The incidence of extrahepatic metastasis was investigated in a cohort of 578 hepatocellular carcinoma patients after curative hepatectomy. A panel of independent risk factors was analyzed, and a predictive scoring system to estimate the risk for EHM was developed and evaluated in a subgroup of early-stage hepatocellular carcinoma patients.
Keywords: Hepatocellular carcinoma, Hepatectomy, Extrahepatic metastasis, Prediction, Risk factor
Abstract
Background.
Postoperative extrahepatic metastasis (EHM) contributes to a poor prognosis in patients with hepatocellular carcinoma (HCC) after hepatectomy. This study was aimed to develop a practical method that can be used to predict postoperative EHM.
Methods.
In total, 578 patients were enrolled. We analyzed the clinicopathological features of the tumors and did a long-term follow-up to observe HCC recurrence. Postoperative EHM was detected in 136 patients, and multivariate analysis was used to confirm independent risk factors for postoperative EHM. After the factors were identified, a predictive scoring system was constructed as a weighted sum of these factors. The cutoff value that determines a high risk for EHM was defined by maximizing the Youden's index of the receiver operating characteristic curve.
Results.
Microvascular invasion, incomplete capsule, and larger tumor diameter were the three independent factors predictive for a high risk for EHM. The scoring system was derived with an area under the curve (AUC) of 0.81 for postoperative 10-year EHM prediction. A cutoff value of 43 was derived and validated with a sensitivity >90% and specificity >60% to predict the development of EHM. This system was further verified in a subgroup of Barcelona Clinic Liver Cancer stage 0–A patients with an AUC of 0.82. When the cutoff value was set at 43, the sensitivity and specificity were 90.38% and 64.88%, respectively.
Conclusions.
Our predictive scoring system may be used to identify HCC patients who have a high risk for EHM following curative hepatectomy.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most commonly diagnosed malignancy worldwide [1]. Although hepatic resection and liver transplantation are accepted first-line curative treatments for well-selected HCC patients, the prognosis of HCC patients following hepatectomy is not always favorable because of a significant chance for intrahepatic recurrence and distant metastasis, which contribute to the high mortality rate of HCC patients [2, 3].
Although intrahepatic relapse is the most common form of recurrence of HCC postoperatively, extrahepatic metastasis (EHM) can count for 14%–25.5% of all recurrent cases [4, 5]. Recently, therapy for intrahepatic lesions was improved because of modalities applicable to primary lesions, such as surgery, transarterial chemoembolization, and radiofrequency ablation [2]. However, treatment options for EHM are relatively limited, especially when metastases present as the “diffuse form” or are accompanied by advanced intrahepatic recurrence [6, 7]. Currently, the detection of EHM is more sensitive and accurate with the development of imaging modalities [8, 9]. Furthermore, the management of some solitary HCC metastases in the lung, adrenal gland, and peritoneum have led to promising outcomes [10–12]. In particular, in the study of Lo et al [13], a mean survival time of 20 months was achieved in 12 HCC patients who received surgical resection for solitary metastasis in the lung or abdomen. Recent studies have shown that the targeted drug sorafenib not only led to longer overall survival times in patients with advanced HCC with EHM in clinical trials but also suppressed postsurgical intrahepatic and distant HCC metastasis in orthotopic mouse models [14–16], making it a potential choice for postoperative EHM therapy. Therefore, it is important to identify HCC patients at high risk for postoperative EHM, which may facilitate not only to find smaller and fewer EHM lesions but also to identify patients who might benefit from potential adjuvant therapies, such as sorafenib. Exploration of biomarkers is a promising way to determine the prognosis of HCC patients [17, 18], but a marker with great clinic implications is still lacking, and accurate and practical methods based on clinicopathological characteristics to predict EHM are still urgently needed.
In this study, we investigated the incidence of EHM in a cohort of 578 HCC patients after curative hepatectomy, analyzed a panel of independent risk factors, and established a predictive scoring system to estimate the risk for EHM. We also evaluated the predictive power of this new system in a subgroup of early-stage (Barcelona Clinic Liver Cancer [BCLC] stage 0–A) [19] HCC patients.
Materials and Methods
Patients
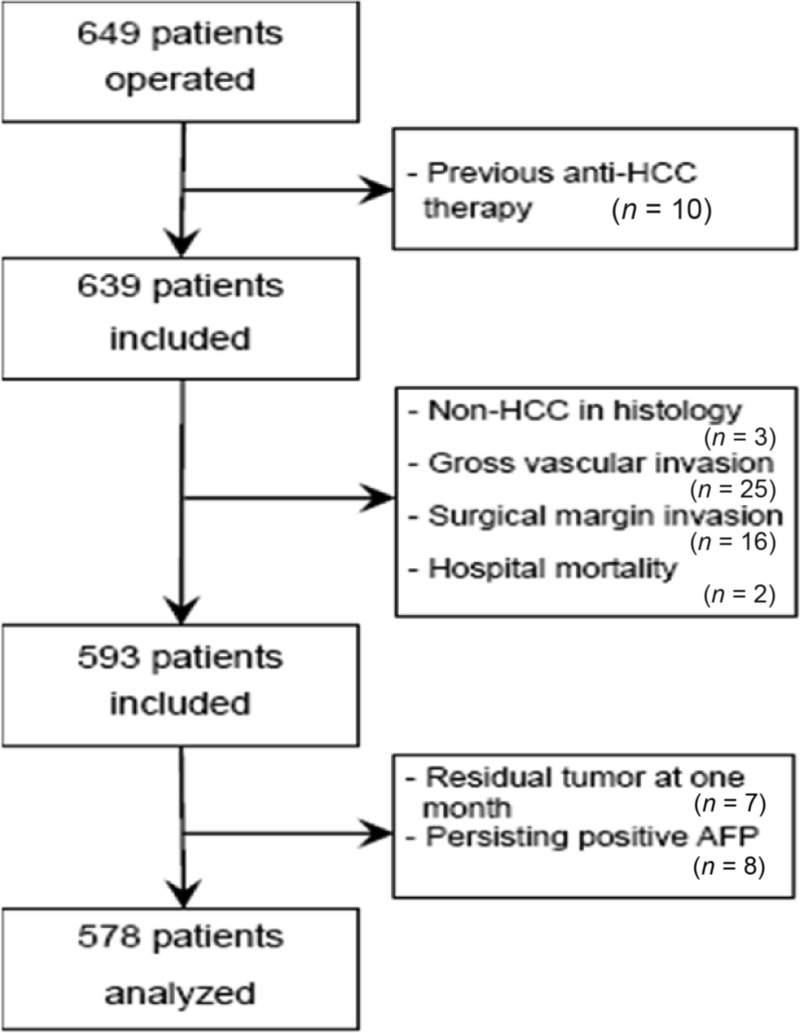
In February 1998 to July 2001, 649 consecutive HCC patients who underwent hepatectomy at the Eastern Hepatobiliary Surgery Hospital were evaluated. Patients who met all the following criteria were enrolled in this study for further analyses: (a) World Health Organization performance status score of 0–1 before treatment, (b) no history of previous anticancer therapy, (c) no distant metastasis at the time of diagnosis, (d) curative hepatectomy, (e) pathological diagnosis of HCC in all resected tumors, and (f) no history of other malignancies. A curative hepatectomy was defined as follows: (a) resection of all macroscopic tumors demonstrated by intraoperative ultrasound; (b) no tumor cells observed at the surgical margin on histological examination; (c) no tumor invasion into the portal vein, hepatic vein, or bile duct; (d) no residual tumors detected using contrast-enhanced computed tomography (CT) within 2 months after surgery; and (e) decrease in postoperative serum α-fetoprotein (AFP) to within the normal range in patients with a positive preoperative AFP. Finally, 578 HCC patients who met the above criteria comprised the study cohort and were followed up for EHM occurrence (Fig. 1).
Figure 1.
Diagram of patient selection flow in the study.
Abbreviations: AFP, α-fetoprotein; HCC, hepatocellular carcinoma.
Clinical staging was determined according to the BCLC staging system. Levels of serological indices were evaluated preoperatively and pathological features of tumors were documented after completion of histopathological study of the resected specimens. The tumor differentiation grade was assigned based on the Edmondson–Steiner classification. This study was approved by the institutional review board. Informed consent was obtained from each patient before surgery.
Follow-Up Studies
Patients were followed up with clinic visits every 2 months during the first 2 years after surgery and then every 3–6 months thereafter. Follow-up ended on June 28, 2009. At each follow-up visit, a complete history and physical examination were obtained, a blood sample was drawn for serum AFP assay and liver function tests, and tumor recurrence was monitored using ultrasonography and chest x-ray. When any recurrence or metastasis was suspected, a CT, hepatic arteriography, or magnetic resonance imaging scan was performed for confirmation. A bone scintigraphy was performed if there was a complaint of local bone pain. Positron emission tomography–CT was also conducted in some patients with suspected metastasis in recent years.
Diagnosis of Postoperative EHM
The diagnosis of EHM was based on the following observations: (a) the development of new lesions that were not found in prior imaging studies or the enlargement of existing lesions detected on dynamic imaging examination following initial resection for HCC, (b) elevation in AFP levels that were high preoperatively but dropped to within the normal range after surgery, (c) histopathological study of extrahepatic lesions in some patients who underwent re-resection for recurrence of HCC, (d) no other malignancies diagnosed on clinical examination when EHM was detected [20, 21]. Only EHM diagnosed prior to or simultaneously with intrahepatic recurrence was recorded for the purpose of this study. EHM that occurred after intrahepatic recurrence was excluded from our analysis because it could be a sequential metastasis from recurrent lesions rather than from the primary tumor that had been resected.
Statistical Analysis
Time to EHM identification prior to or simultaneously with initial intrahepatic recurrence during follow-up and the overall survival duration were considered as endpoints in this study. Time to EHM was calculated from the date of surgery to the date when any initial EHM recurrence of interest was diagnosed or to the last visit before June 28, 2009. The overall survival time was the interval between the date of liver resection and the date of death or last follow-up. The statistical analysis was performed using Stata® 12 for Windows (StataCorp LP, College Station, TX). Categorical variables were compared using the χ2 test or Fisher's exact test, and continuous variables were compared using Student's t-test. Survival curves were calculated using the Kaplan–Meier method and were compared using the log-rank test. Competing-risks Cox regression analysis was used for the multivariate analysis.
A predictive scoring system was formulated following the method described by Hastie et al. [22] and Yuen et al. [23]. Briefly, the scoring system was constructed as a weighted sum of independent risk factors for EHM. The weights were taken as the corresponding estimated coefficients in a Cox regression analysis after being divided by the smallest coefficient and rounded to the nearest integer.
The accuracy of the scoring system for predicting EHM at 10 years was estimated using receiver operating characteristic (ROC) curve analysis [24]. The area under the curve (AUC) was then calculated for measuring the overall prediction accuracy. The predictive ability was evaluated as “poor” when the AUC was 0.6–0.7, “fair” when it was 0.7–0.8, “good” when it was 0.8–0.9, and “excellent” when it was 0.9–1.0 [25]. A 95% confidence interval (CI) for the AUC was obtained by sampling the 589 patients for 1,000 bootstrap samples with the confidence limits calculated as the 2.5th and 97.5th percentiles. The score was assessed using leave-one-out crossvalidation in order to assess the performance in new data [26]. Specifically, the first of the 578 patients was dropped before we redid the determination of the weights for calculating a risk score. The weights were used to calculate the score for the first patient. Similarly, the second patient was dropped before its score was calculated based on the other 577 patients. The process continued until all patients had their score calculated.
The optimal cutoff value for the prediction of EHM development was determined by maximizing the Youden index, that is, sensitivity + specificity − 1, calculated from the ROC analysis. The accuracy of using this value was assessed using the sensitivity, specificity, predictive values, and likelihood ratios. Their 95% CIs were again obtained using 1,000 bootstrap samples. The cutoff value was also crossvalidated by the leave one-out method.
Results
Patient Data and Clinicopathological Characteristics of Tumors
The demographics of the 578 patients and the clinicopathological characteristics of their tumors are summarized in Table 1. The median follow-up time was 5.42 years (range, 0.12–11.70 years) and the median time to EHM was 1.20 years (range, 0.12–8.59 years). Two hundred fifty patients had intrahepatic recurrences alone and 136 patients presented with extrahepatic metastases prior to or simultaneously with intrahepatic recurrence. Of these, pathological examination of the extrahepatic lesions was performed in 15 patients and both types of EHM were identified in six patients. The organs involved with EHM were the lung (n = 57), lymph node (n = 27), abdominal cavity (n = 22), bone (n = 18), brain (n = 8), adrenal gland (n = 5), and other less common sites (n = 5).
Table 1.
Summary of patient demographics and clinical characteristics
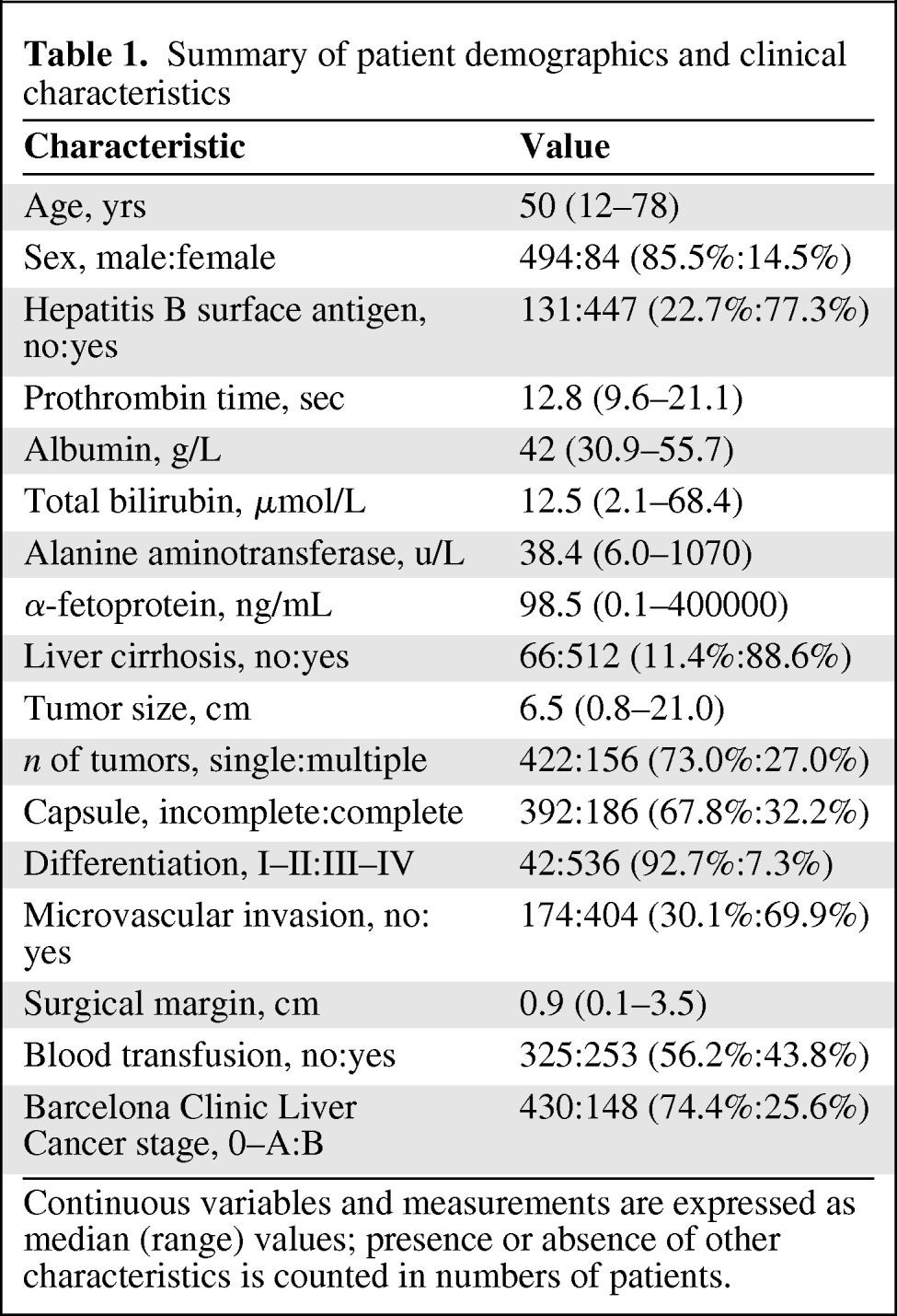
Continuous variables and measurements are expressed as median (range) values; presence or absence of other characteristics is counted in numbers of patients.
The overall survival rates at 1, 3, 5, and 10 years were 71.7%, 24.2%, 10.0%, and 2.6% for patients with extrahepatic metastases and 84.2%, 44.9%, 34.1%, and 15.7% for those with only intrahepatic recurrence, respectively. The differences in survival rates between the two groups were significant at all time points (p = .001), indicating that the prognosis of patients with EHM was significantly poorer than that of patients with intrahepatic recurrence alone.
Factors Associated with a Higher Risk for EHM
Table 2 shows the results of the univariate comparison and multivariate analysis with the Cox proportional hazard competing-risks regression model. Univariate comparison showed that elevated serum AFP (≥400 ng/mL), larger tumor diameter, a tumor with incomplete or no capsule, the presence of microvascular invasion (MVI), and BCLC stage B were all statistically associated with a higher risk for EHM. Multivariate analysis showed that MVI, a tumor with incomplete capsule or no capsule, and larger diameter tumors were independent factors.
Table 2.
Factors associated with extrahepatic metastasis
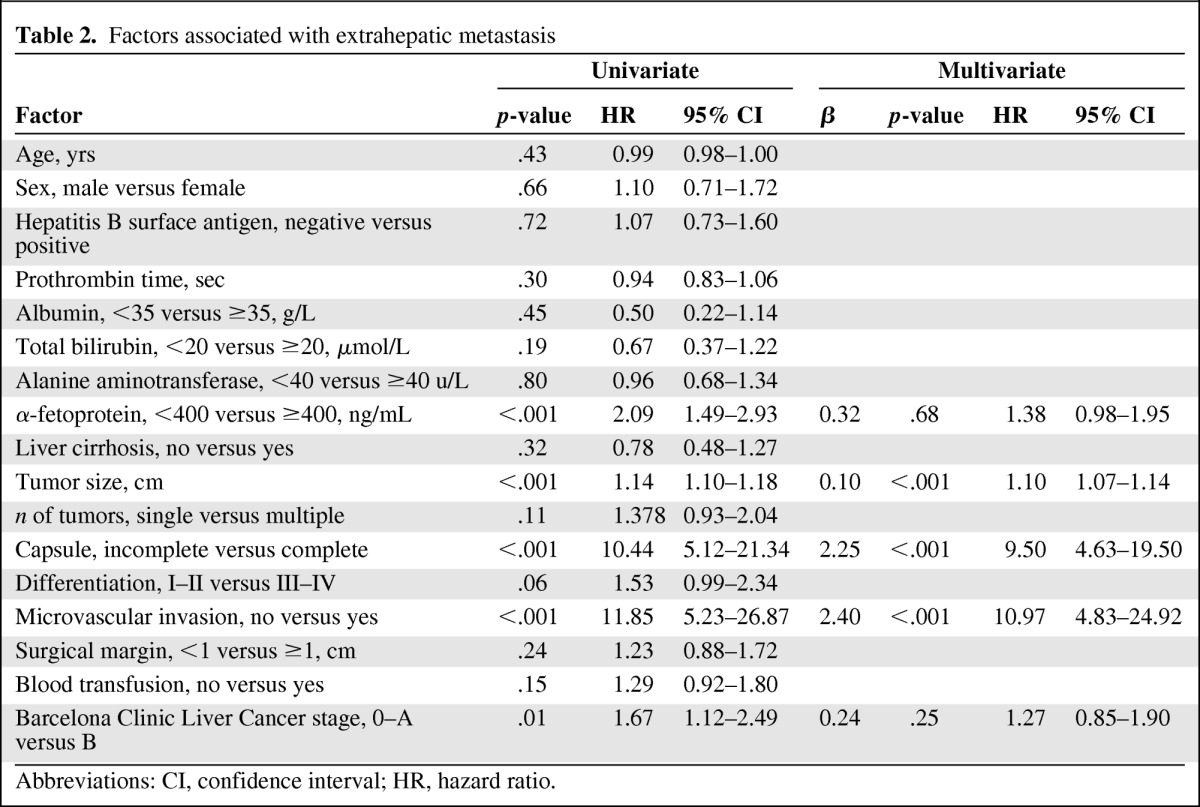
Abbreviations: CI, confidence interval; HR, hazard ratio.
Prediction Scoring System for EHM
A predictive system that integrated all the significant independent factors was formulated as 26 × MVI (presence = 1, absence = 0) + 25 × capsule (incomplete = 1, complete = 0) + diameter (in cm).
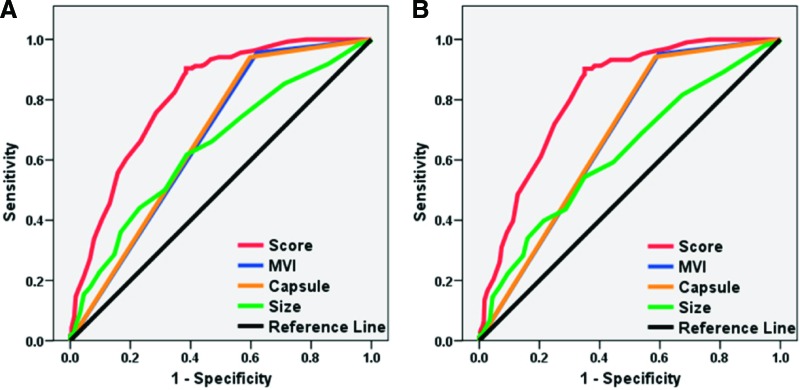
In this scoring system, the hazard ratio (HR) for EHM after resection was 1.10 (95% CI, 1.08–1.12; p < .001), indicating that the risk for developing EHM increases by 10% when the score value increases by one. The AUC was 0.81 (95% CI, 0.81–0.90). The accuracy of this system in predicting the 10-year risk for EHM was evaluated as “good” based on the predictive ability criteria. After optimizing with Youden's index, the optimal cutoff value was determined as 43, suggesting a higher risk for EHM in patients with a score ≥43. The high sensitivity and specificity of this cutoff value in predicting EHM were validated using leave-one-out validation (Table 3). To further confirm that this scoring system is more accurate in assessing the risk for EHM than any single independent factor alone, the AUC of EHM prediction using this system was compared with those using each of the single factors. The AUCs of all three risk factors individually were <0.70, all smaller than the AUC of scoring system (p < .01) (Fig. 2A).
Table 3.
Predictive accuracies for extrahepatic metastasis using the scoring system
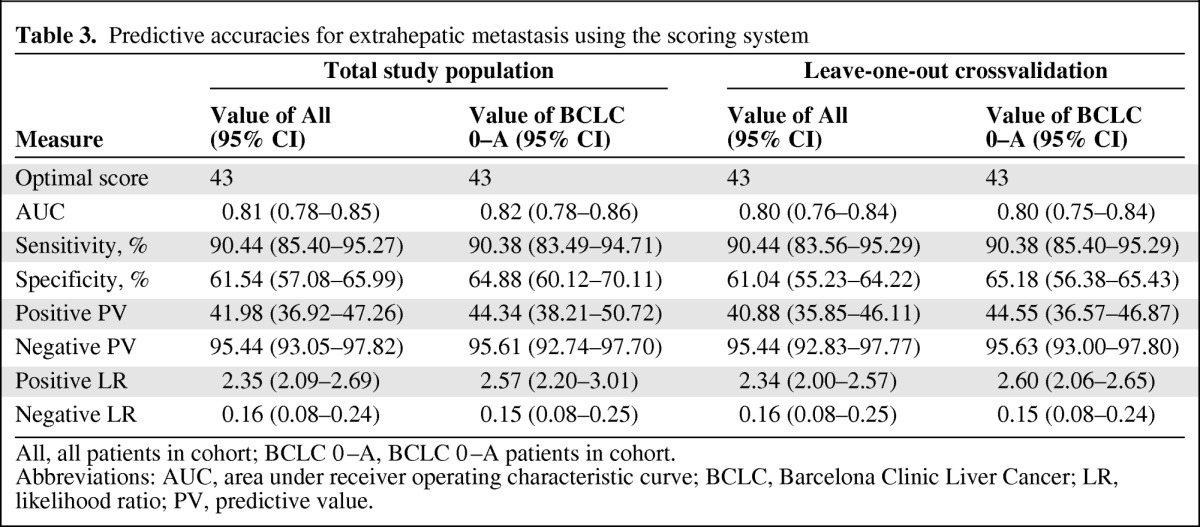
All, all patients in cohort; BCLC 0–A, BCLC 0–A patients in cohort.
Abbreviations: AUC, area under receiver operating characteristic curve; BCLC, Barcelona Clinic Liver Cancer; LR, likelihood ratio; PV, predictive value.
Figure 2.
Receiver operating characteristic curve of the scoring system and the single risk factors in predicting extrahepatic metastasis. The area under the curve for the predictive system was largest in all patients (A) and in Barcelona Clinic Liver Cancer stage 0–A patients (B).
Abbreviation: MVI, microvascular invasion.
Evaluation of the Scoring System in BCLC Stage 0–A Patients
There were 430 BCLC stage 0–A patients in our cohort, and our scoring system was also evaluated by predicting the postoperative risk for EHM in these patients. When the cutoff value was set at 43, the scoring system yielded an AUC of 0.82, a sensitivity of 90.38%, and a specificity of 64.88% in predicting EHM (Table 3). The AUC of this scoring system was larger (p < .01) (Fig. 2B) than that of any single independent factor.
Discussion
In this study, we established a scoring system to predict the risk for EHM following curative resection of resectable HCC. Our results further showed that this scoring system performed efficiently in predicting postoperative EHM probability in patients with BCLC stage 0–A HCC, suggesting that the system might serve as a prognostic reference for early-stage HCC patients. To our knowledge, this is the first clinical scoring system for EHM prediction in HCC patients following hepatectomy.
Some of the predictive factors identified in this study have been reported previously. MVI, for example, was found to be the independent factor with the highest HR value (Table 2). MVI was correlated with intrahepatic metastasis after curative resection and it could predict a poor prognosis [27, 28]. In cancer biology, vascular invasion is a hallmark of tumor progression [29]. In our study, most patients (100 of 136) who developed EHM also had MVI; therefore, patients with MVI might be more susceptible to develop EHM after HCC resection.
Fibrous capsule resulting from expansive growth of cancer cells and condensed collagen or reticulin fibers are unique characteristics of HCC and act as a barricade preventing the spread of cancer cells [30, 31]. Although the influence of the capsule on the extrahepatic spread of HCC has not been well studied, encapsulated HCCs are generally associated with a much lower incidence of direct invasion, tumor microsatellites, and vascular invasion than nonencapsulated HCCs [32]. Consistent with this, our results also showed that an incomplete capsule or the lack of a capsule was an independent risk factor for postoperative extrahepatic HCC metastasis. In tumors with no capsule or an incomplete capsule, it is easier for cancer cells to directly contact the surrounding liver parenchyma, eventually causing the destruction of the extracellular matrix and migration into the circulation [33].
Tumor size is one of the most important predictive factors for HCC progression [34, 35]. It has been included in almost all staging systems for HCC, and it was also identified as the third risk factor in our study. In addition, tumor size may also be correlated with the other two risk factors. The presence of a capsule in HCC is negatively associated with tumor size. A capsule is normally found in 84% tumors of 2–5 cm in diameter [36]. When tumors grow to >5 cm in diameter, the capsule usually disappears and can only be detected in 45% of tumors [36, 37]. In contrast, tumor size is positively correlated with the presence of MVI. The possibility of MVI presence is higher in larger tumors [38]. All these facts may suggest the potential mechanisms by which liver cancer cells migrate extrahepatically with increasing tumor size. Nevertheless, no multicollinearity of these variables included in the present study was verified (data not show), which suggests that our regression model was fitted appropriately.
Our analysis revealed that the pathological features of HCC may be the main predictors of postoperative EHM. However, any single risk factor alone failed to yield a highly accurate prediction of postoperative EHM. For example, in our cohort, 275 patients were MVI positive, and only 31.9% of them developed EHM. Furthermore, none of the single factors yielded an AUC at the level of “fair.” Because HCC patients often possess multiple and coexisting risk factors, our scoring system, constructed with a weighted sum of independent risk factors, predicted postoperative EHM with an AUC at the level of “good.” Because all three variables could be measured on regular pathological examination, the clinician may be able to easily quantify the risk for postoperative primary malignancy of each patient using this system.
Our study is limited in that the diagnosis of EHM was mainly based on clinical examinations and imaging studies (n = 121), and thus we cannot rule out the possibility that extrahepatic primary malignancy (EHPM), non-EHM lesions of HCC might exist in our series. However, the number of patients with EHPM after HCC detected may be small. Previous studies indicated that the mean estimated prevalence of EHPM in patients with HCC was ∼5.8%, and that more than half of these EHPMs occurred prior to the diagnosis of HCC [39–41]. Therefore, the incidence of EHPM after HCC may be <3%, in which case the highest estimated number of these patients in our study would be about four. Furthermore, the following evidence could support the diagnosis of EHM from HCC. Firstly, dynamic changes in serum AFP level are helpful for diagnosis. In the patients with postoperative EHM and highly preoperative level of AFP (≥100 ng/mL) included in this study, a majority of them (66%) presented re-elevated level of AFP when their extrahepatic lesions were detected. Secondly, the clinical or imaging characteristics of most EHPMs can be used in the differential diagnosis of EHM. Finally, the most common organs involved with EHM from HCC are the lungs, adrenal gland, bone, and peritoneum, which are consistent with our results and different from the common site of EHPMs, which is frequently the stomach, colorectum, and breast [39–41]. Therefore, the low incidence of EHPM after a diagnosis of HCC and our stringent diagnostic criteria for EHM in HCC patients make the results of this study reliable.
Postoperative EHM remains a critical factor affecting the prognosis of HCC patients after curative hepatectomy. The proposed predictive scoring model in this study may serve as a useful reference for clinicians to screen high-risk populations and to explore early clinical interventions. With advances in tumor biology and translational medicine, molecular markers with clinical applicability and their combination with the clinicopathological features of the tumor may offer greater predictive power to identify patients at high risk for EHM.
Acknowledgments
This study was supported by the State Key Project on Infectious Diseases of China (No. 2008ZX10002–025 and 2012ZX10002–016 to F.S.) and the National Natural Science Foundation of China (No. 30772141 to F.S. and No. 30540068 and 30700808 to J.L.).
Li Jun, Yan Zhenlin, and Gong Renyan contributed equally to this work.
Author Contributions
Conception/Design: Feng Shen, Zhenlin Yan, Yong Xia, Kui Wang, Hongyang Wang, Mengchao Wu
Provision of study material or patients: Feng Shen, Jun Li, Zhenlin Yan, Renyan Gong, Yizhou Wang, Xuying Wan
Collection and/or assembly of data: Feng Shen, Jun Li, Zhenlin Yan, Renyan Gong, Yizhou Wang, Xuying Wan, Feng Xue, Jian Liu
Data analysis and interpretation: Feng Shen, Jun Li, Zhenlin Yan, Renyan Gong, Yizhou Wang, Xuying Wan, Feng Xue, Yong Xia, Jian Liu, Dong Wu, Lehua Shi
Manuscript writing: Feng Shen, Jun Li, Zhenlin Yan, Renyan Gong, Feng Xue, Kui Wang, Lehua Shi, Hongyang Wang, Mengchao Wu
Final approval of manuscript: Feng Shen, Jun Li, Zhenlin Yan, Renyan Gong, Jian Liu, Dong Wu, Mengchao Wu
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Lopez PM, Villanueva A, Llovet JM. Systematic review: Evidence-based management of hepatocellular carcinoma—an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–1547. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 4.Lee YT, Geer DA. Primary liver cancer: Pattern of metastasis. J Surg Oncol. 1987;36:26–31. doi: 10.1002/jso.2930360107. [DOI] [PubMed] [Google Scholar]
- 5.Hong SS, Kim TK, Sung KB, et al. Extrahepatic spread of hepatocellular carcinoma: A pictorial review. Eur Radiol. 2003;13:874–882. doi: 10.1007/s00330-002-1519-7. [DOI] [PubMed] [Google Scholar]
- 6.Katyal S, Oliver JH, 3rd, Peterson MS, et al. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Nagano H, Ota H, et al. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery. 2007;141:196–202. doi: 10.1016/j.surg.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Yoon KT, Kim JK, Kim do Y, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in detecting extrahepatic metastasis in pretreatment staging of hepatocellular carcinoma. Oncology. 2007;72(suppl 1):104–110. doi: 10.1159/000111715. [DOI] [PubMed] [Google Scholar]
- 9.Sun L, Guan YS, Pan WM, et al. Positron emission tomography/computer tomography in guidance of extrahepatic hepatocellular carcinoma metastasis management. World J Gastroenterol. 2007;13:5413–5415. doi: 10.3748/wjg.v13.i40.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakajima J, Tanaka M, Matsumoto J, et al. Appraisal of surgical treatment for pulmonary metastasis from hepatocellular carcinoma. World J Surg. 2005;29:715–718. doi: 10.1007/s00268-005-7687-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen F, Satok K, Fujinaga T, et al. Pulmonary resection for metastases from hepatocellular carcinoma. World J Surg. 2008;32:2213–2217. doi: 10.1007/s00268-008-9684-8. [DOI] [PubMed] [Google Scholar]
- 12.Shuto T, Hirohashi K, Kubo S, et al. Treatment of adrenal metastases after hepatic resection of a hepatocellular carcinoma. Dig Surg. 2001;18:294–297. doi: 10.1159/000050155. [DOI] [PubMed] [Google Scholar]
- 13.Lo CM, Lai EC, Fan ST, et al. Resection for extrahepatic recurrence of hepatocellular carcinoma. Br J Surg. 1994;81:1019–1021. doi: 10.1002/bjs.1800810730. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 15.Pinter M, Sieghart W, Graziadei I, et al. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. The Oncologist. 2009;14:70–76. doi: 10.1634/theoncologist.2008-0191. [DOI] [PubMed] [Google Scholar]
- 16.Feng YX, Wang T, Deng YZ, et al. Sorafenib suppresses postsurgical recurrence and metastasis of hepatocellular carcinoma in an orthotopic mouse model. Hepatology. 2011;53:483–492. doi: 10.1002/hep.24075. [DOI] [PubMed] [Google Scholar]
- 17.Xiang ZL, Zeng ZC, Tang ZY, et al. Potential prognostic biomarkers for bone metastasis from hepatocellular carcinoma. The Oncologist. 2011;16:1028–1039. doi: 10.1634/theoncologist.2010-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mann CD, Neal CP, Garcea G, et al. Prognostic molecular markers in hepatocellular carcinoma: A systematic review. Eur J Cancer. 2007;43:979–992. doi: 10.1016/j.ejca.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 20.Gwak GY, Jung JO, Sung SW, et al. Long-term survival after pulmonary metastatectomy of hepatocellular carcinoma: Treatment outcome or natural history? Hepatogastroenterology. 2004;51:1428–1433. [PubMed] [Google Scholar]
- 21.Taketomi A, Toshima T, Kitagawa D, et al. Predictors of extrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2010;17:2740–2746. doi: 10.1245/s10434-010-1076-2. [DOI] [PubMed] [Google Scholar]
- 22.Hastie T, Tibshirani R, Friedman J, editors. The Elements of Statistical Learning. First Edition. New York: Springer; 2001. pp. 214–217. [Google Scholar]
- 23.Yuen MF, Tanaka Y, Fong DY, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatology. 2009;50:80–88. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 25.Galen RS. Application of the predictive value model in the analysis of test effectiveness. Clin Lab Med. 1982;2:685–699. [PubMed] [Google Scholar]
- 26.Gould WW. Stata Technical Bulletin 24. College Station, TX: Stata Press; 1995. sg34: Jackknife estimation; pp. 25–29. [Google Scholar]
- 27.Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: Prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cucchetti A, Piscaglia F, Caturelli E, et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. 2009;16:413–422. doi: 10.1245/s10434-008-0232-4. [DOI] [PubMed] [Google Scholar]
- 29.Hart IR. The spread of tumors. In: Franks L, Teich N, editors. Cellular and Molecular Biology of Cancer. Oxford, U.K.: Oxford University Press; 1997. pp. 21–23. [Google Scholar]
- 30.Grigioni WF, D'Errico A, Biagini G, et al. The capsule surrounding primary liver tumors: Wherefrom its prognostic significance? Int J Cancer. 1990;45:637–643. doi: 10.1002/ijc.2910450411. [DOI] [PubMed] [Google Scholar]
- 31.Ishizaki M, Ashida K, Higashi T, et al. The formation of capsule and septum in human hepatocellular carcinoma. Virchows Arch. 2001;438:574–580. doi: 10.1007/s004280000391. [DOI] [PubMed] [Google Scholar]
- 32.Ng IO, Lai EC, Ng MM, et al. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer. 1992;70:45–49. doi: 10.1002/1097-0142(19920701)70:1<45::aid-cncr2820700108>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Iguchi T, Aishima S, Sanefuji K, et al. Both fibrous capsule formation and extracapsular penetration are powerful predictors of poor survival in human hepatocellular carcinoma: A histological assessment of 365 patients in Japan. Ann Surg Oncol. 2009;16:2539–2546. doi: 10.1245/s10434-009-0453-1. [DOI] [PubMed] [Google Scholar]
- 34.Edge SB, Carducci MA, Compton CC, et al., editors. AJCC Cancer Staging Manual. Seventh Edition. Chicago: Springer; 2009. p. 237. [Google Scholar]
- 35.Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: The BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 36.Nagao T, Inoue S, Goto S, et al. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg. 1987;205:33–40. doi: 10.1097/00000658-198701000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka T, Yamanaka N, Oriyama T, et al. Factors regulating tumor pressure in hepatocellular carcinoma and implications for tumor spread. Hepatology. 1997;26:283–287. doi: 10.1002/hep.510260205. [DOI] [PubMed] [Google Scholar]
- 38.Cucchetti A, Piscaglia F, Grigioni AD, et al. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: A pilot study. J Hepatol. 2010;52:880–888. doi: 10.1016/j.jhep.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 39.Koide N, Hanazaki K, Fujimori Y, et al. Synchronous gastric cancer associated with hepatocellular carcinoma: A study of 10 patients. Hepatogastroenterology. 1999;46:3008–3014. [PubMed] [Google Scholar]
- 40.Wong LL, Lurie F, Takanishi DM., Jr Other primary neoplasms in patients with hepatocellular cancer: Prognostic implications? Hawaii Med J. 2007;66:204, 206–208. [PubMed] [Google Scholar]
- 41.Fernandez-Ruiz M, Guerra-Vales JM, Castelbon-Fernandez FJ, et al. Multiple primary malignancies in Spanish patients with hepatocellular carcinoma: Analysis of a hospital-based tumor registry. J Gastroenterol Hepatol. 2009;24:1424–1430. doi: 10.1111/j.1440-1746.2009.05793.x. [DOI] [PubMed] [Google Scholar]