This study identifies independent factors prognostic for survival in patients with hepatocellular carcinoma diagnosed with Barcelona Clinic Liver Cancer stage C and Child-Pugh class A disease who are candidates for therapeutic clinical trials.
Keywords: Hepatocellular carcinoma, Prognosis, Clinical trial, Systemic therapy, Staging systems
Abstract
Background.
The purpose of this study was to determine the prognostic significance of clinical factors and staging systems for survival of hepatocellular carcinoma (HCC) patients who are candidates for therapeutic clinical trials.
Methods.
From December 1990 to July 2005, 236 patients with unresectable HCC were enrolled into six published phase II trials assessing various therapeutic regimens. Of these, 156 chemotherapy-naive patients with Child-Pugh class A and Barcelona Clinic Liver Cancer stage C disease were included in this analysis. Twenty-seven relevant clinical characteristics were analyzed to identify prognostic factors of survival. Beyond these prognosticators, the predictive ability of eight staging systems (the tumor–node–metastasis, Okuda, Cancer of the Liver Italian Program [CLIP], Chinese University Prognostic Index, Japanese Integrated Staging, Tokyo, National Taiwan University Risk Estimation, and Advanced Liver Cancer Prognostic System [ALCPS] score) were compared using the Akaike information criteria.
Results.
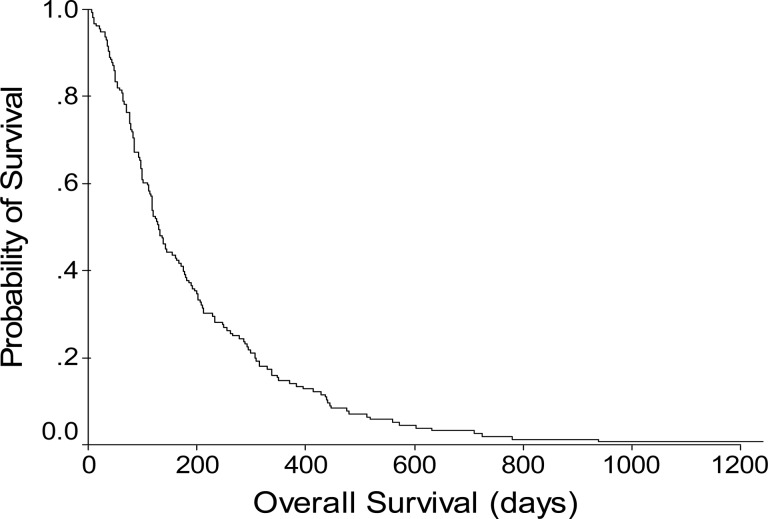
The median overall survival time was 129 days (95% confidence interval, 111–147 days). Significant predictors of a shorter overall survival time were an Eastern Cooperative Oncology Group performance status score ≥2, the presence of symptoms, ascites, an aspartate transaminase level more than two times the upper limit of normal, and regional lymph node involvement. The ALCPS and CLIP scores were superior to the other systems for predicting survival.
Conclusions.
The prognosis of patients with advanced HCC who are candidates for therapeutic clinical trials is affected by several factors related to the patient, liver function, and the tumor. The ALCPS and CLIP scores appear to be superior to the other systems for predicting survival.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer-related mortality globally [1, 2]. Surgical resection and liver transplantation may provide curative opportunities, but these options benefit <20% of HCC patients [3]. Traditionally, patients with unresectable or metastatic disease that is not suitable for locoregional therapies are candidates for systemic chemotherapy; however, conventional cytotoxic chemotherapy has shown little effect on patient survival outcomes. Recently, two large-scale, placebo-controlled, randomized, phase III studies demonstrated that sorafenib provides a survival advantage for patients with advanced HCC [4, 5]. Nevertheless, sorafenib has only modest anti-HCC activity [4, 5]. Therefore, clinical trials exploring novel therapeutic agents for the treatment of patients with advanced HCC are still urgently needed.
Prognosis prediction is important in guiding treatment options for cancer patients. Factors affecting the overall survival outcomes of HCC patients can be classified into three groups: tumor factors (such as the α-fetoprotein [AFP] level, tumor size, tumor extent, and portal vein thrombosis), liver function reserve (using Child-Pugh classification), and patient factors (age, performance status) [6]. Several staging and prognostic systems have been proposed and incorporate a variety of the above factors. Some of these systems include the tumor–node–metastasis (TNM) stage [7], Okuda stage [8], Cancer of the Liver Italian Program (CLIP) score [9], Barcelona Clinic Liver Cancer (BCLC) stage [10], Chinese University Prognostic Index (CUPI) stage [11], Japanese Integrated Staging (JIS) score [12], Tokyo score [13], National Taiwan University Risk Estimation (NATURE) score [6], and Advanced Liver Cancer Prognostic System (ALCPS) score [14]. These staging systems are derived from different patient populations and may be useful for categorizing HCC patients into various risk groups [6–15]. Of the above-mentioned staging systems, only the ALCPS score were constructed to predict the survival outcome of patients with advanced HCC.
The overall survival time of patients with unresectable HCC is short, with a median survival duration <1 year. Because HCC is a heterogeneous disease, the clinical courses of patients with similarly advanced disease are diverse. The 1-year survival rates of untreated patients with unresectable HCC in 25 randomized control trials were in the range of 10%–72% [16]. The BCLC classification stratifies patients with unresectable HCC into three categories: intermediate (stage B), advanced (stage C), and end stage (stage D). However, the survival times of patients categorized in the same BCLC stage are still widely variable. Survival-related endpoints are important elements in the design of HCC clinical trials exploring novel therapeutic agents [17]. Although homogeneous patient populations are often selected for the evaluation of new agents [16], variations in the survival outcomes of patients in clinical trials are still significant. We conducted six prospective systemic therapeutic trials (supplemental online Fig. S1) for patients with advanced HCC before the era of sorafenib [18–23]. Despite similar eligibility criteria, obvious variations in the survival outcomes of patients were observed in the six trials, with the median survival time in the range of 96–137 days (supplemental online Table S1). Therefore, factors determining the survival outcome of patients with advanced HCC who are eligible for participating in therapeutic drug trials are important in trial design and data interpretation. Nevertheless, prognostic information is scarce in the literature for this specific patient population.
The Panel of Experts in HCC-Design Clinical Trials recommended including patients with Child-Pugh class A disease and a specific BCLC stage disease in HCC clinical trials [16], and patients with BCLC stage C HCC constitute the vast majority of patients enrolled in HCC drug trials [17]. Therefore, HCC patients with BCLC stage C and Child-Pugh class A disease are an ideal target population for participating in novel therapeutic trials and can also be used to identify prognostic factors for this group of patients. The purpose of this study was to identify independent prognostic factors of survival in HCC patients diagnosed with BCLC stage C and Child-Pugh class A disease who are candidates for therapeutic clinical trials. The prognostic values of various staging systems were also explored. The authors hypothesized that better patient selection and clinical trial design may be achieved on the basis of this prognostic information.
Methods
Study Population
From December 1990 to July 2005, data from 236 patients with advanced HCC enrolled in six prospective systemic therapeutic trials (supplemental online Table S1) were reviewed [18–23]. Each of the trials was approved by the local institutional review boards and written informed consent was obtained before trial enrollment. The diagnosis of HCC was established either by histological examination or by fulfilling all the following four criteria: (a) the presence of liver cirrhosis and/or chronic viral hepatitis infection, (b) the presence of hepatic tumor(s) with imaging findings (i.e., ultrasonography, computed tomography) compatible with HCC, (c) persistent AFP elevation ≥400 ng/mL, and (d) no evidence of gastrointestinal or other primary cancer. Patients included in this study were not candidates for definitive surgical resection or local therapies of higher priority. All patients had radiographically measurable disease and acceptable bone marrow (hemoglobin ≥10 g/dL, WBC ≥3,000/μL, platelet count ≥75,000/μL), liver (bilirubin ≤4.0 mg/dL), and renal (creatinine ≤2.0 mg/dL) function. All patients included in the current analysis had Child-Pugh class A and BCLC stage C disease. Patients previously treated with systemic anticancer therapy before enrollment in the six clinical trials were excluded from this analysis.
Treatment Plan, Response Assessment, and Survival Evaluation
The protocol treatments of the six trials are shown in supplemental online Table S1. Four of the six clinical trials involved traditional chemotherapeutic agents. The first trial involved oral etoposide (VP-16) and low-dose tamoxifen (VP-16 trial) [18]. The second trial studied doxorubicin and high-dose tamoxifen (HTD trial) [19]. The third and fourth trials studied doxorubicin, high-dose tamoxifen, and interferon-α2b (I-HTD trial) and doxorubicin encapsulated with pegylated liposome (PLD trial), respectively [20, 21]. In the fifth and sixth trials, patients received oral thalidomide (Thalidomide trial) [22] or i.v. arsenic trioxide (AS2O3 trial) [23]. Each of the six study treatments were continued until disease progression or the development of intolerable toxicity.
Clinical responses were evaluated using routine history, physical examination, laboratory tests, and imaging studies, including computed tomography performed every 4–8 weeks. Objective tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) [24] in the AS2O3 trial and the World Health Organization (WHO) criteria [25] in the remaining five trials. The overall survival time was calculated from the date that the systemic anticancer therapy was started until the date of death or last follow-up. At the time of analysis (June 2011), 234 of the 236 patients (99%) had died.
Prognostic Factors and Staging Systems
Twenty-seven clinical factors relating to the patient, organ function reserve, and the tumor were evaluated to determine their prognostic value for predicting overall survival outcome. Cirrhosis was defined on the basis of either histologic or radiologic evidence. Prior local treatment included transarterial chemoembolization, local ablative therapy, and radiotherapy. Vascular invasion was determined based on imaging studies. Portal hypertension was defined as the presence of splenomegaly and thrombocytopenia (platelet count <1 × 105/μL). The TNM stage [7], Okuda stage [8], CLIP score [9], CUPI stage [11], JIS score [12], Tokyo score [13], NATURE score [6], and ALCPS score [14] were calculated for each included patient using 19 of our 27 clinical factors.
Statistical Analysis
Data analyses were performed using SAS 9.1 software (SAS Institute, Inc., Cary, NC). In statistical testing, a two-tailed p-value ≤.05 was considered statistically significant. The survival curve was plotted using the Kaplan–Meier method. The log-rank test was used for univariate analyses of the potential predictors of overall survival outcomes. Multivariate analyses were conducted to evaluate the prognostic value of the available 27 clinical factors by fitting a Cox proportional hazards model. Then, the prognostic value of each of the eight staging or scoring systems was assessed. After fitting a simple Cox proportional hazards model, their discriminatory abilities for predicting overall survival outcome were compared based on the values of the Akaike information criteria (AIC), with smaller AIC values indicating that the system was more favorable for predicting the overall survival outcome. To ensure quality of the analyses, basic model-fitting techniques for (a) variable selection, (b) goodness-of-fit assessment, and (c) regression diagnostics (including residual analysis, influence analysis, and check of multicollinearity) were used in our regression analyses.
Results
Patients Characteristics
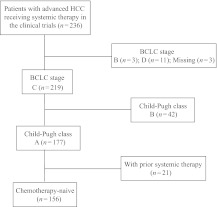
Of the 236 patients included in the six clinical trials, 156 were included in this analysis. As described in Figure 1 and Table 1, there were 126 male and 30 female patients. The median age was 52 years (range, 21–82 years). One hundred twelve (72%) patients were positive for the hepatitis B surface antigen and 26 (17%) patients had antibodies against the hepatitis C virus (HCV). A serum AFP level ≥400 ng/mL was measured in 109 (70%) patients. Portal vein thrombosis and extrahepatic metastasis was present in 79 (51%) and 93 (60%) patients, respectively. The majority of patients had tumors classified as TNM stage IV (60%), Okuda stage II–III (50%), and CLIP score 4–6 (21%). Fifty-six (36%) of the 156 patients had disease recurrence after prior surgery. Ninety-two (59%) patients underwent radiotherapy or transarterial chemoembolization prior to drug treatment.
Figure 1.
Patient flow diagram.
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; HCC, hepatocellular carcinoma.
Table 1.
Baseline demographic and clinical characteristics of patients
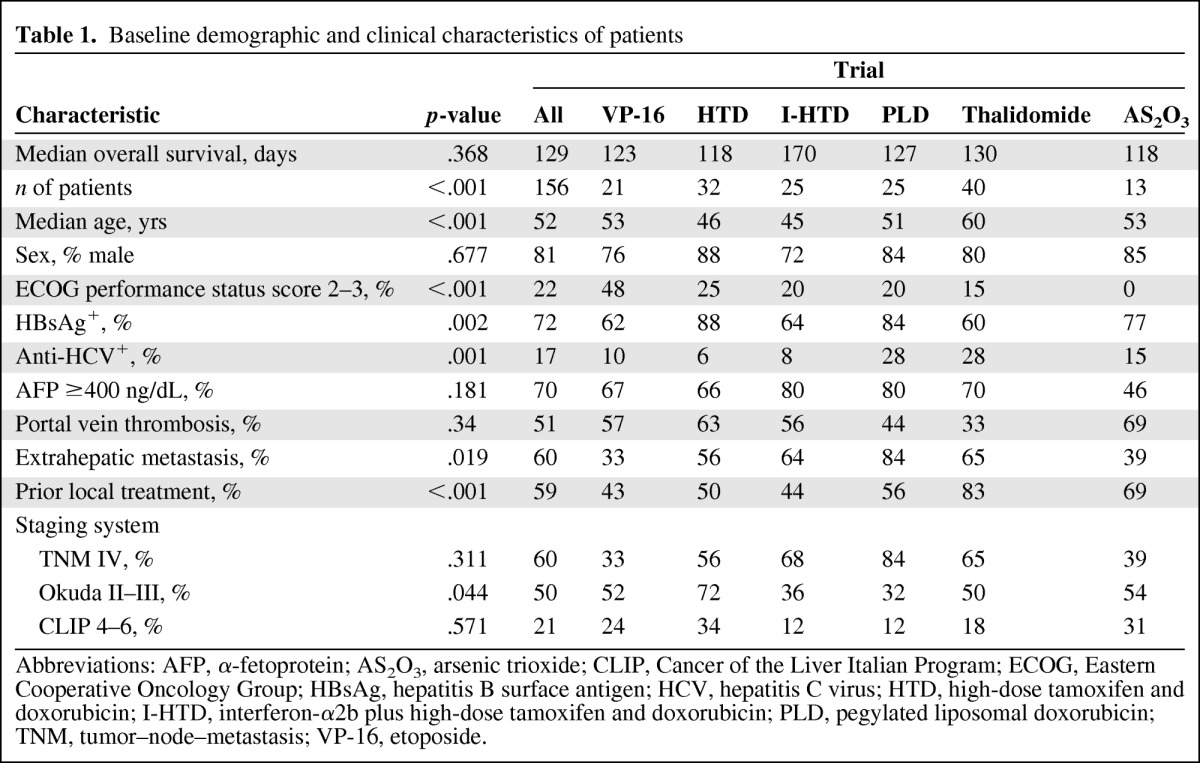
Abbreviations: AFP, α-fetoprotein; AS2O3, arsenic trioxide; CLIP, Cancer of the Liver Italian Program; ECOG, Eastern Cooperative Oncology Group; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HTD, high-dose tamoxifen and doxorubicin; I-HTD, interferon-α2b plus high-dose tamoxifen and doxorubicin; PLD, pegylated liposomal doxorubicin; TNM, tumor–node–metastasis; VP-16, etoposide.
Variation in Survival Outcomes and Baseline Clinical Characteristics
All six clinical trials had similar patient eligibility criteria. Specifically, eligible patients had advanced HCC not amenable to either surgical resection or local therapies. All included patients had an adequate performance status and adequate bone marrow, liver, and kidney function. Although the eligibility criteria restrained the heterogeneity of the enrolled patients, the survival rates of patients in these six phase II trials were significantly different (e.g., PLD versus thalidomide, p = .0376; PLD versus AS2O3, p = .0405) (supplemental online Fig. S1). The median survival in the six trials, ranged from 96 days in the PLD trial to 137 days in the I-HTD trial (supplemental online Table S1), was 119 days (Fig. 2). To further investigate the variation in patient survival outcomes in different trials, the baseline clinical characteristics of the patients were analyzed. As shown in Table 1, all selected parameters were significantly different among the six trials (despite their similar eligibility criteria) except for the male-to-female ratio, rate of AFP elevation (≥400 ng/mL), and the presence of portal vein thrombosis. Further, the stage distribution of patients was uneven among the six trials (Table 1). The percentages of patients with Okuda stage II–III disease (p = .044) were significantly different among the trials; however, the numbers of patients with advanced TNM (stage IV) and CLIP stage (score 4–6) were not statistically significant.
Figure 2.
Overall survival probability of chemotherapy-naive hepatocellular carcinoma patients with Barcelona Clinic Liver Cancer stage C and Child-Pugh class A disease in the therapeutic drug trials (n = 156).
Identification of Prognostic Factors for Survival Outcome
To explore the determinants of survival outcome for HCC patients diagnosed with BCLC stage C and Child-Pugh class A disease who were candidates for therapeutic clinical trials, the prognostic significance of the 27 clinical factors related to the patient, major organ function, and the tumor was examined using univariate analyses (Table 2). Fourteen factors were significantly associated with a worse overall survival outcome. These included an Eastern Cooperative Oncology Group (ECOG) performance score ≥2, the presence of symptoms, the presence of ascites, abdominal pain, weight loss, albumin <2.8 g/dL, ALP ≥240 IU/L, aspartate transaminase (AST) more than two times the upper limit of normal, a tumor size >5 cm, tumor extent >50% of the liver, tumor number greater than three, portal vein thrombosis, infiltrative tumor, and regional lymph node involvement (Table 2).
Table 2.
Univariate analysis of clinical factors associated with overall survival
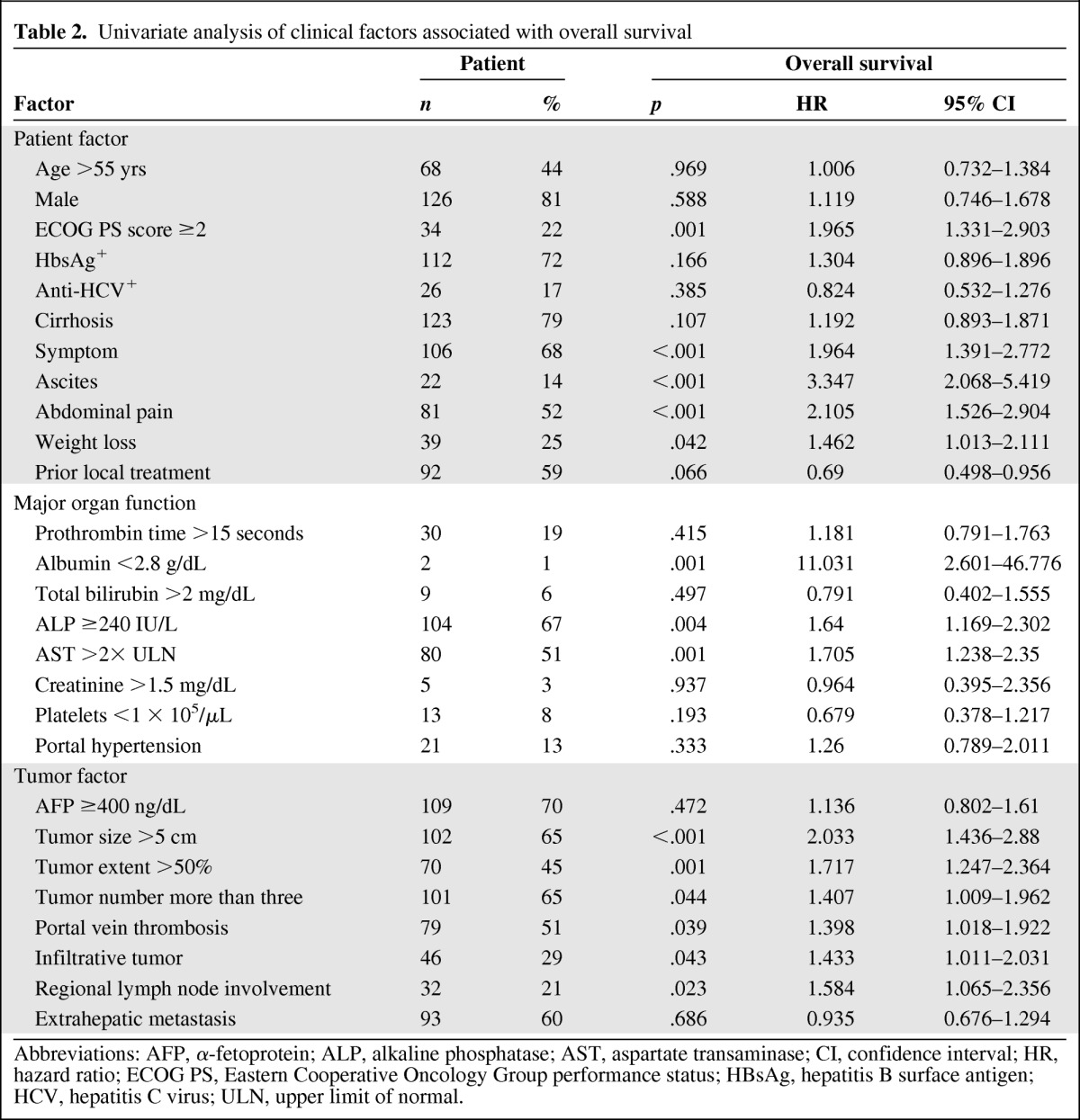
Abbreviations: AFP, α-fetoprotein; ALP, alkaline phosphatase; AST, aspartate transaminase; CI, confidence interval; HR, hazard ratio; ECOG PS, Eastern Cooperative Oncology Group performance status; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; ULN, upper limit of normal.
Multivariate analysis identified five statistically significant independent predictors of a worse overall survival outcome in the Cox proportional hazards model (Table 3). For patient factors, the presence of symptoms (hazard ratio [HR], 1.524; 95% confidence interval [CI], 1.009–2.301; p = .045) and the presence of ascites (HR, 2.79; 95% CI, 1.621–4.801; p < .001) were significant. For organ function–related factors, an AST level more than two times the upper limit of normal (HR, 1.527; 95% CI, 1.031–2.261; p = .035) was significant. For tumor factors, only regional lymph node involvement (HR, 1.997; 95% CI, 1.259–3.167; p = .003) was significant. In addition, better patient performance status (ECOG score ≤1) was associated with a better overall survival outcome (HR, 0.562; 95% CI, 0.355–0.889; p = .014).
Table 3.
Multivariate analysis of prognostic factors associated with overall survival probability assessed by fitting a Cox proportional hazards model with the stepwise variable selection method
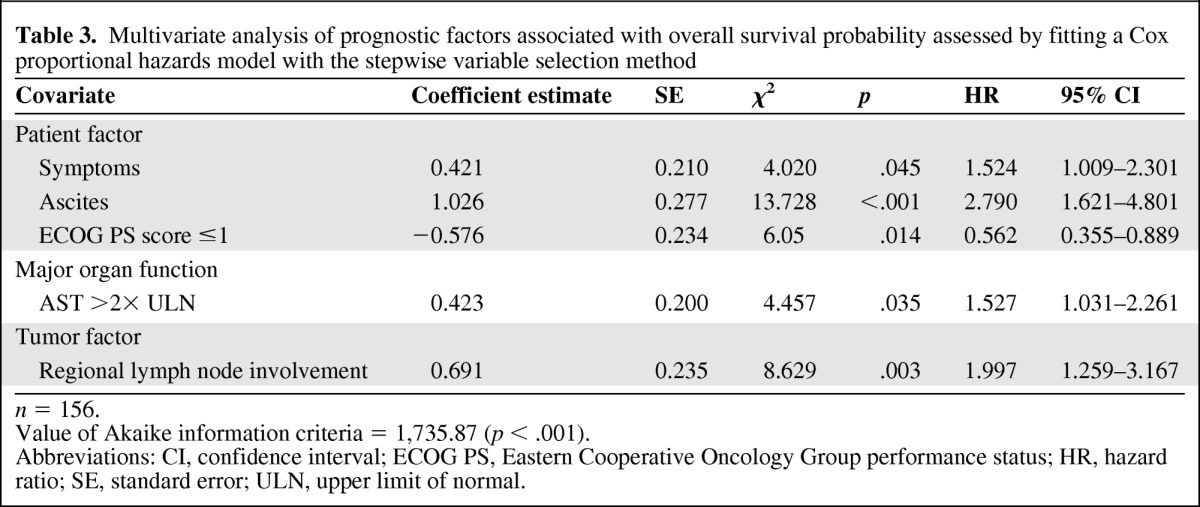
n = 156.
Value of Akaike information criteria = 1,735.87 (p < .001).
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; SE, standard error; ULN, upper limit of normal.
Prognostic Value of Staging Systems
In addition to the above-mentioned independent prognostic factors, statistical analyses were performed to establish or verify the predictive abilities of the eight staging systems that are commonly used in HCC patients. We found that the ACLPS and CLIP scores had the lowest AIC values (Table 4), indicating that they had relatively better predictive abilities for overall survival outcome than the other systems for the HCC patients included in drug trials. As shown in Table 4, three of the five identified prognostic factors in our multivariate analysis (symptoms, ascites, regional lymph node involvement) were also included in some of the staging systems. Because each staging system had some deficiencies to cover the five prognostic factors, the AIC values of these staging systems were slightly higher than that of our model (AIC, 1735.87; p < .001) (Table 3), which plausibly fitted our patients and had the best ability in predicting their survival.
Table 4.
Comparison of prognostic values among staging and scoring systems for hepatocellular carcinoma
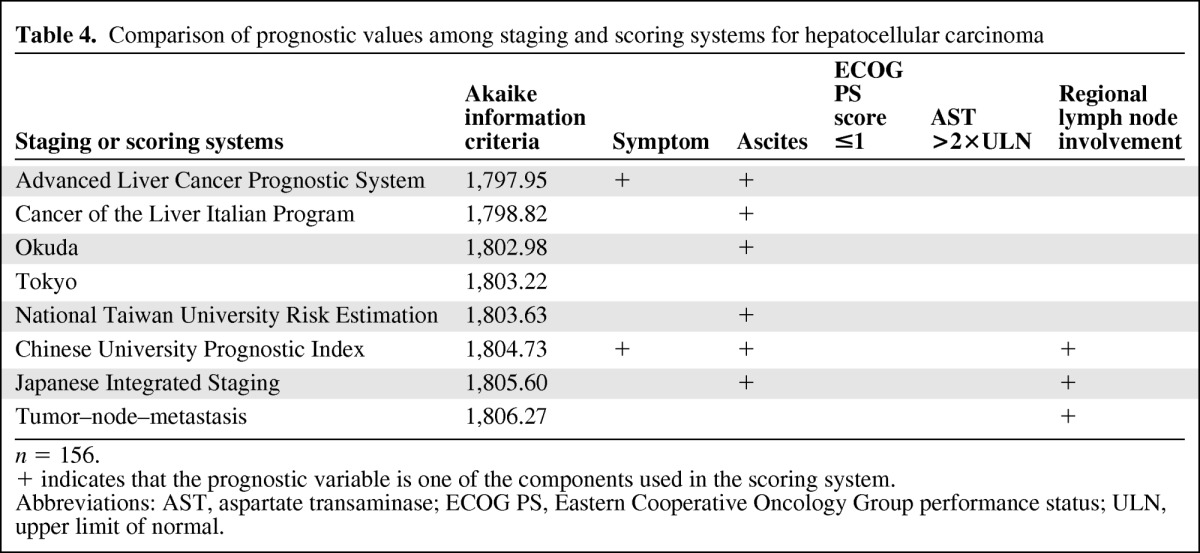
n = 156.
+ indicates that the prognostic variable is one of the components used in the scoring system.
Abbreviations: AST, aspartate transaminase; ECOG PS, Eastern Cooperative Oncology Group performance status; ULN, upper limit of normal.
Discussion
To the best of our knowledge, this is the first prognostic analysis of HCC patients with BCLC stage C and Child-Pugh class A disease enrolled in prospective clinical trials. The selected patients represent a large target population for HCC drug trials. This study cohort also represents a group of patients with relatively homogeneous clinical features, compared with other patients with HCC, which is a disease notorious for its heterogeneity.
In the present study, a variety of prognostic factors were associated with the survival outcome of patients with advanced HCC who were candidates for prospective therapeutic clinical trials. These included patient factors such as performance status (ECOG score ≤1 or >1), the presence of symptoms, and the presence of ascites; one major organ function related factor (AST more than two times the upper limit of normal); and one tumor factor (regional lymph node involvement). In addition, this study demonstrated that the ALCPS and CLIP scores were relatively better than the other commonly employed staging/scoring systems in predicting the overall survival outcome of advanced HCC patients eligible for therapeutic clinical trials.
Predicting prognosis allows the patient and physician to make decisions for optimal therapies. Nevertheless, prognostic factors for HCC patients are often challenging to establish because of the heterogeneous patient populations. Prognostic factors for HCC patients receiving surgical resection have been extensively studied before [26–33]. On the other hand, studies of prognostic factors for patients with advanced HCC are few. In a retrospective single-institutional study of 149 patients with unresectable HCC after combination chemotherapy, vascular involvement and liver cirrhosis were related to a shorter survival time [34]. Fifty (34.0%) of the 149 patients were ultimately recruited into a prospective therapeutic drug trial. In another study, including 233 patients with unresectable HCC enrolled in two phase III clinical trials of palliative chemotherapy or hormonal therapy, higher total bilirubin level and worse quality-of-life scores were associated with a shorter survival time [35]. In the current study, we found that five prognostic factors (ECOG score >1, symptoms, ascites, AST more than two times the upper limit of normal, and regional lymph node involvement) were independently associated with a worse survival outcome in patients with advanced HCC with BCLC stage C and Child-Pugh class A liver function, the most representative patient cohort for therapeutic clinical trials. Therefore, those five prognostic factors should be considered when interpreting the results of phase II clinical trials for advanced HCC.
A number of staging systems have been proposed for predicting survival outcomes and guiding therapeutic options for patients with HCC [6–14]. In this study, the ALCPS and CLIP scores were superior to the other systems in predicting the overall survival outcome of HCC patients with BCLC stage C and Child-Pugh class A disease enrolled in prospective clinical trials (Table 4). Nevertheless, the differences among the various scores were only marginal in terms of the AIC value, indicating that the predictive powers of these commonly used staging systems are not significantly distinct. Because some of the scoring systems, including the TNM, CUPI, JIS, and Tokyo systems, were established for patients treated using medical ablation or surgical resection [6], it is therefore not unexpected that the predictive power of these systems is unsatisfactory. The NATURE system was established to guide treatment options such as surgery, chemoembolization, and systemic therapy. Therefore, this staging system is especially applicable to the population of patients with early to advanced stage HCC. The predictive power of the NATURE system could be lower in a narrow spectrum of HCC patients (e.g., those with BCLC stage C and Child-Pugh class A disease). In a French study of patients with advanced HCC who were enrolled in therapeutic trials, the CLIP, BCLC, and Okuda staging systems were compared for predicting survival [36]. The CLIP score appeared to be the best staging system to predict prognosis in patients with advanced HCC receiving trial therapy in the French study [36]. Similarly, Huitzil-Melendez et al. [37] compared the accuracy at predicting survival outcome of the TNM (sixth edition), Okuda, BCLC, CLIP, CUPI, JIS, and Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire systems for patients with advanced HCC. They found that the CLIP system was again the most informative staging system.
The ALCPS is a novel prognostic system designed for HCC patients not amenable to surgical intervention, chemoembolization, or local ablative procedures and was established based on a large hepatitis B virus (HBV) prevalent Chinese population [14]. The patients included in the study used to construct ALCPS scores were similar to those in the present study because both cohorts consisted of patients with advanced HCC not suitable for local therapies but with relatively good organ function reserve. In addition, the majority of patients in both groups had chronic HBV infection. Nevertheless, the patients included in the current study tended to be younger (median age, 52 years versus 59 years), had fewer symptoms (68% versus 87%), and had better liver function (Child-Pugh class A, 100% versus 49%) because they were selected to fit the eligibility criteria for HCC drug trials. These minor differences in patient characteristics may explain why the pattern of independent risk factors predicting patient survival outcome in the current study was distinct from that of the ALCPS investigation [14].
There are several limitations to the current study. First, the prognostic factors revealed in this study may not be the same as those for patients with advanced HCC in Japan and western countries because the majority of our patients had HBV-associated HCC (72%), whereas HCV and alcoholism are the main etiologies for HCC in Japan and western countries. Another limitation of this study is the lack of a validation set of patients with advanced HCC to confirm the predictive capacity and accuracy of the prognostic factors found in the current study. Furthermore, not all the therapeutic regimens in this analysis were proven to produce survival benefits for patients with advanced HCC. The prognostic factors for sorafenib-based and other novel regimens may be largely different from those found in the current study. Nonetheless, all the patients included in this analysis were recruited into prospective clinical trials and all prognostic factors were prospectively collected. The prognostic information collected in this analysis is undoubtedly valuable for future prospective clinical trials of systemic therapies for patients with advanced HCC in Asian countries.
In summary, this study demonstrates that the overall survival probability of HCC patients with BCLC stage C and Child-Pugh class A disease in prospective clinical trials is affected by factors related to the patients themselves, their liver function, and the tumors. The ALCPS and CLIP scores appear to be mildly superior to the other staging or scoring systems for predicting the overall survival outcome in this HCC patient population. Establishment of a trial dataset including patients from different ethnic backgrounds and etiologies is required for better estimating prognosis, which is the basis of good trial design, in patients with advanced HCC receiving systemic therapy in prospective clinical trials.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The authors would like to thank Ms. Chia-Chi Cheng (National Taiwan University Hospital) for assistance with the statistical analysis.
This study was funded by grant DOH99-TD-B-111–001 from the Department of Health (Taiwan).
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Zhong-Zhe Lin, Chiun Hsu, Chih-Hung Hsu, Ann L. Cheng
Provision of study material or patients: Zhong-Zhe Lin, Chiun Hsu, Chih-Hsin Yang, Ruey-Long Hong, Chih-Hung Hsu, Ann L. Cheng
Collection and/or assembly of data: Zhong-Zhe Lin, Yu-Yun Shao, Dwan-Ying Chang
Data analysis and interpretation: Zhong-Zhe Lin, Chiun Hsu, Fu-Chang Hu, Chih-Hung Hsu, Ann L. Cheng
Manuscript writing: Zhong-Zhe Lin, Chiun Hsu, Chih-Hung Hsu, Ann L. Cheng
Final approval of manuscript: Zhong-Zhe Lin, Chiun Hsu, Fu-Chang Hu, Yu-Yun Shao, Chih-Hsin Yang, Chih-Hung Hsu, Ann L. Cheng
References
- 1.International Agency for Cancer Research: GLOBOCAN 2008. [accessed May 31, 2012]; Available at: http://globocan.iarc.fr/factsheets/cancers/liver.asp. [Google Scholar]
- 2.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: Diagnosis and treatment. Gastroenterology. 2002;122:1609–1619. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bruix J, Gores GJ. Surgical resection versus transplantation for early hepatocellular carcinoma: Clues for the best strategy. Hepatology. 2000;31:1019–1021. doi: 10.1053/he.2000.6959. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Kang Y, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen CH, Hu FC, Huang GT, et al. Applicability of staging systems for patients with hepatocellular carcinoma is dependent on treatment method—analysis of 2010 Taiwanese patients. Eur J Cancer. 2009;45:1630–1639. doi: 10.1016/j.ejca.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. Sixth Edition. Chicago, IL: Springer; 2002. pp. 1–469. [Google Scholar]
- 8.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Cancer of the Liver Italian Program (CLIP) investigators. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Brû C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 11.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: A study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 12.Kudo M, Chung H, Haji S, et al. Validation of a new prognostic staging system for hepatocellular carcinoma: The JIS score compared with the CLIP score. Hepatology. 2004;40:1396–1405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 13.Tateishi R, Yoshida H, Shiina S, et al. Proposal of a new prognostic model for hepatocellular carcinoma: An analysis of 403 patients. Gut. 2005;54:419–425. doi: 10.1136/gut.2003.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yau T, Yao TJ, Chan P, et al. A new prognostic score system in patients with advanced hepatocellular carcinoma not amendable to locoregional therapy: Implication for patient selection in systemic therapy trials. Cancer. 2008;113:2742–2751. doi: 10.1002/cncr.23878. [DOI] [PubMed] [Google Scholar]
- 15.Sala M, Forner A, Varela M, et al. Prognostic prediction in patients with hepatocellular carcinoma. Semin Liver Dis. 2005;25:171–180. doi: 10.1055/s-2005-871197. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng AL, Chen YC, Yeh KH, et al. Chronic oral etoposide and tamoxifen in the treatment of far-advanced hepatocellular carcinoma. Cancer. 1996;77:872–877. [PubMed] [Google Scholar]
- 19.Cheng AL, Yeh KH, Fine RL, et al. Biochemical modulation of doxorubicin by high-dose tamoxifen in the treatment of advanced hepatocellular carcinoma. Hepatogastroenterology. 1998;45:1955–1960. [PubMed] [Google Scholar]
- 20.Lu YS, Hsu C, Li CC, et al. Phase II study of combination doxorubicin, interferon-alpha, and high-dose tamoxifen treatment for advanced hepatocellular carcinoma. Hepatogastroenterology. 2004;51:815–819. [PubMed] [Google Scholar]
- 21.Hong RL, Tseng YL. A phase II and pharmacokinetic study of pegylated liposomal doxorubicin in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2003;51:433–438. doi: 10.1007/s00280-003-0583-2. [DOI] [PubMed] [Google Scholar]
- 22.Hsu C, Chen CN, Chen LT, et al. Low-dose thalidomide treatment for advanced hepatocellular carcinoma. Oncology. 2003;65:242–249. doi: 10.1159/000074477. [DOI] [PubMed] [Google Scholar]
- 23.Lin CC, Hsu C, Hsu CH, et al. Arsenic trioxide in patients with hepatocellular carcinoma: A phase II trial. Invest New Drugs. 2007;25:77–84. doi: 10.1007/s10637-006-9004-9. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 27.Poon RT, Fan ST. Evaluation of the new AJCC/UICC staging system for hepatocellular carcinoma after hepatic resection in Chinese patients. Surg Oncol Clin N Am. 2003;12:35–50. doi: 10.1016/s1055-3207(02)00086-8. [DOI] [PubMed] [Google Scholar]
- 28.Ueno S, Tanabe G, Nuruki K, et al. Prognosis of hepatocellular carcinoma associated with Child class B and C cirrhosis in relation to treatment: A multivariate analysis of 411 patients at a single center. J Hepatobiliary Pancreat Surg. 2002;9:469–477. doi: 10.1007/s005340200058. [DOI] [PubMed] [Google Scholar]
- 29.Chen MF, Tsai HP, Jeng LB, et al. Prognostic factors after resection for hepatocellular carcinoma in noncirrhotic livers: Univariate and multivariate analysis. World J Surg. 2003;27:443–447. doi: 10.1007/s00268-002-6708-7. [DOI] [PubMed] [Google Scholar]
- 30.Poon RT, Fan ST, Lo CM, et al. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol. 2000;18:1094–1101. doi: 10.1200/JCO.2000.18.5.1094. [DOI] [PubMed] [Google Scholar]
- 31.Poon RT, Fan ST, Ng IO, et al. Significance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann Surg. 2000;231:544–551. doi: 10.1097/00000658-200004000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grazi GL, Cescon M, Ravaioli M, et al. Liver resection for hepatocellular carcinoma in cirrhotics and noncirrhotics. Evaluation of clinicopathologic features and comparison of risk factors for long-term survival and tumour recurrence in a single centre. Aliment Pharmacol Ther. 2003;17(suppl 2):119–129. doi: 10.1046/j.1365-2036.17.s2.9.x. [DOI] [PubMed] [Google Scholar]
- 33.Wayne JD, Lauwers GY, Ikai I, et al. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235:722–730. doi: 10.1097/00000658-200205000-00015. discussion 730–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung TW, Tang AM, Zee B, et al. Factors predicting response and survival in 149 patients with unresectable hepatocellular carcinoma treated by combination cisplatin, interferon-alpha, doxorubicin and 5-fluorouracil chemotherapy. Cancer. 2002;94:421–427. doi: 10.1002/cncr.10236. [DOI] [PubMed] [Google Scholar]
- 35.Yeo W, Mo FK, Koh J, et al. Quality of life is predictive of survival in patients with unresectable hepatocellular carcinoma. Ann Oncol. 2006;17:1083–1089. doi: 10.1093/annonc/mdl065. [DOI] [PubMed] [Google Scholar]
- 36.Collette S, Bonnetain F, Paoletti X, et al. Prognosis of advanced hepatocellular carcinoma: Comparison of three staging systems in two French clinical trials. Ann Oncol. 2008;19:1117–1126. doi: 10.1093/annonc/mdn030. [DOI] [PubMed] [Google Scholar]
- 37.Huitzil-Melendez FD, Capanu M, O'Reilly EM, et al. Advanced hepatocellular carcinoma: Which staging systems best predict prognosis? J Clin Oncol. 2010;28:2889–2895. doi: 10.1200/JCO.2009.25.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.