The prognostic and predictive value of the circulating tumor cell count was assessed in a subgroup of patients involved in the Spanish Cooperative Group for the Treatment of Digestive Tumors Maintenance in Colorectal Cancer trial. The circulating tumor cell count was a strong prognostic factor for progression-free survival and overall survival outcomes.
Keywords: Circulating tumor cells, Prognostic factor, Veridex CellSearch®
Abstract
Background.
The Maintenance in Colorectal Cancer trial was a phase III study to assess maintenance therapy with single-agent bevacizumab versus bevacizumab plus chemotherapy in patients with metastatic colorectal cancer. An ancillary study was conducted to evaluate the circulating tumor cell (CTC) count as a prognostic and/or predictive marker for efficacy endpoints.
Patients and Methods.
One hundred eighty patients were included. Blood samples were obtained at baseline and after three cycles. CTC enumeration was carried out using the CellSearch® System (Veridex LLC, Raritan, NJ). Computed tomography scans were performed at cycle 3 and 6 and every 12 weeks thereafter for tumor response assessment.
Results.
The median progression-free survival (PFS) interval for patients with a CTC count ≥3 at baseline was 7.8 months, versus the 12.0 months achieved by patients with a CTC count <3 (p = .0002). The median overall survival (OS) time was 17.7 months for patients with a CTC count ≥3, compared with 25.1 months for patients with a lower count (p = .0059). After three cycles, the median PFS interval for patients with a low CTC count was 10.8 months, significantly longer than the 7.5 months for patients with a high CTC count (p = .005). The median OS time for patients with a CTC count <3 was significantly longer than for patients with a CTC count ≥3, 25.1 months versus 16.2 months, respectively (p = .0095).
Conclusions.
The CTC count is a strong prognostic factor for PFS and OS outcomes in metastatic colorectal cancer patients.
Introduction
The CellSearch® System (Veridex LLC, Raritan, NJ) was approved by the U.S. Food and Drug Administration for the detection and enumeration of circulating tumor cells (CTCs) in the peripheral blood of patients with breast, prostate, and colorectal cancer. This semiautomated immunomagnetic method for quantification of CTCs has also been validated by three centers in the U.S. and Europe [1]. Allard et al. [2], using the CellSearch® System in healthy volunteers, patients with nonmalignant diseases, and patients with metastatic carcinomas, established that a CTC count ≥2 was only present in patients with malignant epithelial tumors. In advanced colorectal cancer patients, a CTC count ≥2 was detected in 60.7% of patients, and no correlation was found with other clinical or pathological variables such as grade of differentiation, lactate dehydrogenase (LDH) level, or carcinoembryonic antigen level [3]. A threshold CTC count ≥3 was chosen and validated in a prospective multicenter study conducted in 430 patients with metastatic colorectal cancer to stratify them into favorable and unfavorable prognostic groups [4]. The number of CTCs before and during treatment was an independent predictor of progression-free survival (PFS) and overall survival (OS) outcomes. Similar outcomes were recently reported in a more selected patient population in which the CTC count was determined at baseline and during first-line treatment with chemotherapy plus targeted agents [5].
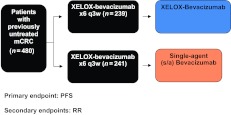
The Spanish Cooperative Group for the Treatment of Digestive Tumors (TTD) Maintenance in Colorectal Cancer (MACRO) trial [6] (Fig. 1), a randomized phase III study in which patients were treated first line with six cycles of capecitabine and oxaliplatin (XELOX) plus bevacizumab followed by either single-agent bevacizumab or XELOX plus bevacizumab, suggested that maintenance therapy with single-agent bevacizumab may be an appropriate treatment option following induction chemotherapy with XELOX plus bevacizumab. We assessed the prognostic and predictive value of CTC enumeration in a subgroup of patients involved in that trial.
Figure 1.
The Maintenance in Colorectal Cancer trial design.
Abbreviations: mCRC, metastatic colorectal cancer; PFS, progression-free survival; q3w, every 3 weeks; RR, response rate; XELOX, capecitabine and oxaliplatin.
Patients and Methods
Patient Population
One hundred eighty patients of the 480 patients involved in the MACRO trial were included in this biological marker substudy. Patients aged ≥18 years with histologically confirmed metastatic colorectal cancer, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score ≤2, measurable disease, no previous chemotherapy for advanced disease, and adequate hepatic and renal function as well as no contraindications to receiving bevacizumab were included. The primary endpoint of the MACRO trial was the PFS interval; secondary endpoints included the OS time, response rate, toxicity, and several translational research assessments.
Local ethics committee approval was obtained before enrollment of any patient into the study, which was performed in accordance with the Declaration of Helsinki and its subsequent amendments as well as the Good Clinical Practice Guidelines. Signed informed consent was obtained from all patients before study entry.
Imaging
Computed tomography scans were performed at the end of cycle 3 and cycle 6 and every 9 weeks thereafter for tumor response assessment according to the Response Evaluation Criteria in Solid Tumors [7]. The PFS interval was defined as the interval between chemotherapy initiation and the date of documented disease progression, death, or loss to follow-up, whichever occurred first. The OS duration was defined from the date of chemotherapy initiation to the date of death.
Isolation and Enumeration of CTCs
Peripheral blood samples were obtained at baseline, after cycle 3 when the first tumor assessment was planned, and after disease progression. CTC isolation and enumeration were centralized at the Laboratory Department of San Carlos Hospital and were carried out in 7.5 mL of whole blood using the CellSearch® System as previously described [3]. A CTC threshold ≥3 was chosen following previous publications [4, 5].
Statistical Analysis
Assessment of the CTC count as a prognostic and predictive factor in patients with metastatic colorectal cancer treated with chemotherapy and bevacizumab was selected as the primary objective of this ancillary study in the setting of the MACRO trial. PFS and OS curves according to the presence of a CTC count <3 and a CTC count ≥3 CTCs at baseline were made using the Kaplan–Meier method. Their comparison using the log-rank test defined the prognostic value of the biologic marker. The predictive role of the CTC count was explored through correlation between CTC count change during treatment (baseline and cycle 3 time points) and the response rate, PFS and OS curves, and their respective comparisons. Univariate and multivariate Cox proportional hazards models were built with the following variables: ECOG PS score, 0–1 versus 2; age, <70 years versus ≥70 years; number of affected organs, one versus two or more; LDH level, within the normal range versus high; alkaline phosphatase, within the normal range versus high; gender, male versus female; CTC count at baseline, <3 versus ≥3; and treatment arm.
Results
Patient Characteristics
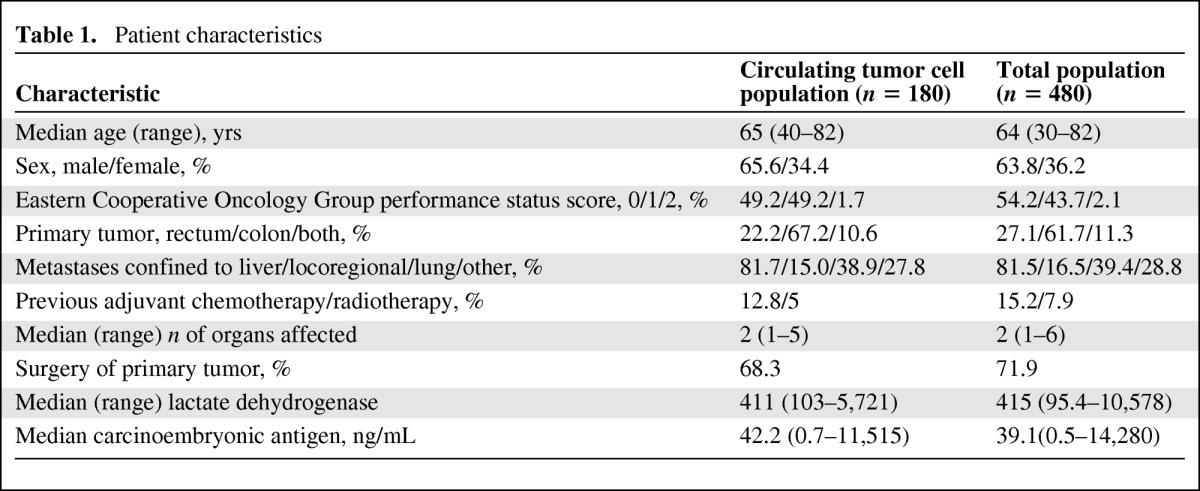
Blood samples for CTC enumeration were collected at baseline in 180 of 480 patients randomized in the MACRO trial and after three cycles of XELOX plus bevacizumab first-line chemotherapy in 147 patients. Characteristics of this subset and the total population were comparable and are shown in Table 1. The median CTC count was two (range, 0–1042) at baseline and zero at cycle 3 (range, 0–46).
Table 1.
Patient characteristics
Prognostic Value of the CTC Count
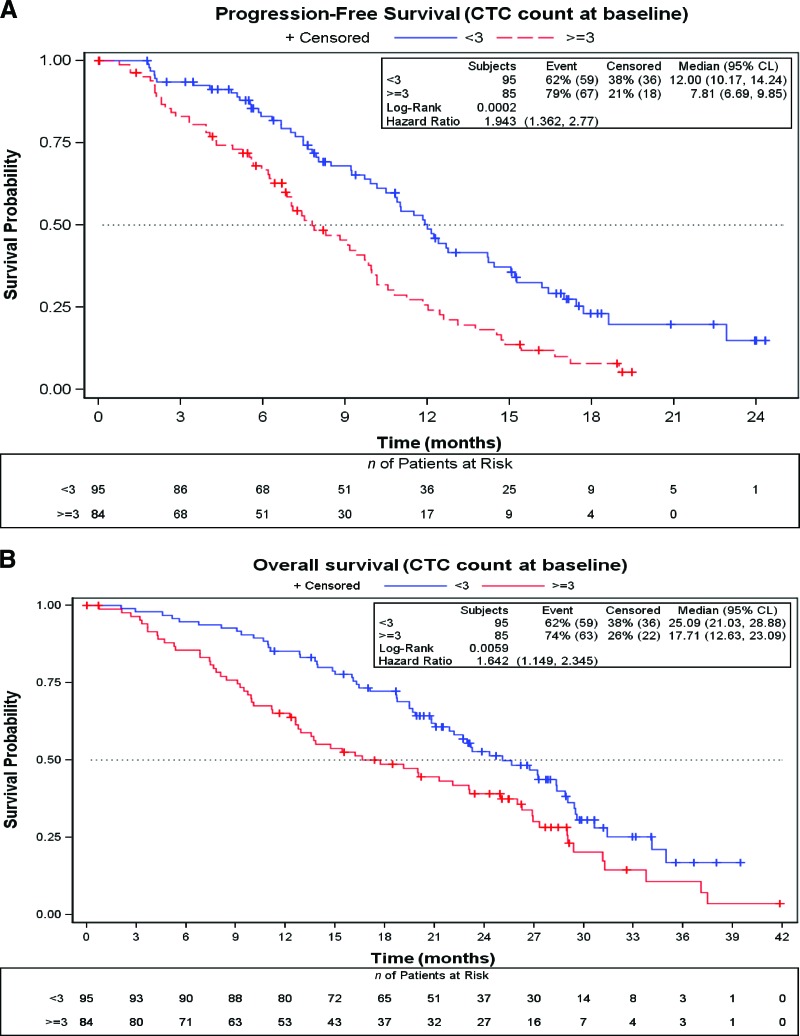
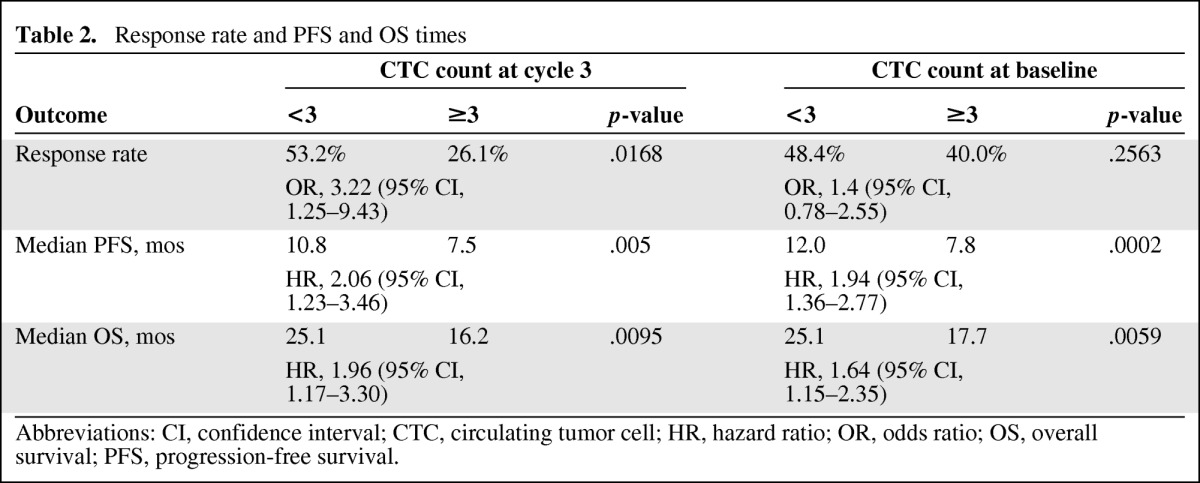
In 85 (47.2%) of the 180 patients in which the CTC count was determined at baseline, the count was ≥3. The median PFS interval for this subgroup was 7.8 months (95% confidence interval [CI], 6.7–9.9 months), significantly shorter than the median PFS time of 12.0 months (95% CI 10.2–14.2 months) achieved by patients with a CTC count <3 (p = .0002; hazard ratio [HR], 1.94; 95% CI, 1.36–2.77) (Fig. 2A). The median OS time was also shorter for patients with a CTC count ≥3 than for patients with a lower count—17.7 months (95% CI, 12.6–23.1 months) versus 25.1 months (95% CI, 21.0–28.9 months (p = .0059; HR, 1.64; 95% CI, 1.15–2.34) (Fig. 2B).
Figure 2.
Survival outcomes according to CTC count at baseline using the Kaplan–Meier method. (A): Progression-free survival probability. (B): Overall survival probability.
Abbreviations: CL, confidence limits; CTC, circulating tumor cell.
Predictive Value of the CTC Count
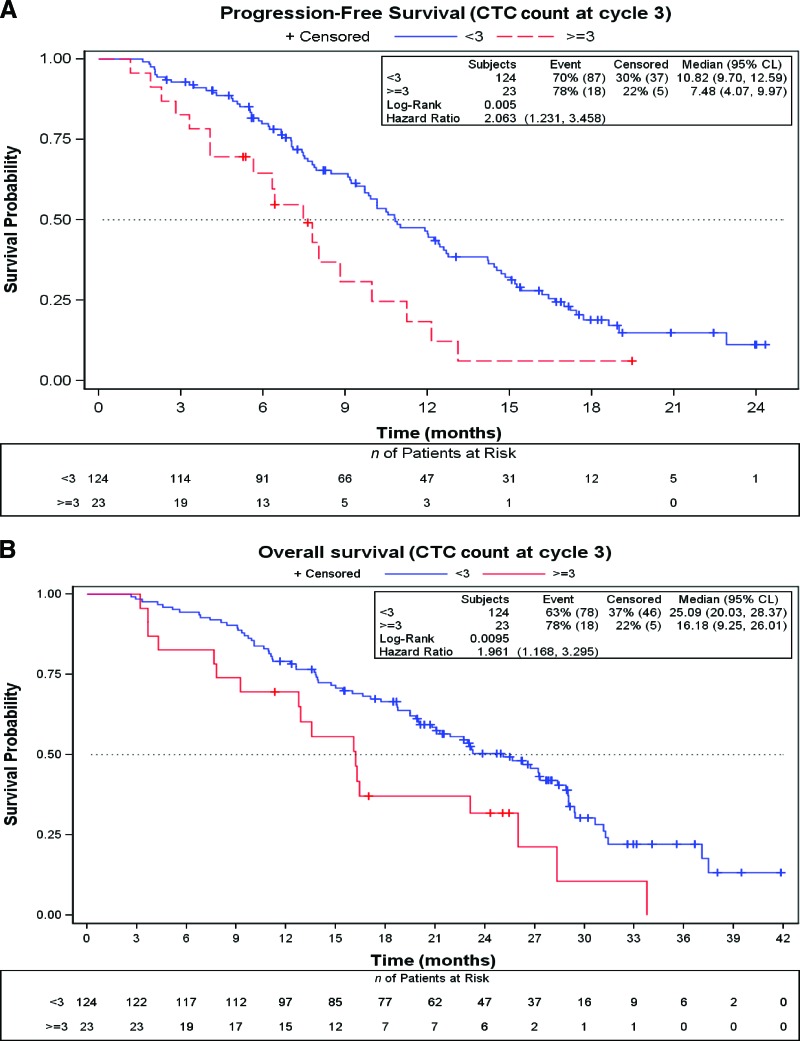
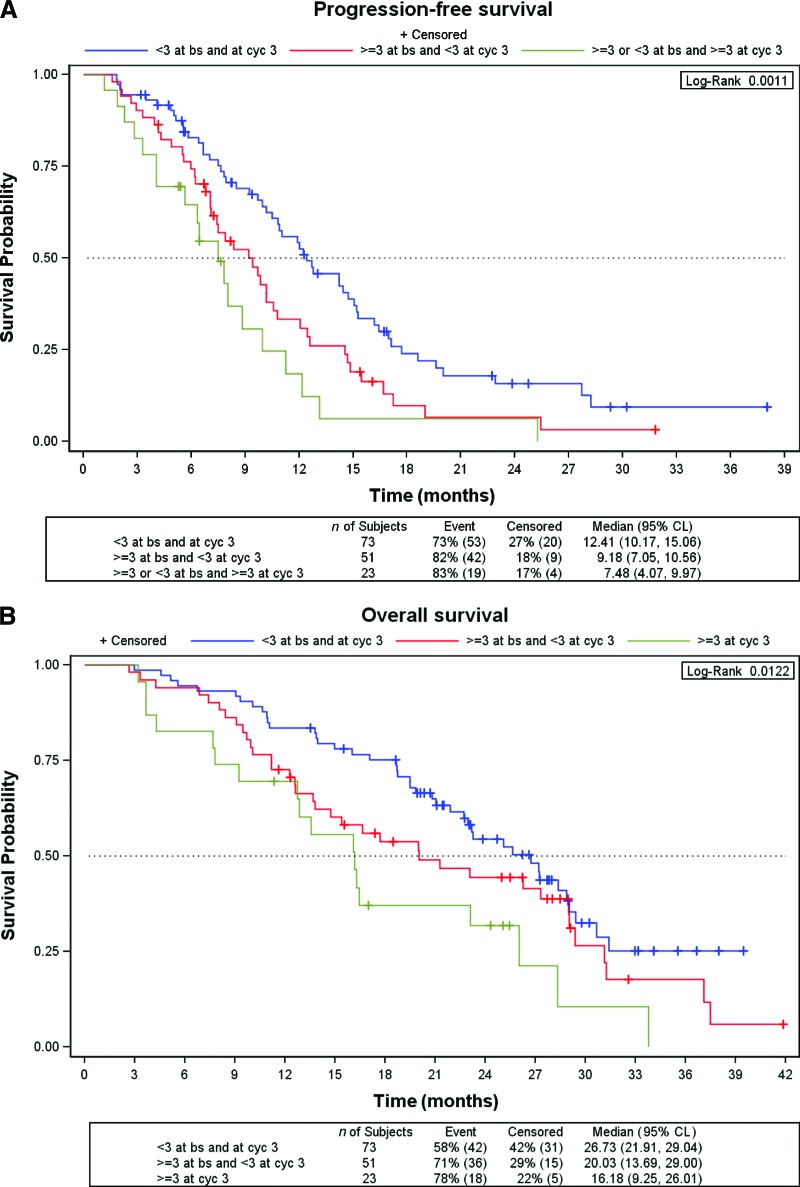
The number of CTCs at baseline did not predict the response to chemotherapy plus bevacizumab. The response rates in the low and high CTC count groups were 48.4% and 40.0%, respectively (p = .25). After three cycles of treatment, only 23 (15.7%) of the 147 patients in whom the CTC count was determined had a CTC count ≥3. The response rate for patients with a CTC count <3 was significantly higher than the response rate for those with a CTC count ≥3 after three cycles (53% versus 26%; p = .017; odds ratio, 3.22; 95% CI, 1.25–9.43). The median PFS interval for patients with a low CTC count after cycle 3 was 10.8 months (95% CI, 9.7–12.6 months), significantly longer than that observed in the group of patients with a high CTC count at this time point (7.5 months; 95% CI, 4.1–10.0 months; p = .005; HR, 2.06; 95% CI, 1.23–3.46). Similarly, the median OS time of patients with a CTC count <3 after three cycles of chemotherapy plus bevacizumab was significantly longer than that achieved by patients with a CTC count ≥3—25.1 months (95% CI, 20.0–28.4 months) versus 16.2 months (95% CI, 9.3–26.0 months); p = .0095; HR, 1.96 (95% CI 1.17–3.29) (Fig. 3A, 3B). Figures 4A and 4B show the PFS and OS curves according to changes in the CTC count after treatment. Patients with a low CTC count at baseline and after treatment had significantly longer PFS and OS times than those with a high CTC count at baseline and low CTC count after treatment. A tendency for better PFS and OS outcomes for patients in whom the CTC count declined to less than three than in those with a high CTC count after treatment was observed, but this did not reach statistical significance.
Figure 3.
Survival outcomes according to CTC count at cycle 3 using the Kaplan–Meier method. (A): Progression-free survival probability. (B): Overall survival probability.
Abbreviations: CL, confidence limits; CTC, circulating tumor cell.
Figure 4.
Survival outcomes according to the change in the CTC count at cycle 3 using the Kaplan–Meier method. (A): Progression-free survival probability. (B): Overall survival probability.
Abbreviations: bs, baseline; CL, confidence limits; cyc, cycle; CTC, circulating tumor cell.
Three groups of patients were identified: a good prognostic group, consisting of patients with a low CTC count both at baseline and after cycle 3 (median PFS interval, 12.4 months; median OS time, 26.7 months); an intermediate group, including patients with a high CTC count at baseline that converted to a low CTC count after treatment (median PFS interval, 9.2 months; median OS time, 20.0 months); and a poor prognostic group, involving patients with a high CTC count after treatment (median PFS interval, 7.5 months; median OS time, 16.2 months).
Summaries of the response rate, PFS time, and OS time are shown in Table 2.
Table 2.
Response rate and PFS and OS times
Abbreviations: CI, confidence interval; CTC, circulating tumor cell; HR, hazard ratio; OR, odds ratio; OS, overall survival; PFS, progression-free survival.
Univariate and Multivariate Analyses
Results of the univariate and multivariate analyses for PFS and OS outcomes are shown in Table 3. In the univariate analysis for the PFS outcome, only a baseline CTC count ≥3 and a high LDH level were significantly associated with a poor PFS outcome. Both factors remained independent poor prognostic markers in the multivariate analysis (HR, 1.47 and 1.65, respectively). For the OS outcome, a CTC count >3, high LDH level, and a number of affected organs of two or more were independent poor prognostic factors in the multivariate analysis (HR, 1.54, 1.62, and 1.70, respectively).
Table 3.
Univariate and multivariate analyses for progression-free survival and overall survival outcomes
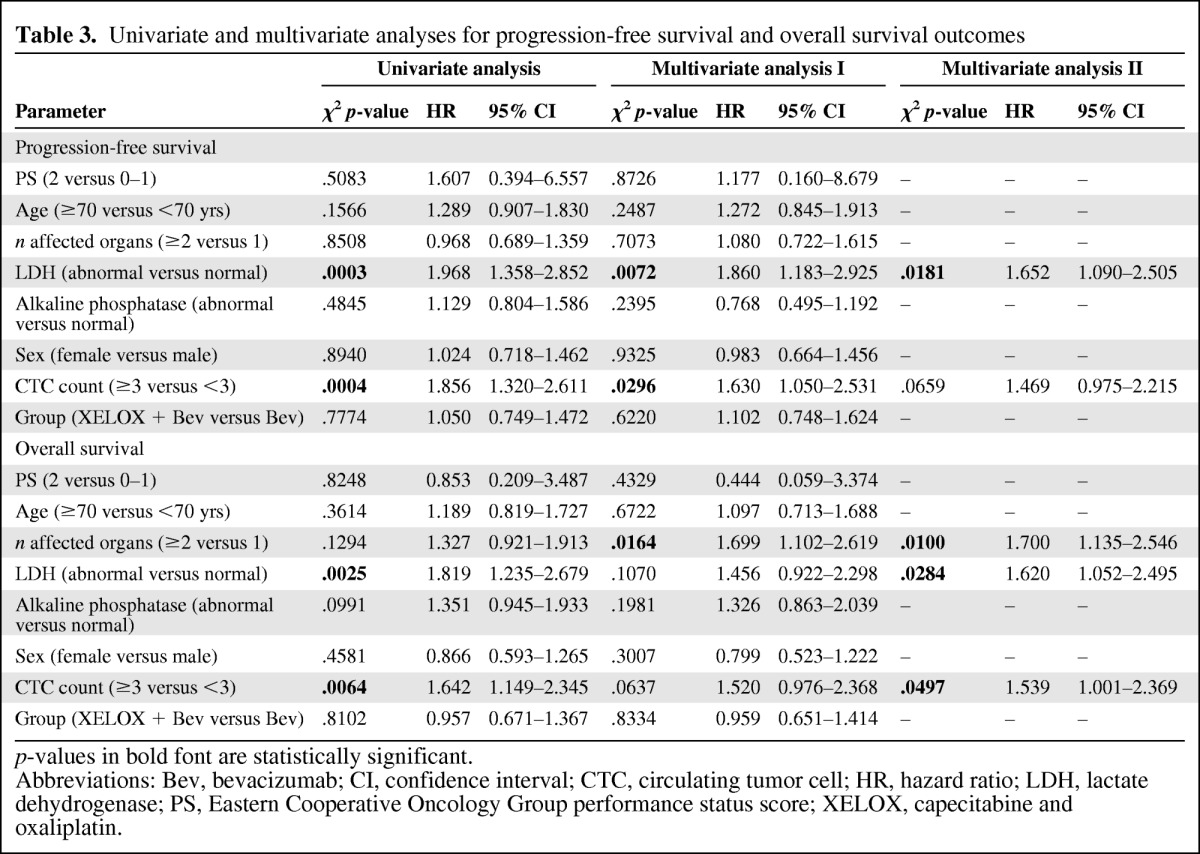
p-values in bold font are statistically significant.
Abbreviations: Bev, bevacizumab; CI, confidence interval; CTC, circulating tumor cell; HR, hazard ratio; LDH, lactate dehydrogenase; PS, Eastern Cooperative Oncology Group performance status score; XELOX, capecitabine and oxaliplatin.
Discussion
The CellSearch® System is a validated semiautomated technique to detect CTCs in patients with colorectal, breast, and prostate cancer. In healthy volunteers and patients without cancer, no more than one CTC is found [2, 3]. Nevertheless, a threshold CTC count of three or more has been established as a predictive and prognostic factor in patients with advanced colorectal cancer [4, 5]. Taking into account this cutoff, our ancillary study demonstrated the prognostic role of CTC assessment in patients with advanced colorectal cancer treated with chemotherapy plus bevacizumab.
Until now, only one trial had investigated the role of the CTC count as an independent prognostic and predictive factor before and after first-line chemotherapy plus targeted agents in patients with metastatic colorectal cancer [5]. Basically, that trial and the study presented here came to similar conclusions: (a) the CTC count before treatment is a strong and independent prognostic factor for PFS and OS outcomes and (b) a change in the CTC counts during treatment can define three different prognostic subgroups: patients who never had a CTC count ≥3, with the longest PFS and OS times; those in whom the CTC count was high at baseline and/or during treatment, with the shortest PFS and OS times; and those in whom the CTC count was converted from high to low, with intermediate outcomes. Nevertheless, some differences should be noted between these studies. First, the percentages of patients with a CTC count ≥3 at baseline were different. In our trial, 47% of patients had a CTC count ≥3 at baseline, which is in accordance with our previous experience [3], whereas in the Dutch trial only 29% of patients had a CTC count ≥3 at that time point.
Hepatic filtration via the portal circulation has been suggested to explain the relatively low number of CTCs found in patients with gastrointestinal tumors, compared with patients with other carcinomas [2]. Thus, a higher proportion of patients with intermediate and lower third rectum carcinoma might explain differences in the CTC count. However, no significant differences were found between our trial and the Dutch study in that respect (22% and 26% of primary tumors located in the rectum, respectively), so variations in this phenotypic feature according to geographic areas cannot be ruled out and should be explored in the future. Second, the sample size was larger in the Dutch study, allowing stronger conclusions. Consistent with the results of the Dutch study, we found a clear tendency to worse PFS and OS outcomes for patients who had a high CTC count after three cycles of treatment than for patients whose CTC count was converted from high to low, but in the current study this did not reach statistical significance. On the other hand, Tol et al. [5] found a correlation between a low CTC count at baseline and the response rate that we could not confirm. In our trial, a low CTC count after treatment was a predictive factor for response, which was also true in the Dutch study, but the CTC count at baseline did not predict response to chemotherapy plus bevacizumab. Finally, in both studies the CTC count remained a strong prognostic factor for PFS and OS outcomes in the multivariate analysis, but in contrast with the Dutch trial, we found that the number of affected organs was also an independent prognostic factor for the OS outcome. Another trial conducted in 430 patients with advanced colorectal cancer had previously shown the prognostic and predictive value of the CTC count regardless of the line of chemotherapy that patients were receiving [4].
Our results, together with those previously reported, clearly establish a subgroup of patients with more aggressive colorectal cancer cells that may be a result of a higher capacity for migration and invasion throughout the bloodstream. Nevertheless, this hypothesis is limited by the technique itself. Detected cells cannot be collected later on, characterized, and used to investigate their ability to growth in preclinical models.
From a clinical perspective, CTC enumeration may be useful to stratify patients included in phase III trials of metastatic colorectal cancer. In the near future, new questions should be addressed, such as: Should patients with a CTC count ≥3 at baseline receive more aggressive chemotherapeutic regimens upfront? Should chemotherapy be modified early in patients with an unfavorable CTC count during treatment? Is the CTC count a prognostic factor for relapse or survival in patients with stage II or stage III colorectal cancer? To answer the last question, the Spanish TTD group recently recruited >500 patients with stage III colon cancer, collecting blood samples for CTC enumeration after surgery to correlate with cancer relapse and survival.
In summary, our results confirm that the CTC count at baseline is a strong prognostic factor for PFS and OS outcomes in patients with metastatic colorectal cancer and it would be of interest to implement this test in clinical practice.
Acknowledgments
The following physicians cared for the patients in this study. The authors thank them for their cooperation and support: E. Aranda and A. Gómez. (H. Reina Sofía); E. Díaz Rubio and J. Sastre (H. Universitario Clínico San Carlos); F. Rivera (H. Marqués de Valdecilla); M. Valladares (C. H. Universitario La Coruña); B. Massutí and A. Yuste (H. General Universitario de Alicante); M. Benavides (H. Universitario Carlos Haya); M. Gallén (H. del Mar); E. Marcuello and M. Tobeña (H. Santa Creu i Sant Pau); A. Abad (ICO. H. Germans Trias i Pujol); A. Arrivi (F. H. Son Llatzer); C. Fernández-Martos (Instituto Valenciano de Oncología); E. González (H. Virgen de las Nieves); J. Ma. Tabernero (H. Universitari Vall d'Hebrón); Ma. J. Safont (H. General Universitario de Valencia); P. Escudero (H. C. Universitario Lozano Blesa); A. López-Ladrón (H. Nuestra Señora de Valme); J. Remón (H. de Mataró); T. García (H. Morales Meseguer); J. L. García López and Ma. L. Garcia de Paredes (H. Ramón y Cajal); A. Lacasta (H. de Donostia); A. Velasco (H. Universitario de la Princesa); A. Carrato and A. Rodriguez Lescure (H. General Universitario de Elche); A. Ruiz (H. de Fuenlabrada); H. Manzano (H. Son Dureta); J. García-Foncillas (C. Universitaria de Navarra); M. Llanos (H. Universitario de Canarias); A. Anton (H. Miguel Servet); Ma. J. Gómez (H. Puerta del Mar); J. Alfaro (C. Sanitari de Terrasa); E. Jiménez (H. Jerez de la Frontera); D. Almenar (H. Dr. Peset); A. Etxeberría (Instituto Oncológico); E. Cabrera, L. del Río, J. Ponce, and A. Oltra (H. Virgen de los Lirios); T. Checa (I. de Oncología Corachán).
TTD Data Center: I. Ruiz de Mena, S. Rodríguez, and M. García.
Statistics and Data Management: Pivotal: N. Martin and J.J. García.
Monitoring: Dynamic Solutions: A. Sotés y R. Colorado.
Sponsors: Roche: P. Romero.
Supported by the Spanish Cooperative Group for the Treatment of Digestive Tumors (TTD), Madrid, Spain.
Financial support for this research was provided by Roche.
Presented in part at the 2010 35th European Society for Medical Oncology Congress, Milan, Italy, October 8–12, 2010. (Ann Oncol 2010;21(suppl 8):173PD).
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: M. Luisa Maestro, Javier Sastre, Enrique Aranda, Eduardo Díaz-Rubio
Provision of study material or patients: Manuel Valladares, Auxiliadora Gómez-España, M. Luisa Maestro, Javier Sastre, Fernando Rivera, Bartomeu Massuti, Manuel Benavides, Manuel Gallén, Eugenio Marcuello, Albert Abad, Antonio Arrivi, Carlos Fernández-Martos, Encarnación González, Josep M. Tabernero, Marta Vidaurreta, Enrique Aranda, Eduardo Díaz-Rubio
Collection and/or assembly of data: Manuel Valladares, Auxiliadora Gómez- España, M. Luisa Maestro, Javier Sastre, Fernando Rivera, Bartomeu Massuti, Manuel Benavides, Manuel Gallén, Eugenio Marcuello, Albert Abad, Antonio Arrivi, Carlos Fernández-Martos, Encarnación González, Josep M. Tabernero, Marta Vidaurreta, Enrique Aranda, Eduardo Díaz-Rubio
Data analysis and interpretation: M. Luisa Maestro, Javier Sastre, Enrique Aranda, Eduardo Díaz-Rubio
Manuscript writing: Javier Sastre, Enrique Aranda, Eduardo Díaz-Rubio
Final approval of manuscript: Manuel Valladares, Auxiliadora Gómez-España, M. Luisa Maestro, Javier Sastre, Fernando Rivera, Bartomeu Massuti, Manuel Benavides, Manuel Gallén, Eugenio Marcuello, Albert Abad, Antonio Arrivi, Carlos Fernández-Martos, Encarnación González, Josep M. Tabernero, Marta Vidaurreta, Enrique Aranda, Eduardo Díaz-Rubio
References
- 1.Riethdorf S, Fritsche H, Ml̈ler V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch System. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 2.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with non-malignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 3.Sastre J, Maestro ML, Puente J, et al. Circulating tumor cells in colorectal cancer: Correlation with clinical and pathological variables. Ann Oncol. 2008;19:935–938. doi: 10.1093/annonc/mdm583. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 5.Tol J, Koopman M, Miller MC, et al. Circulating tumor cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol. 2010;21:1006–1012. doi: 10.1093/annonc/mdp463. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Rubio E, Gómez-España A, Massuti B, et al. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: The phase III MACRO TTD Study. The Oncologist. 2012;17:15–25. doi: 10.1634/theoncologist.2011-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]