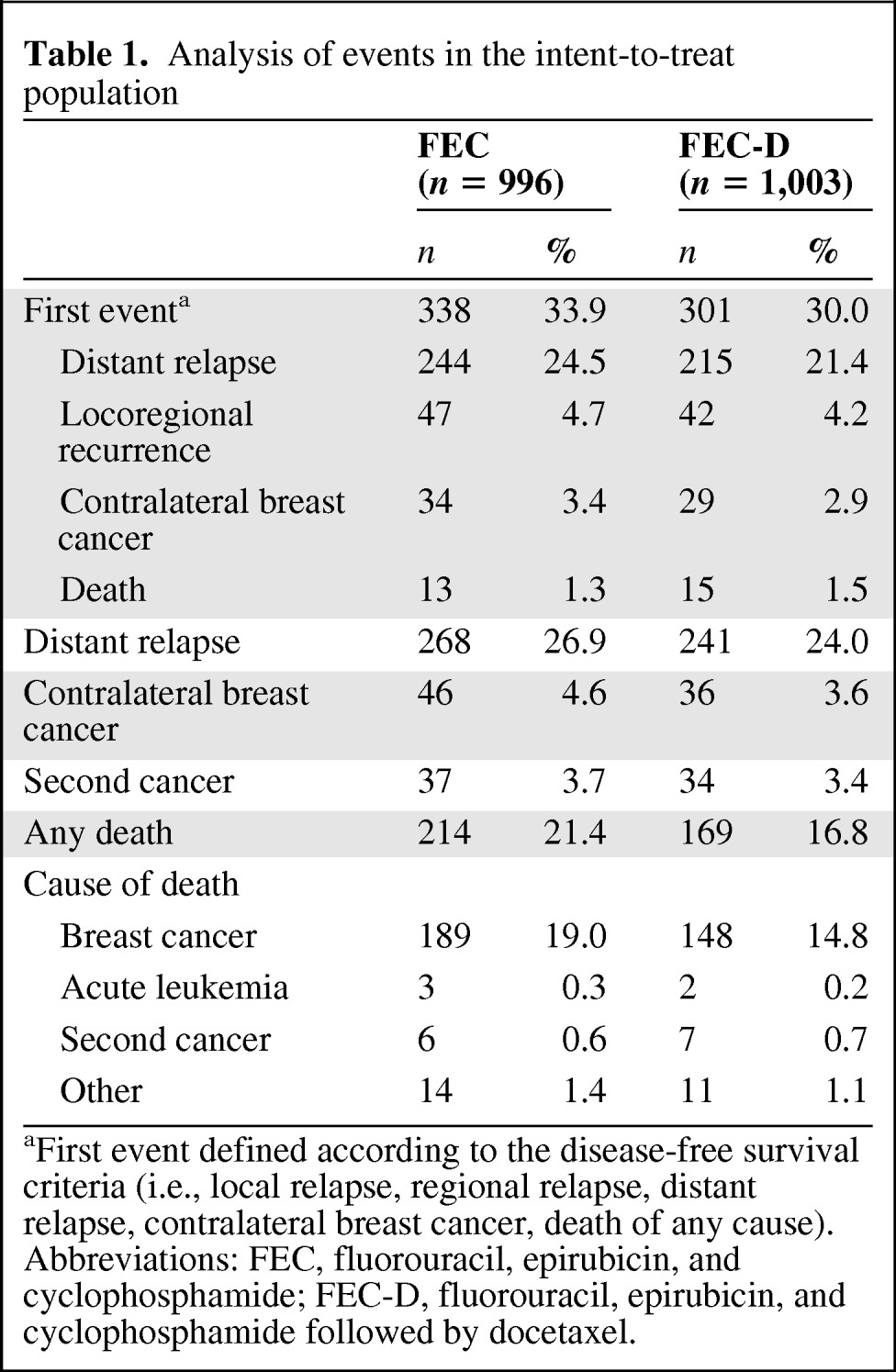
aFirst event defined according to the disease-free survival criteria (i.e., local relapse, regional relapse, distant relapse, contralateral breast cancer, death of any cause).
Abbreviations: FEC, fluorouracil, epirubicin, and cyclophosphamide; FEC-D, fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel.
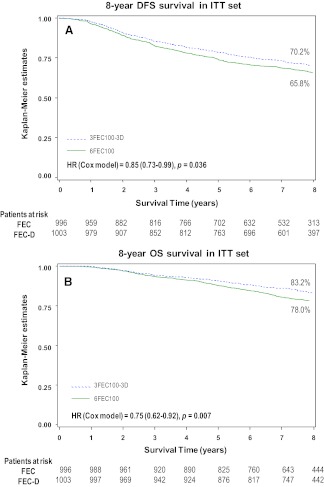
Survival analysis is reported in Figure 1A and 1B. Cox regression analysis, adjusted for age and number of positive nodes, showed a 15% reduction in the relative risk of relapse (hazard ratio = 0.85, 95% confidence interval [CI] = 0.73–0.99, adjusted log-rank p = .036; Fig. 1A) and a 25% reduction in the relative risk of death (hazard ratio = .75, 95% CI = 0.62–0.92, p = .007; Fig. 1B) with FEC-D. This difference was due mainly to the reduction of distant metastasis (Table 1).
Figure 1.
Kaplan-Meier 8-year estimates in the intent-to-treat population. (A): Disease-free survival rates were 65.8% with fluorouracil-epirubicin-cyclophosphamide (FEC) and 70.2% with fluorouracil-epirubicin-cyclophosphamide with docetaxel (FEC-D). (B): Overall survival rates were 78% with FEC and 83.2% with FEC-D.
Abbreviations: D, docetaxel; DFS, disease-free survival; FEC, fluorouracil, epirubicin, and cyclophosphamide; HR, hazard ratio; ITT, intent-to-treat; OS, overall survival.
The multivariate analysis adjusted for prognostic factors (age, nodal status, tumor size, grading, hormone receptors; Table 2) showed 13% and 21% reductions in the relative risk of relapse (DFS) and death (OS), respectively, with FEC-D (hazard ratio = 0.87, 95% CI = 0.75–1.02, p = .086 for DFS; hazard ratio = 0.79, 95% CI = 0.65–0.97, p = .024 for OS; Table 2). Introducing the variable (arm × time) in our Cox multivariate model has allowed us to verify that the hypothesis of hazards proportionality was achieved for both DFS (hazard ratio = 1.022; 95% CI = 0.96–1.09; p = .53) and OS (hazard ratio = 0.98; 95% CI = 0.89–1.07; p = .63).
Table 2.
Complete Cox regression model for disease-free survival and overall survival rates in the intent-to-treat population
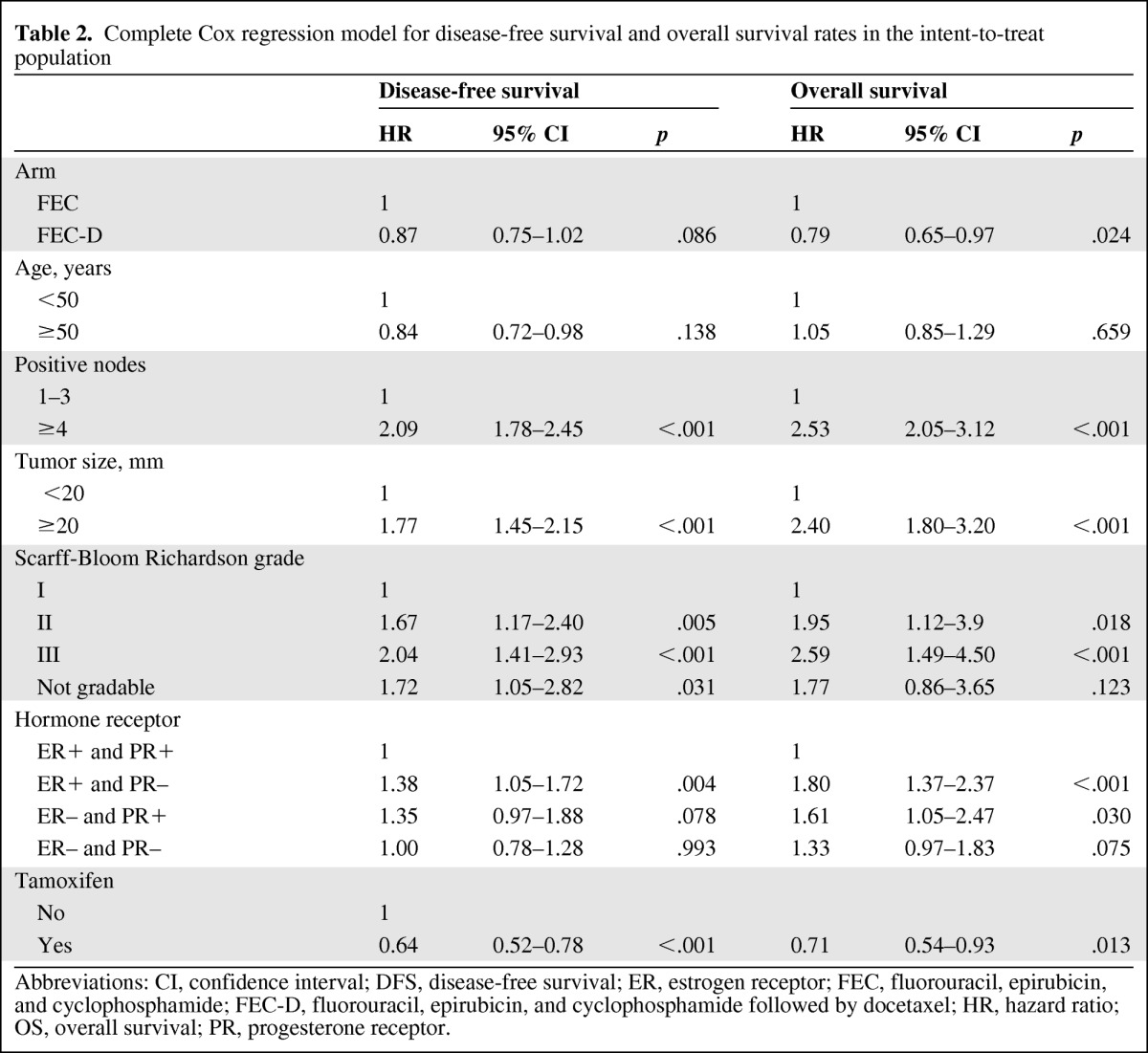
Abbreviations: CI, confidence interval; DFS, disease-free survival; ER, estrogen receptor; FEC, fluorouracil, epirubicin, and cyclophosphamide; FEC-D, fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel; HR, hazard ratio; OS, overall survival; PR, progesterone receptor.
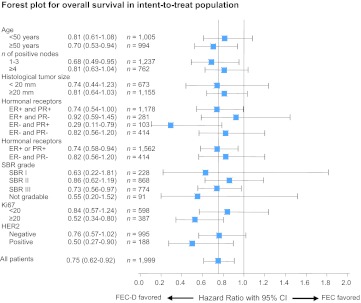
Figure 2 shows the treatment effect on OS in different subgroups (Forest plot). The risks of relapse and death were reduced in almost all the subgroups of patients. Women aged ≥50 years seem to have more benefit from treatment with FEC-D, as did patients with 1–3 positive nodes compared with patients with >3 positive nodes (Fig. 2), but interactions between age and treatment as well as node status and treatment were not significant. The benefit of FEC-D was indifferent to the tumor size or the type of positivity of hormonal receptors. The benefit from FEC-D also seems to be more statistically significant in high-grade tumors than in lower-grade tumors.
Figure 2.
Analysis of treatment effect by subgroups in the intent-to-treat population. Hazard ratios and 95% confidence intervals are reported in different subgroups (Forest plot analysis) for overall survival rates.
Abbreviations: CI, confidence interval; D, docetaxel; ER, estrogen receptor; FEC, fluorouracil, epirubicin, and cyclophosphamide; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
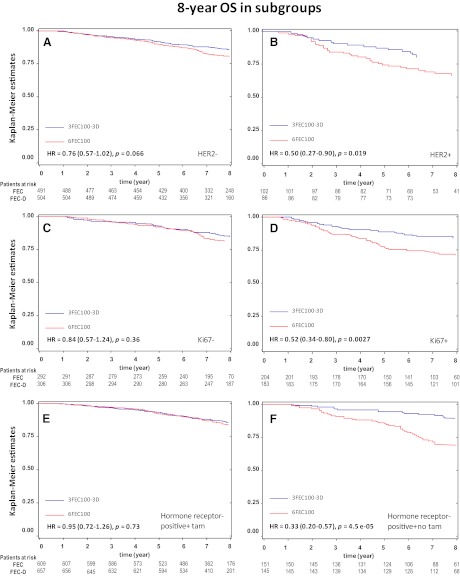
HER2-positive tumors (log-rank value p = .01) and high Ki67 tumors (log-rank value p = .005) derived more benefit from the FEC-D regimen than the HER2-negative and low Ki67 subgroups (Figs. 2 and 3A–D). Of note, a complete Cox regression model for DF5 and OS in the intent-to-treat population (including the covariate HER2 and Ki67 as categorical variables) is given as supplemental online Table 1.
Figure 3.
Kaplan-Meier survival curves for overall survival rates are shown for the patient subgroups of (A) HER2-negative, (B) HER2-positive, (C) Ki67-negative, (D) Ki67-positive, (E) hormone receptor (HR)-positive with tamoxifen, and (F) HR-positive without tamoxifen.
Abbreviations: D, docetaxel; FEC, fluorouracil, epirubicin, and cyclophosphamide; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival.
The use of tamoxifen, which was mandatory for patients with HR-positive tumors, was beneficial for DFS and OS (p < .001 and p = .013, respectively; Table 2). Interaction between the treatment arm and tamoxifen was tested for DFS. The benefit of FEC-D could be hampered when tamoxifen is used in the HR-positive (i.e., ER-positive or PR-positive) subgroup (n = 1,366; hazard ratio = 1.29, 95% CI = 0.94–1.77; p = .11; Fig. 3E). On the contrary, DFS analysis at 8 years shows a statistically significant difference in the risk of relapse in favor of the FEC-D arm for the HR-positive subgroup (n = 296) of patients who did not receive tamoxifen (hazard ratio = 0.69, 95% CI = 0.48–0.98, p = .036; Fig. 3F).
Long-Term Toxicity and Adverse Effects
The additional cardiac toxicities encountered beyond 5 years of follow-up were observed only in the 6-cycle FEC100 arm (Table 3) with additional cardiogenic shock leading to cardiac death (1 patient), cardiomyopathy (1 patient), clinical heart failure (2 patients), myocardial infarction (1 patient), and asymptomatic decline of left ventricular ejection fraction (2 patients). During the overall follow-up period (8 years), cardiac toxicity affected only 1% of the ITT population in the 6-cycle FEC100 arm. The rate of leukemia was nearly identical in the 6 FEC100 arm and in the FEC-D arm (0.3% vs. 0.2%, respectively). The rate of second cancer also was nearly identical in the 6 FEC100 arm and in the FEC-D arm (3.7% vs. 3.5%, respectively).
Table 3.
Analysis of cardiac toxicity
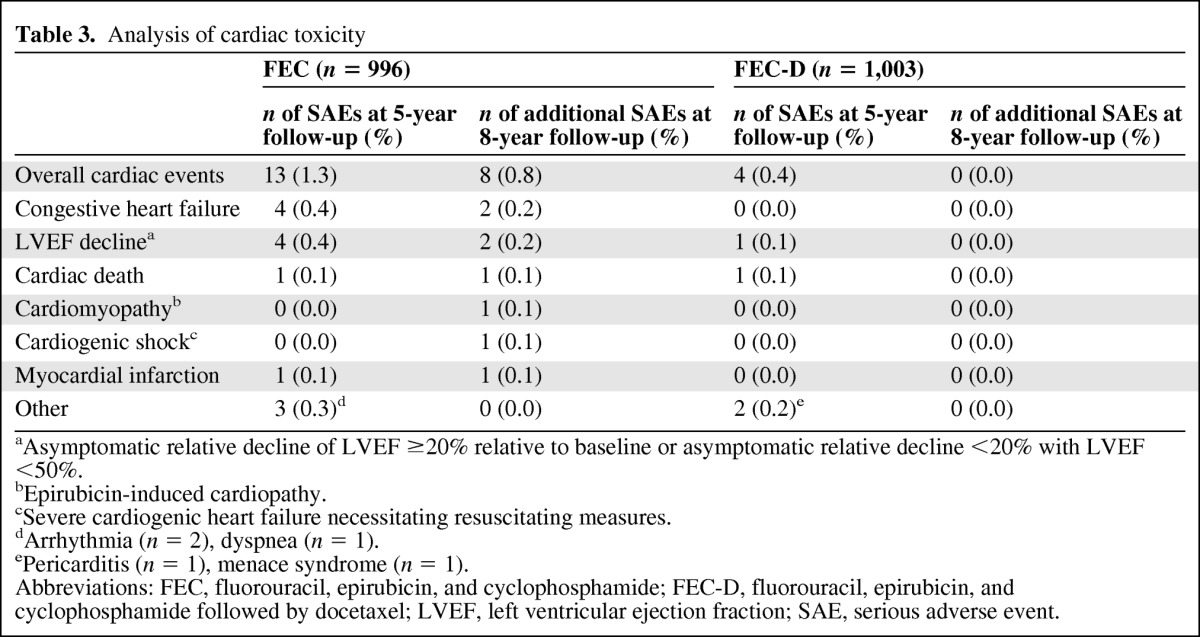
aAsymptomatic relative decline of LVEF ≥20% relative to baseline or asymptomatic relative decline <20% with LVEF <50%.
bEpirubicin-induced cardiopathy.
cSevere cardiogenic heart failure necessitating resuscitating measures.
dArrhythmia (n = 2), dyspnea (n = 1).
ePericarditis (n = 1), menace syndrome (n = 1).
Abbreviations: FEC, fluorouracil, epirubicin, and cyclophosphamide; FEC-D, fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel; LVEF, left ventricular ejection fraction; SAE, serious adverse event.
Discussion
With 8 years of median follow-up, the PACS01 trial confirms that substituting 3 cycles of docetaxel for 3 cycles of FEC100 following 3 cycles of FEC100 significantly improves DFS and OS for patients with node-positive early-stage breast cancer. Survival benefits obtained in the previously published 5-year results are maintained in the long-term 8-year analysis with FEC-D, leading to 15% and 25% reductions in the relative risks of relapse and death, respectively. The absence of interaction between treatment arm and time reasonably indicates that the risk reductions for relapse and death versus the comparator anthracycline arm are maintained over 8 years when docetaxel is sequentially incorporated in the adjuvant treatment of node-positive early breast cancer.
Similar to the 5-year results, this benefit is more significant for patients older than 50 years than it is for younger patients; it is also more significant for patients with 1–3 positive nodes than for patients with 4 or more positive nodes. Other specific subgroups of patients—HER2-positive and high Ki67—show DFS and OS differences in favor of the FEC-D regimen.
Note that these improved rates occur with the FEC-D regimen, which also demonstrated less cardiac toxicity than FEC100 at the 8-year follow-up. The cardiac toxicity associated with the use of anthracyclines has been described in many trials, reviews, and the Early Breast Cancer Trialists Collaborative Group meta-analysis, which demonstrated that the cardiac mortality risk ratio for any anthracycline-based regimen is in favor of the other treatment [4]. Therefore, the benefit-to-risk ratio of the use of an anthracycline regimen in the adjuvant treatment of breast cancer must be individually and carefully weighed. Otherwise, the long-term safety profile of the FEC-D and FEC regimens are similar, with ∼3.5% risk of second malignancies (FEC-D), and there is no indication of significant differences in long-term toxicities.
Individual data collected from 44,000 women in 33 studies showed that adding taxanes, in combination, sequentially or additionally to an anthracycline-based regimen significantly reduced breast cancer mortality versus an anthracycline-based regimen alone [4]. However, additional phase III studies and more long-term (∼10 years) follow-up results are needed to ascertain the meta-analysis results and validate whether the two drugs are necessary. Their most effective administration (sequential vs. concurrent) and the optimal number of cycles are still pending investigation.
Long-term follow-up of docetaxel-based adjuvant chemotherapy (docetaxel, doxorubicin, and cyclophosphamide [TAC]) on DFS rates for patients with high-risk node-negative breast cancer showed added benefit regardless of HR status, menopausal status, or number of high-risk factors compared with standard fluorouracil-doxorubicin-cyclophosphamide [8]. On the contrary, results at 5 years for the PACS04 trial, which evaluated the concurrent administration of docetaxel and epirubicin versus FEC100 for patients with node-positive early-stage breast cancer, did not evidence any advantage for DFS and OS rates compared with standard FEC100 [9]. Moreover, doxorubicin-docetaxel was not superior to doxorubicin-cyclophosphamide in terms of DFS and OS and doxorubicin-cyclophosphamide/docetaxel was significantly inferior to (cyclophosphamide, epirubicin, and fluorouracil [CEF]) in terms of recurrence-free survival [10, 11]. Similarly, 4 cycles of FEC60 (epirubicin 60 mg/m2, fluorouracil 500 mg/m2, cyclophosphamide 500 mg/m2) followed by 4 cycles of docetaxel was not superior to taxane-free regimens [12].
In the randomized U.S. Oncology Trial 9735, the head-to-head benefit of taxanes with respect to anthracyclines was evaluated by substituting docetaxel for doxorubicin in the standard doxorubicin-cyclophosphamide regimen over 4 cycles of treatment. DFS rates at 7 years improved significantly in the docetaxel-cyclophosphamide arm (81% vs. 75%, p = .033), as did OS rates (87% vs. 82%; p = .032) [13, 14].
Sequential administration of docetaxel seems to be superior to concomitant administration [5, 15–17]. Recently, the 8-year results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-30 trial demonstrated that 4 cycles of doxorubicin-cyclophosphamide followed by 4 cycles of docetaxel improves survival rates compared to either the same regimen without docetaxel or 4 cycles of concurrent doxorubicin-cyclophosphamide/docetaxel [15]. In the Breast Cancer International Research Group (BCIRG) 005 trial, the sequential regimen of doxorubicin with cyclophosphamide followed by docetaxel was compared to the combination regimen of TAC. The sequential and combination regimens incorporating three drugs were equally effective in terms of progression-free survival and OS rates, but their toxicity profiles differed [18].
Long-term follow-up of other trials evaluating adjuvant taxanes in early breast cancer have been published recently with outcomes significantly in favor of regimens containing docetaxel [14, 15]. Several recent meta-analyses suggest taxanes are beneficial in the adjuvant setting, irrespective of their type, schedule of administration, patient age and menopausal status, lymph node involvement, and HR expression [4, 5, 8, 19–22].
However, it is controversial whether taxanes produce consistent benefit across specific subgroups of patients. Identifying the patients who would better benefit from taxanes is relevant to optimize the therapeutic choice, but such identification has not yet been successful. HER2 and HR positivity have been scrutinized at several occasions, showing no particular benefit in favor of the taxane-containing regimen [1, 3–6, 13, 14].
The question of whether the ER and PR status of a tumor's hormone receptors can define subpopulations that may differentially derive benefit from taxanes arises continuously with conflicting results [23–27]. In a pooled analysis of BCIRG001 and PACS01, including 3,323 patients with node-positive breast cancer and assessed ER status, the benefit of docetaxel on the risk of recurrence or death was the same in ER-positive and ER-negative patients [6]. However, this analysis did not take into account HER2 and other proliferative markers. Oncotype DX (Genomic Health, Inc., Redwood City, CA) results demonstrated also that biological heterogeneity might influence the degree of responsiveness in ER-positive patients with high recurrence score tumors deriving the most benefit from polychemotherapy [28, 29].
Overall, these results suggest that adding taxanes to anthracyclines could partly overcome the relative chemorefractoriness of the more proliferative HR-positive tumors. One additional difficulty in deciphering the role of taxanes in ER-positive patients is that two endocrine factors can interfere: the ovarian function suppressing effects of chemotherapy in premenopausal women and/or the use of tamoxifen [15]. In PACS01, we observed that treating HR-positive patients with tamoxifen could hamper the beneficial role of FEC-D. However, tamoxifen was extremely beneficial to HR-positive patients for whom the two therapies add to each other [30]. These data support the hypothesis presented by Berry et al. that “in the ER positive tumors the large benefit provided by tamoxifen overwhelms the potential benefit of chemotherapy, or that the prognosis associated to these subtypes of tumors is so good it is difficult to detect a difference between the two chemotherapy arms” [24].
Both Ki67, a cell-cycle protein associated with short-term prognosis and higher sensitivity to chemotherapy, and HER2 amplification are detected in 12%–18% of all breast cancers; they could become relevant tumor biomarkers to identify specific subgroups of patients sensitive to the addition of docetaxel [31–33]. In the PACS01 8-year follow-up, Ki67 >20% seems to be a significant predictor of response to docetaxel when the entire ITT set is considered, irrespective of the ER status, in agreement with the 5-year results. Unfortunately, the HER2 status influence could not be completely tested because of the small number of HER2-positive tumors [7].
In the BCIRG 001 trial, which compared 6 cycles of TAC to 6 cycles of fluorouracil-doxorubicin-cyclophosphamide, TAC significantly complemented endocrine therapy in patients with luminal B tumors (ER-positive and/or PR-positive and either HER2-positive and/or high Ki67) [34]. However, a retrospective analysis of BCIRG 001, which tested multiple biologic parameters, showed that Ki67 was associated with both DFS and OS improvement but no significant interaction was evidenced between Ki67 and treatment allocation [33].
A retrospective analysis of Cancer and Leukemia Group B 9344, which included 1,322 randomly selected node-positive women, demonstrated a significant interaction between HER2 positivity and treatment arm favoring the paclitaxel-containing (175 mg/m2) regimen (hazard ratio = 0.59, p = .01) [35]. Conversely, no interaction was observed between HER2 positivity and doxorubin doses ≥60 mg/m2. Complicating matters, HER2 status is a positive predictor for the benefit of both taxane and anthracycline therapy [35, 36]. However, HER2-positive tumors would not currently be eligible for treatment with any of the nontrastuzumab-based regimens investigated in PACS01, and conclusions that might be drawn from this study are thus irrelevant for this important subgroup.
In summary, these prognostic factors (HR, HER2, Ki67) should not yet be used in clinical practice to identify patients who may or may not benefit from a taxane-based regimen [5, 19]. Differences in the type of regimen (concurrent vs. sequential), schedule, and dosage are such that the true role of taxanes in the adjuvant management of early-stage breast cancer remains a relevant subject of study, especially in biologically defined subgroups of tumors [19].
It is confirmed that the sequential taxane-containing regimen FEC-D is significantly more active than the standard 6 cycles of FEC100 in the treatment of node-positive early breast cancer. Long-term impact on safety and cardiac toxicity are limited. Among the anthracycline-taxane regimens, FEC-D compares favorably with a good efficacy/toxicity profile [1, 16, 17].
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank Valère Lounnas who provided medical writing services on behalf of the UNICANCER Breast Group and Meredith Charpantier for editing grammar and syntax.
The study was supported by the Ligue Nationale Contre le Cancer and Sanofi-Aventis.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Bruno Coudert, Bernard Asselain, Marc Spielmann, Frédérique Pénault-Llorca, Gilles Romieu, Jean-Luc Canon, Thierry Petit, Guy Jerusalem, Henri Roché
Provision of study material or patients: Bruno Coudert, Mario Campone, Marc Spielmann, Jean-Pascal Machiels, Daniel Serin, Christelle Lévy, Gilles Romieu, Jean-Luc Canon, Hubert Orfeuvre, Gilles Piot, Thierry Petit, Guy Jerusalem, Bruno Audhuy, Corinne Veyret, Marc Beauduin, Jean-Christophe Eymard, Henri Roché
Collection and/or assembly of data: Mario Campone, Marc Spielmann, Jean-Pascal Machiels, Frédérique Pénault-Llorca, Daniel Serin, Christelle Lévy, Gilles Romieu, Jean-Luc Canon, Hubert Orfeuvre, Gilles Piot, Thierry Petit, Guy Jerusalem, Bruno Audhuy, Corinne Veyret, Marc Beauduin, Jean-Christophe Eymard, Anne-Laure Martin, Henri Roché
Data analysis and interpretation: Bruno Coudert, Bernard Asselain, Mario Campone, Jean-Pascal Machiels, Frédérique Pénault-Llorca, Daniel Serin, Christelle Lévy, Gilles Romieu, Jean-Luc Canon, Hubert Orfeuvre, Gilles Piot, Thierry Petit, Guy Jerusalem, Bruno Audhuy, Corinne Veyret, Marc Beauduin, Jean-Christophe Eymard, Anne-Laure Martin, Henri Roché
Manuscript writing: Bruno Coudert, Bernard Asselain, Mario Campone, Marc Spielmann, Jean-Pascal Machiels, Frédérique Pénault,-Llorca, Daniel Serin, Christelle Lévy, Gilles Romieu, Jean-Luc Canon, Hubert Orfeuvre, Gilles Piot, Thierry Petit, Guy Jerusalem, Bruno Audhuy, Corinne Veyret, Marc Beauduin, Jean-Christophe Eymard, Anne-Laure Martin, Henri Roché
Final approval of manuscript: Bruno Coudert, Bernard Asselain, Mario Campone, Marc Spielmann, Jean-Pascal Machiels, Frédérique Pénault-Llorca, Daniel Serin, Christelle Lévy, Gilles Romieu, Jean-Luc Canon, Hubert Orfeuvre, Gilles Piot, Thierry Petit, Guy Jerusalem, Bruno Audhuy, Corinne Veyret, Marc Beauduin, Jean-Christophe Eymard, Anne-Laure Martin, Henri Roché
References
- 1.Roché H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24:5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 2.Gianni L, Baselga J, Eiermann W, et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol. 2009;27:2474–2481. doi: 10.1200/JCO.2008.19.2567. [DOI] [PubMed] [Google Scholar]
- 3.Martin M, Rodriguez-Lescure A, Ruiz A, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100:805–814. doi: 10.1093/jnci/djn151. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists Collaborative Group (EBCTCG) Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Laurentiis M, Cancello G, D'Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: A meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 6.Andre F, Broglio K, Roché H, et al. Estrogen receptor expression and efficacy of docetaxel-containing adjuvant chemotherapy in patients with node-positive breast cancer: Results from a pooled analysis. J Clin Oncol. 2008;26:2636–2643. doi: 10.1200/JCO.2007.14.9146. [DOI] [PubMed] [Google Scholar]
- 7.Penault-Llorca F, Andre F, Sagan C, et al. Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2809–2815. doi: 10.1200/JCO.2008.18.2808. [DOI] [PubMed] [Google Scholar]
- 8.Martín M, Seguí MA, Antón A, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363:2200–2210. doi: 10.1056/NEJMoa0910320. [DOI] [PubMed] [Google Scholar]
- 9.Roché H, Allouache D, Romieu G, et al. Five-year analysis of the FNCLCC-PACS04 trial: FEC100 vs ED75 for the adjuvant treatment of node positive breast cancer. Paper presented at: 32nd Annual San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, TX. [Google Scholar]
- 10.Goldstein LJ, O'Neill A, Sparano JA, et al. Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E 2197. J Clin Oncol. 2008;26:4092–4099. doi: 10.1200/JCO.2008.16.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margot Burnell M, Levine MN, Chapman JW, et al. Cyclophosphamide, epirubicin, and fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by paclitaxel versus doxorubicin and cyclophosphamide followed by paclitaxel in node-positive or high-risk node-negative breast cancer. J Clin Oncol. 2010;28:77–82. doi: 10.1200/JCO.2009.22.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis P, Barrett-Lee P, Johnson L, et al. Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): An open-label, phase III, randomised controlled trial. Lancet. 2009;373:1681–1692. doi: 10.1016/S0140-6736(09)60740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 14.Jones SE, Holmes FA, O'Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 15.Swain SM, Jeong JH, Geyer CE, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis P, Crown J, Di Leo A, et al. Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: Breast International Group 02–98 randomized trial. J Natl Cancer Inst. 2008;100:121–133. doi: 10.1093/jnci/djm287. [DOI] [PubMed] [Google Scholar]
- 17.Loesch D, Greco FA, Senzer NN, et al. Phase III multicenter trial of doxorubicin plus cyclophosphamide followed by paclitaxel compared with doxorubicin plus paclitaxel followed by weekly paclitaxel as adjuvant therapy for women with high-risk breast cancer. J Clin Oncol. 2010;20:2958–2965. doi: 10.1200/JCO.2009.24.1000. [DOI] [PubMed] [Google Scholar]
- 18.Eiermann W, Pienkowski T, Crown J, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol. 2011;29:3877–84. doi: 10.1200/JCO.2010.28.5437. [DOI] [PubMed] [Google Scholar]
- 19.Bedard PL, Di Leo A, Piccat-Gebhart MJ. Taxanes. Optimizing adjuvant chemotherapy for early-stage breast cancer. Nat Rev Clin Oncol. 2010;7:22–36. doi: 10.1038/nrclinonc.2009.186. [DOI] [PubMed] [Google Scholar]
- 20.Bria E, Nistico C, Cuppone F, et al. Benefit of taxanes as adjuvant chemotherapy for early breast cancer: Pooled analysis of 15,500 patients. Cancer Treat Rev. 2007;33:474–483. doi: 10.1002/cncr.21886. [DOI] [PubMed] [Google Scholar]
- 21.Laporte S, Jones S, Chapelle C, et al. Consistency of effect of docetaxel-containing adjuvant chemotherapy in patients with early stage breast cancer independent of nodal status: Meta-analysis of 12 randomized clinical trials. Cancer Res. 2009;69(24 Suppl) doi: 10.1007/s10549-011-1933-0. Abstract 605. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson T, Wilcken N, Vagg R, et al. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Sys Rev. 2007;4:CD004421. doi: 10.1002/14651858.CD004421.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Conforti R, Boulet T, Tomasic G, et al. Breast cancer molecular subclassification and estrogen receptor expression to predict efficacy of adjuvant anthracyclines-based chemotherapy: A biomarker study from two randomized trials. Ann Oncol. 2007;18:1477–1483. doi: 10.1093/annonc/mdm209. [DOI] [PubMed] [Google Scholar]
- 24.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buzdar AU, Singletary SE, Valero V, et al. Evaluation of paclitaxel in adjuvant chemotherapy for patients with operable breast cancer: Preliminary data of a prospective randomized trial. Clin Cancer Res. 2002;8:1073–1079. [PubMed] [Google Scholar]
- 26.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 2003; 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 27.Fountzilas G, Skarlos D, Dafni U, et al. Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high-risk operable breast cancer: A randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 2005; 2005;16:1762–1771. doi: 10.1093/annonc/mdi366. [DOI] [PubMed] [Google Scholar]
- 28.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 29.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Early Breast Cancer Trialists Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 31.de Azambuja E, Cardoso F, de Castro G, Jr., et al. Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504–1513. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent-Salomon A, Rousseau A, Jouve M, et al. Proliferation markers predictive of the pathological response and disease outcome of patients with breast carcinomas treated by anthracycline-based preoperative chemotherapy. Eur J Cancer. 2004;40:1502–1508. doi: 10.1016/j.ejca.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Dumontet C, Krajewska M, Treilleux I, et al. BCIRG 001 molecular analysis: Prognostic factors in node-positive breast cancer patients receiving adjuvant chemotherapy. Clin Cancer Res. 2010;16:3988–3997. doi: 10.1158/1078-0432.CCR-10-0079. [DOI] [PubMed] [Google Scholar]
- 34.Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: Use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168–1176. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 36.Muss HB, Thor AD, Berry DA, et al. c-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med. 1994;330:1260–1266. doi: 10.1056/NEJM199405053301802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.