The results of a questionnaire that examined the referral practices of health care professionals who treat women with breast cancer in the United Kingdom and investigated their understanding and knowledge of available fertility preservation options are presented.
Keywords: Breast cancer, Fertility preservation, Adjuvant chemotherapy, U.K.
Abstract
Objective.
Fertility preservation is an important survivorship issue for women treated for breast cancer. The aim of this work was to examine the referral practices of health care professionals who treat women with breast cancer in the United Kingdom, and to investigate their understanding and knowledge of the fertility preservation options available.
Method.
An invitation to participate in a confidential, online questionnaire was e-mailed to surgeons, oncologists, and clinical nurse specialists who manage patients with breast cancer in the United Kingdom.
Results.
n = 306 respondents. Factors which influenced whether fertility preservation options were discussed with a patient included the following: patient's age (78%), final tumor/nodes/metastasis status (37.9%); concern that fertility preservation would delay chemotherapy (37.3%); whether the patient had children (33.5%) or a partner (24.7%); estrogen receptor expression (22.6%), lack of knowledge regarding the available options (20.9%); and concern that fertility preservation would compromise the success of cancer treatment (19.8%). Twenty-seven percent did not know whether fertility preservation was available for their patients on the National Health Service. Nearly half (49.4%) of respondents said that gonadotropin-releasing hormone agonists were used for fertility preservation outside the setting of a clinical trial. Knowledge regarding the available options varied according to different members of the multidisciplinary team, with consultant oncologists better informed than consultant surgeons or clinical nurse specialists (p < .05).
Conclusions.
Many health care professionals have incomplete knowledge regarding the local arrangements for fertility preservation for patients with breast cancer. This may result in patients receiving inadequate or conflicting information regarding fertility preservation.
Introduction
Breast cancer is the most common cancer diagnosed in women of reproductive age, with 4,901 new diagnoses in women under 45 years in the United Kingdom (U.K.) in 2007 [1]. Younger women have specific concerns that need to be addressed, especially regarding the effects of treatment on fertility and premature menopause, and they are at higher risk for emotional distress [2–4]. With the age of first delivery increasing, patients may not have completed their families at the time of their breast cancer diagnosis, making fertility after cancer treatment an important survivorship issue. Pregnancy is contraindicated while taking adjuvant tamoxifen, which is recommended in women with estrogen receptor positive (ER+) breast cancer for 5 years to reduce the risk of recurrence [5, 6]. However, during this time there would be a natural age-related decline in fertility. Younger women are also more likely to be offered adjuvant chemotherapy, which reduces the risk of breast cancer recurrence in high-risk patients, but will also have a negative impact on ovarian reserve and may cause infertility or premature menopause [7–10]. This has a significant impact on patients' quality of life, with breast cancer survivors rating infertility, premature menopause, and sexual dysfunction highest of the problems experienced since diagnosis [11]. Loss of fertility has been shown to be of greater concern to women who have not completed their families or have had prior difficulty conceiving [12–14]. In a survey of 657 breast cancer survivors who were under 40 years at the time of diagnosis, 29% of women reported that concerns regarding fertility impacted their treatment decision, with only 51% reporting that their fertility concerns had been adequately addressed [12].
Guidance on how to manage the effect of cancer treatment on fertility has been published by the Royal College of Obstetricians and Gynaecologists [15] and a Joint Working Party of the Royal Colleges of Physicians, Radiologists, and Obstetricians and Gynaecologists [16]. They recommended that all patients with reproductive potential requiring anticancer treatment should be fully informed of the risk of early menopause and infertility, that referral for embryo cryopreservation should be considered, and that designated pathways should exist for prompt referral to a fertility specialist.
There is a lack of data regarding the rate of referral for fertility preservation in breast cancer patients in the U.K. and regarding health care professionals' knowledge of, and attitudes toward, fertility preservation in these patients. The aim of this research was to address these issues.
Methods
Participants
There is no central database of health care professionals who manage breast cancer in the U.K. Therefore, an e-mail inviting respondents to participate in an anonymous online survey was distributed via the National Cancer Research Institute breast group, the Cancer Research UK breast group, the British Association of Surgical Oncologists breast group, and the Breast Cancer Care Nursing Network. All cancer centers and cancer units who treat women with breast cancer in the U.K. were contacted via the Patient Advice and Liaison office at each hospital trust, requesting that the survey invitation be forwarded to the local breast multidisciplinary team. All postgraduate deaneries in the U.K. were contacted via their Websites, requesting that the survey invitation be forwarded to all surgical and oncology trainees in their region. Only National Health Service (NHS) Hospital Trusts were contacted; primary care and private health care providers were excluded. Although it was acknowledged that this methodology would yield an incomplete picture of U.K. clinical practice, the aim was to get a broad overview and identify issues that would become the focus of future work and research.
Study Questionnaire
The development of the questionnaire was informed by a panel of five oncologists, surgeons, and fertility specialists with an interest in fertility preservation in breast cancer. The questionnaire was piloted with 10 oncologists, surgeons, and clinical nurse specialists and modified where appropriate.
Data Analysis
Responses to questions were analyzed using two-tailed Fisher's exact test or χ2 test.
Results
Three hundred and six questionnaires were completed. The response rate among clinical nurse specialists was 22.5%, among Consultant (attending) surgeons 7%, and surgical SAS/SpRs (residents) 7%. The overall response rate is unknown because the total number of oncologists and research nurses is unknown. External agencies were forwarding the invitation to complete the questionnaire on our behalf, and there would have been a degree of overlap on the e-mail distribution lists.
Respondent Characteristics
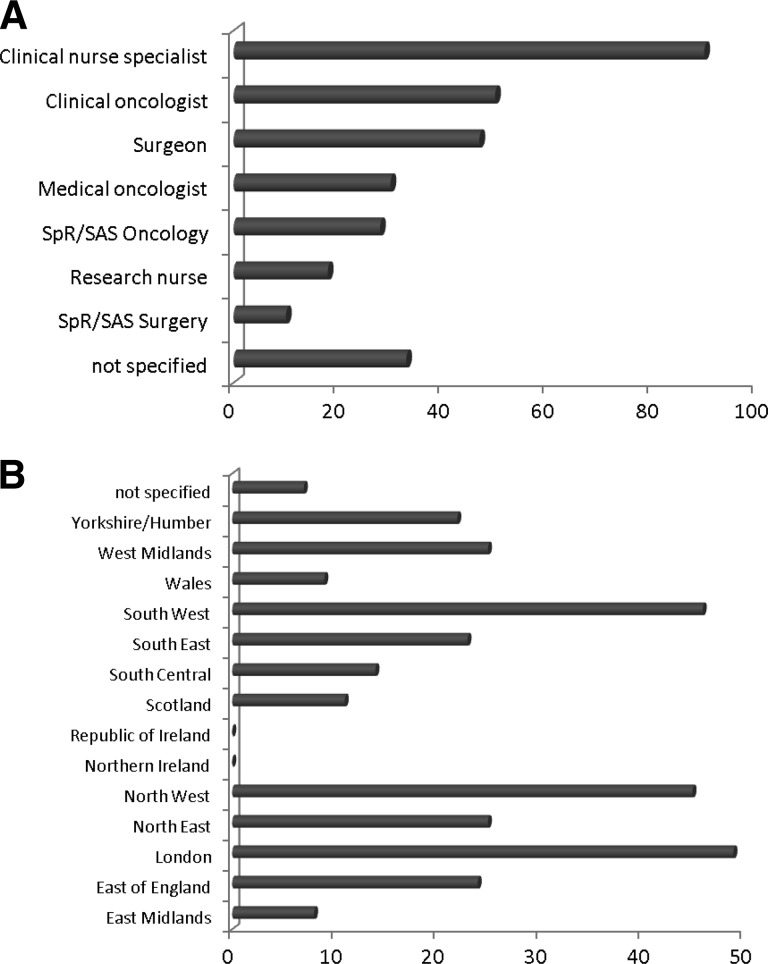
The respondents were comprised of 90 (29%) clinical nurse specialists (key workers involved in the patient's care from the point of diagnosis onward), 50 (16.3%) Consultant (attending) clinical (radiation) oncologists, 47 (15.4%) Consultant surgeons, 30 (9.8%) Consultant medical (non-radiation) oncologists, 28 (9.2%) oncology registrar/staff grade doctors (residents), 18 (5.9%) research nurses (key workers for patients recruited to clinical trials), 10 (3.3%) surgery registrar/staff grade doctors, and 33 (10.8%) not specified (Fig. 1A). Responses were received from all regions of the U.K. except Ireland (Fig. 1B); 39.1% of respondents worked primarily in a cancer center, 41.4% worked in a cancer unit, 17.4% worked in both, and 3.6% did not specify. There were no statistical differences in responses from those working in cancer centers versus cancer units. Nor did responses differ significantly according to geography, although this may reflect low response rates from some regions.
Figure 1.
Respondent characteristics. A: Occupation. Figures indicate number of respondents. Clinical nurse specialists are key workers who are involved with breast cancer patients from the time of diagnosis onward. Research nurses are key workers for patients enrolled in clinical trails. Consultant = attending equivalent. SpR/SAS grades are approximately equivalent to that of resident. Clinical oncologist = radiation oncologist. B: The region where respondents worked (figures indicate number of respondents).
Abbreviations: SAS, staff and associate specialist grade; SpR, registrar.
Referral for Consideration of Fertility Preservation
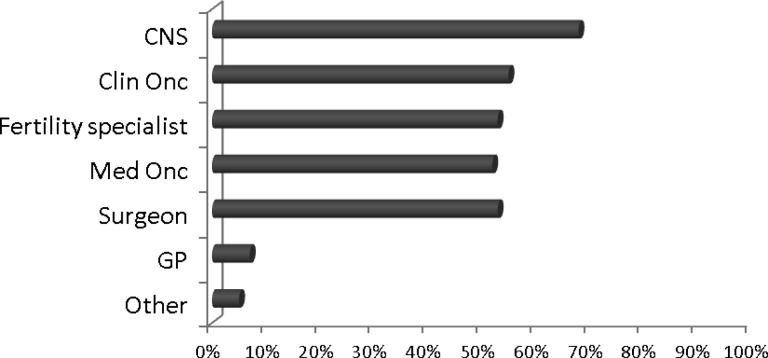
Forty-eight percent of respondents reported “always” discussing the risk of treatment-related infertility with breast cancer patients, with 34% reporting “most of the time.” Several members of the multidisciplinary team discuss fertility preservation options with breast cancer patients. Clinical nurse specialists were most likely to be involved in the discussion (68%), followed by clinical oncologists (55%), fertility specialists (53%), medical oncologists (52%), surgeons (40%), general practitioners (7%), and others (5%) (Fig. 2).
Figure 2.
“Who discusses fertility preservation with breast cancer patients? Tick all that apply. ” Figures indicate the percentage of respondents who reported that, for example, the CNS discussed fertility preservation with patients.
Abbreviations: Clin Onc, clinical oncologist; CNS, clinical nurse specialist; GP, general practitioner; Med Onc, medical oncologist.
Respondents were asked which factors would influence whether or not they discussed fertility preservation options with their patients (Table 1). Overall, the majority (77%) stated that patient's age would influence this, with only 9.8% reporting that time constraints in the clinic were a factor. A large minority agreed or strongly agreed that the following factors would influence whether they discussed fertility preservation: final tumor/nodes/metastasis status (37.9%); concern that fertility preservation would delay chemotherapy (37.3%); whether the patient had children (33.5%); whether the patient had a partner (24.7%); estrogen receptor expression (22.6%); a lack of knowledge regarding available options (20.9%); and concern that fertility preservation would compromise the success of cancer treatment (19.8%). There was no statistical difference between those who would refer patients with ER+ (62.4%) versus ER− (67.4%) cancer, although more respondents strongly agreed that they would refer ER− cancer than ER+ cancer (17.1% vs. 9.5%) (Table 2).
Table 1.
Factors influencing whether fertility preservation is discussed: “The following factors influence whether I discuss fertility options with breast cancer patients of reproductive age (tick one box only)”
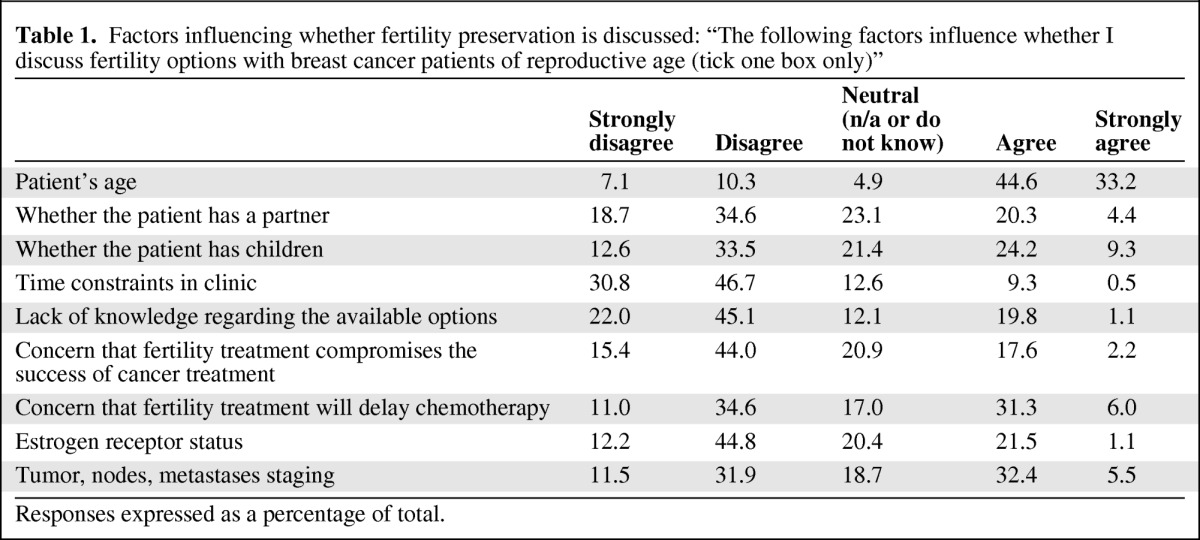
Responses expressed as a percentage of total.
Table 2.
“Regarding embryo cryopreservation in women with breast cancer (tick one box only)”
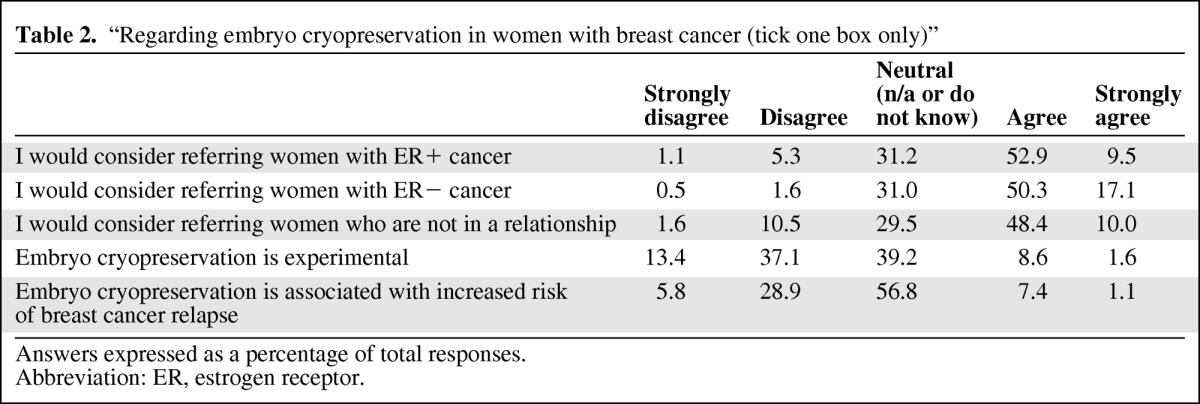
Answers expressed as a percentage of total responses.
Abbreviation: ER, estrogen receptor.
Sixty-two percent of respondents were aware of an established referral pathway to a local fertility unit for breast cancer patients requiring fertility preservation. The median number of women referred to the local fertility unit in the last 12 months was 3 per respondent (range 0–25). Where the average time to be seen from referral was known (n = 106 responses), 48% patients were seen within a week of referral, 44% within 2 weeks, and 8% in over 2 weeks.
Fertility Preservation Options Available
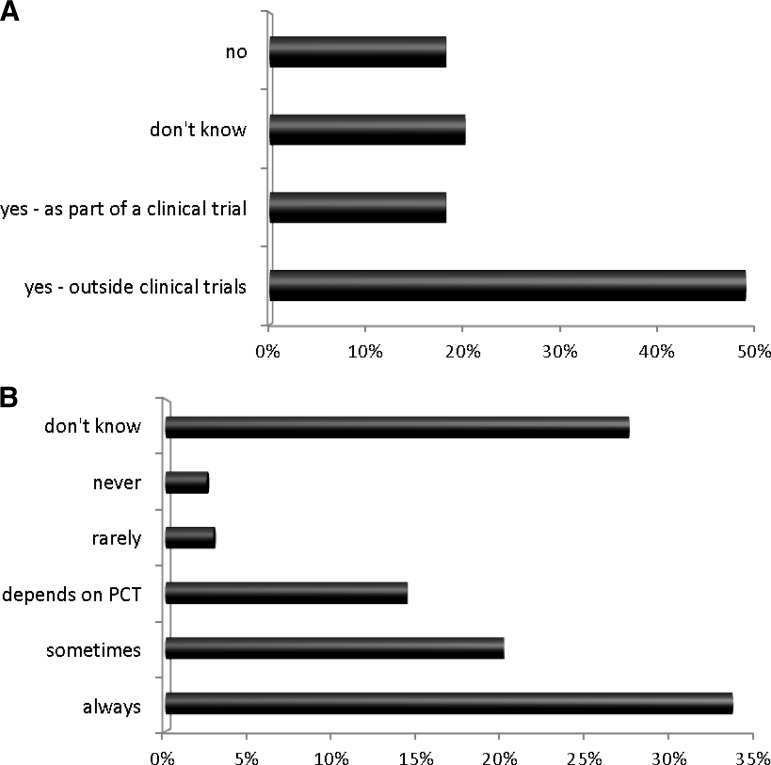
The use of gonadotropin-releasing hormone (GnRH) agonists was variable. Nearly half (49.4%) reported the use of GnRH agonists for fertility preservation in standard practice, 18.3% reported their use as part of clinical trials, 18.3% said that they were not used, and 20.3% did not know (Fig 3A). There were statistically significant differences between responses from different health care professionals: Consultant surgeons (31.1%) and clinical nurse specialists (18%) were statistically more likely to say they did not know if GnRH agonists were used compared with Consultant medical/clinical oncologists (3%) (two-tailed χ2, p < .001).
Figure 3.
Fertility options available locally. A: “Are GnRH agonists offered for fertility preservation?” B: “Does your local fertility preservation unit provide services for cancer patients on the NHS?”
Abbreviations: GnRH, gonadotropin-releasing hormone; NHS, National Health Service; PCT, Primary Care Trust.
There was also variation in whether local fertility preservation units provided services for cancer patients on the NHS: overall, 34% of respondents said fertility preservation was always available on the NHS, with 20% reporting “sometimes,” an additional 15% reporting that it depended on the primary care trust (local funding arrangements), and 28% saying that they did not know (Fig 3B). There was a statistical difference between the proportion of Consultant surgeons (16.3%) who did not know compared with surgical registrars (83.3%) (p = .02); the difference between Consultant medical/clinical oncologists (15.5%) who did not know and oncology registrars (30%) was not significant (p = .12).
Knowledge regarding the options available at local fertility units was also variable. Overall, only 53% of respondents reported that embryo cryopreservation was available; 40%, 43%, and 66% of total respondents reported that they did not know whether embryo cryopreservation, oocyte cryopreservation, and ovarian tissue cryopreservation were available, respectively (Table 3). Junior doctors were statistically less likely to know whether embryo cryopreservation was available than their Consultants: more surgical registrars (100%) did not know than Consultant surgeons (42%) (p = .01), and more oncology registrars (40%) did not know than Consultant oncologists (14%) (p = .02). Consultant oncologists were also more likely to know if embryo cryopreservation was available than Consultant surgeons (p = .0015) or clinical nurse specialists (p = .01). Overall, 10% thought that embryo cryopreservation was an experimental treatment and 39% said they did not know, of whom Consultant surgeons (40%) and clinical nurse specialists (62%) were more likely to report that they did not know than Consultant oncologists (11%) (p < .001). Overall, 8.5% thought that undergoing embryo cryopreservation increased the subsequent risk of breast cancer relapse, with the majority saying that they did not know (57%) (Table 2).
Table 3.
“Which fertility preservation options does your local fertility unit offer cancer patients?”
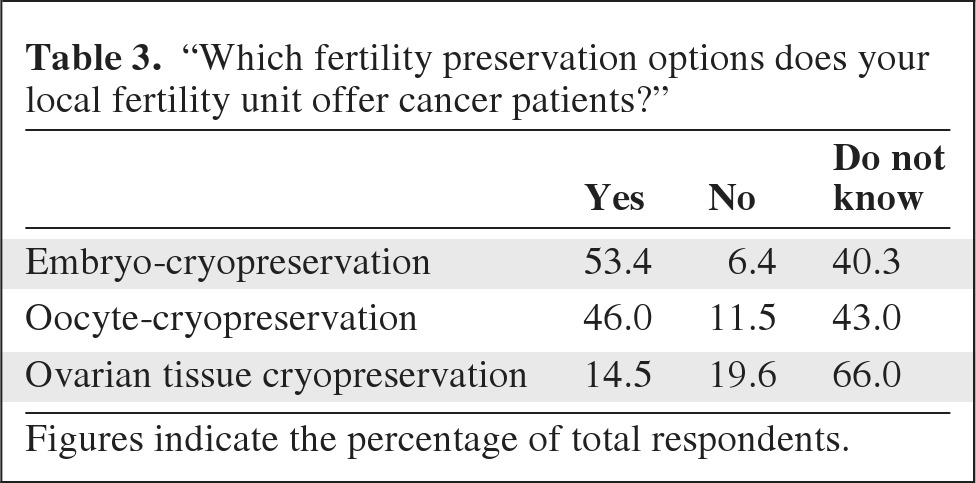
Figures indicate the percentage of total respondents.
Discussion
Advances in treatment have improved the life expectancy for young women with breast cancer, thus putting increased emphasis on survivorship issues, including fertility. The National Cancer Survivorship Initiative Vision has highlighted the importance of fertility preservation for healthy survivorship [17].
Fertility preservation is an important concern of many young women diagnosed with breast cancer. These women require well-informed discussions regarding the risks of cancer treatment on fertility, as well as fertility preservation options. This in turn requires that the health care professionals who manage these patients are able to facilitate these discussions and refer to a fertility specialist as appropriate. However, this work has demonstrated variability in the knowledge and attitudes of different health care professionals involved in the care of these women.
Factors Influencing Whether Fertility Preservation Is Discussed
With the exception of the patient's age, the majority of respondents reported that neither patient factors (such as whether they had children or a partner), tumor factors (such as ER expression), nor clinician factors (such as a lack of knowledge regarding fertility preservation) influenced whether they discussed fertility preservation with breast cancer patients. Several respondents commented that they understood the importance of having an informed discussion with all patients who had not completed their families, despite any concerns they might personally have that fertility therapy might delay or influence the efficacy of systemic cancer treatment. However, this practice is not uniform, with a substantial minority (19.8%–37.9%) reporting that at least one of these factors did influence whether they discussed fertility issues with their patients.
Some respondents raised concerns about fertility therapy delaying the onset of chemotherapy in those with a high risk of relapse. However, early referral to a fertility specialist can reduce these delays [10]. Referral prior to surgery has been shown to significantly reduce the time from initial diagnosis to commencing chemotherapy, and may also allow more than one fertility preservation cycle, enabling a larger number of embryos to be cryopreserved, which may increase the chance of a future successful pregnancy [18]. This makes it important that surgeons and specialist nurses, who interact with patients earlier in the pathway, are well informed and offer early referral to fertility specialists. However, our data demonstrated that surgeons and clinical nurse specialists were less likely to know about fertility preservation than oncologists, who usually interact with patients later in the care pathway.
Knowledge Regarding the Fertility Preservation Options Available and Variations in Practice
Knowledge varied widely across the health care professionals surveyed. There were several instances where Consultant surgeons and clinical nurse specialists were more likely to answer that they did not know than Consultant oncologists. There were also occasions where Consultants were more likely to know than their registrars, which may represent a training issue that could be addressed during specialty-specific induction.
There is no data on the impact of ovarian stimulation on the risk of breast cancer recurrence, and long-term follow-up is required [19]. Despite this, the majority of respondents (65.3%) either agreed that embryo cryopreservation was associated with an increased risk of breast cancer relapse or did not know. The RCOG (Royal College of Obstetricians and Gynaecologists) guidelines state that careful discussion is required given the unknown long-term risks, and that alternative stimulation approaches using letrozole or tamoxifen, which are associated with reduced estrogen levels, should be considered in ER+ breast cancer [15, 20, 21].
The use of GnRH agonists for fertility preservation outside the setting of clinical trials was 49.4% overall, and highest in London at 59.5%. This is despite conflicting data regarding the efficacy of GnRH agonists in this setting. Randomized trial data (for example, UK OPTION trial) are awaited, and although nonrandomized data are suggestive of benefit [22–24], there is currently insufficient evidence to support the routine use of GnRHa for ovarian protection [15].
Limitations of the Data
Caution is required when interpreting data from a questionnaire such as this. Sample size (for example, trainees are under-represented), responder bias (those with an interest in the subject may be more likely to complete the questionnaire), self-reporting bias (reported practice may differ from actual clinical practice), and recall bias (inaccurate reporting) may affect the results [1, 25]. In addition, these results do not demonstrate causality, and must be interpreted with caution. For example, although a surgeon reports incomplete knowledge regarding fertility therapy, this may not be the reason they do not discuss fertility with patients (for example, they may consider it to be someone else's role). Attitudes and practice varied within each subgroup (for example, among medical oncologists), making it difficult to generalize these results. Whether these results would be obtained in other health care systems where, for example, fertility therapy is more widely available is also questionable.
Conclusion
Previous surveys of breast cancer survivors have demonstrated that a large proportion of patients do not recall their doctors having discussed with them the risks of early menopause and infertility prior to the onset of adjuvant cancer treatment [26]. Such retrospective surveys are subject to recall bias, especially as discussions take place at a time when patients may not be able to process the information they receive [27]. Breast cancer patients' recall of discussions has been found to differ from their physicians' [28]. However, this work demonstrates that not all health care professionals are initiating these discussions with their patients, in keeping with previous data showing that physicians do not always offer sperm banking to male cancer patients undergoing chemotherapy [29]. Variability in the knowledge, attitudes, and practice of those who manage young women with breast cancer in the U.K. may translate into a lack of standardized information given to patients, and may mean that referral to a fertility specialist is not always being offered. Not all women will accept referral, and not all those referred will go on to have fertility preservation. Overall, less than 10% of women diagnosed with breast cancer will go on to become pregnant. However, the risk of infertility should be discussed with all women of reproductive age at an early stage, to allow maximum time for referral to a fertility specialist prior to the onset of adjuvant therapy. It is crucial that health care professionals are aware of the fertility preservation options available if they are to be effective advocates for their patients, as a working knowledge of the available technologies is a prerequisite for an informed discussion [30].
Acknowledgment
The abstract has been published at the National Cancer Research Institute 2011 Conference, http://www.ncri.org.uk/ncriconference/2011abstracts/abstracts/A123.html.
Author Contributions
Conception/Design: Judy W. King, Alison L. Jones
Collection and/or assembly of data: Judy W. King
Data analysis and interpretation: Judy W. King
Manuscript writing: Judy W. King, Melanie C. Davies, Nicola Roche, Jacinta M. Abraham, Alison L. Jones
Final approval of manuscript: Judy W. King, Melanie C. Davies, Nicola Roche, Jacinta M. Abraham, Alison L. Jones
References
- 1.Cancer Research UK Statistics, CRUK 2007. [Accessed November 18, 2011]. Available at http://info.cancerresearchuk.org/cancerstats/types/breast/incidence/#trends.
- 2.Ganz PA, Greendale GA, Petersen L, et al. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21:4184–4193. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 3.Partridge A, Gelber S, Gelber RD, et al. Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer. 2007;43:1646–1653. doi: 10.1016/j.ejca.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Bakewell RT, Volker DL. Sexual dysfunction related to the treatment of young women with breast cancer. Clin J Oncol Nurs. 2005;9:697–702. doi: 10.1188/05.CJON.697-702. [DOI] [PubMed] [Google Scholar]
- 5.Barthelmes L, Gateley CA. Tamoxifen and pregnancy. Breast. 2004;13:446–451. doi: 10.1016/j.breast.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 8.Reichman BS, Green KB. Breast cancer in young women: effect of chemotherapy on ovarian function, fertility, and birth defects. J Natl Cancer Inst Monogr. 1994;(16):125–129. [PubMed] [Google Scholar]
- 9.Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. The Oncologist. 2006;11:422–434. doi: 10.1634/theoncologist.11-5-422. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 11.Avis NE, Crawford S, Manuel J. Psychosocial problems among younger women with breast cancer. Psychooncology. 2004;13:295–308. doi: 10.1002/pon.744. [DOI] [PubMed] [Google Scholar]
- 12.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 13.Fallowfield L, McGurk R, Dixon M. Same gain, less pain: potential patient preferences for adjuvant treatment in premenopausal women with early breast cancer. Eur J Cancer. 2004;40:2403–2410. doi: 10.1016/j.ejca.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Thewes B, Meiser B, Taylor A, et al. Fertility- and menopause-related information needs of younger women with a diagnosis of early breast cancer. J Clin Oncol. 2005;23:5155–5165. doi: 10.1200/JCO.2005.07.773. [DOI] [PubMed] [Google Scholar]
- 15.Royal College of Obstetricians and Gynaecologists. Breast Cancer Pregnancy: Green-top guideline No. 12. [Accessed May 28, 2011]. Available at http://www.rcog.org.uk/womens-health/clinical-guidance/pregnancy-and-breast-cancer-green-top-12.
- 16.Royal College of Physicians, Royal College of Radiologists, Royal College of Obstetricians and Gynaecologists. The effects of cancer treatment on reproductive function. Guidance on management. [Accessed February 2, 2012]. Available at http://www.rcog.org.uk/womens-health/clinical-guidance/effects-cancer-treatment-reproductive-function-guidance-management.
- 17.Department of Health, National Cancer Survivorship Initiative (NCSI) Vision. [Accessed February 2, 2011]. Available at http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111230.
- 18.Lee S, Ozkavukcu S, Heytens E, et al. Value of early referral to fertility preservation in young women with breast cancer. J Clin Oncol. 2010;28:4683–4686. doi: 10.1200/JCO.2010.30.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahill DJ, Wardle PG, Harlow CR, et al. Expected contribution to serum oestradiol from individual ovarian follicles in unstimulated cycles. Hum Reprod. 2000;15:1909–1912. doi: 10.1093/humrep/15.9.1909. [DOI] [PubMed] [Google Scholar]
- 20.Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol. 2005;23:3858–3859. doi: 10.1200/JCO.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Oktay K, Buyuk E, Libertella N, et al. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Maisano R, Caristi N, Mare M, et al. Protective effect of leuprolide on ovarian function in young women treated with adjuvant chemotherapy for early breast cancer: a multicenter phase II study. J Chemother. 2008;20:740–743. doi: 10.1179/joc.2008.20.6.740. [DOI] [PubMed] [Google Scholar]
- 23.Maltaris T, Weigel M, Mueller A, et al. Cancer and fertility preservation: fertility preservation in breast cancer patients. Breast Cancer Res. 2008;10:206. doi: 10.1186/bcr1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recchia F, Saggio G, Amiconi G, et al. Gonadotropin-releasing hormone analogues added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer. 2006;106:514–523. doi: 10.1002/cncr.21646. [DOI] [PubMed] [Google Scholar]
- 25.Adams AS, Soumerai SB, Lomas J, et al. Evidence of self-report bias in assessing adherence to guidelines. Int J Qual Health Care. 1999;11:187–192. doi: 10.1093/intqhc/11.3.187. [DOI] [PubMed] [Google Scholar]
- 26.Duffy C, Allen S, Clark M. Discussings regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol. 2005;23:766–773. doi: 10.1200/JCO.2005.01.134. [DOI] [PubMed] [Google Scholar]
- 27.Fallowfield L, Jenkins V. Effective communication skills are the key to good cancer care. Eur J Cancer. 1999;35:1592–1597. doi: 10.1016/s0959-8049(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 28.Keating NL, Weeks JC, Borbas C, et al. Treatment of early stage breast cancer: do surgeons and patients agree regarding whether treatment alternatives were discussed? Breast Cancer Res Treat. 2003;79:225–231. doi: 10.1023/a:1023903701674. [DOI] [PubMed] [Google Scholar]
- 29.Schover LR, Brey K, Lichtin A, et al. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20:1880–1889. doi: 10.1200/JCO.2002.07.175. [DOI] [PubMed] [Google Scholar]
- 30.Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res. 2007;138:3–11. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]