5α-Reductase inhibitors (5ARIs) are commonly used to treat benign prostate hyperplasia. This study suggests a possible association between the use of the 5ARIs finasteride and dutasteride and the subsequent risk of prostate cancer or other cancers.
Keywords: 5α-Reductase inhibitors, Benign prostate hyperplasia, Prostate cancer
Learning Objectives:
After completing this course, the reader will be able to:
Describe the effect of finasteride use on the incidence of prostate cancer and overall cancer.
Describe the effect of dutasteride use on the incidence of renal cancer.
Explain the relationship between finasteride dosage and risk of prostate cancer and overall cancer risk.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
5α-Reductase inhibitors (5ARIs) are commonly used to treat benign prostate hyperplasia (BPH) by blocking the conversion of testosterone into the more potent dihydrotestosterone. This study explored a possible association between the use of the 5ARIs finasteride and dutasteride and the subsequent risk of prostate cancer or other cancers.
Methods.
We analyzed data from the Taiwanese National Health Insurance system. In a BPH cohort, we identified 1,489 patients with cancer and included them in our study group. For the control group, 3 patients without cancer were frequency matched with each BPH case for age, BPH diagnosis year, index year, and month. Information regarding past 5ARI use was obtained from the Taiwanese National Health Insurance Research Database (NHIRD). Multivariate logistic regression analysis was conducted, and odds ratio (OR) and 95% confidence interval (CI) were estimated.
Results.
Finasteride use marginally increased the incidence of prostate and overall cancer at a level of statistical significance (prostate cancer: OR = 1.90; 95% CI: 1.00–3.59; overall cancer: OR = 1.51; 95% CI: 1.00–2.28). Dutasteride use significantly increased kidney cancer risk (OR = 9.68, 95% CI: 1.17–80.0). Dosage analysis showed that lower doses of finasteride were associated with higher overall and prostate cancer risks. The major limitation is the lack of important data in the NHIRD, such as prostate cancer histologic grades, smoking habits, alcohol consumption, body mass index, socioeconomic status, and family history of cancer.
Conclusions.
This population-based nested case-control study suggested that finasteride use may increase prostate and overall cancer risks for patients with BPH. The effects were more prominent for patients using lower doses of finasteride.
Introduction
Benign prostate hyperplasia (BPH) is the most common prostate disease in aging men, and patients may suffer considerably from related urinary symptoms. Androgens play an essential role in prostatic growth and development [1], but they also contribute to prostate disease pathogenesis [2]. One of the major roles of 5α-reductase is to convert testosterone into the more potent dihydrotestosterone and then enhance the androgenic signal in tissues. Finasteride and dutasteride are two well-known 5α-reductase inhibitors (5ARIs) that are commonly used to treat BPH by blocking this conversion. Both agents result in similar prostate gland volume reduction and symptom improvement. Both agents also achieve reductions in the long-term risk of BPH development for symptom progression, acute urinary retention, and BPH-related surgery [3].
A currently debated issue is the use of 5ARIs to prevent prostate cancer [4]. Two landmark large randomized clinical trials, namely the Prostate Cancer Prevention Trial (PCPT) with finasteride and the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, aroused great interest in the public health field [5, 6]. These studies revealed a 23%–25% reduction in prostate cancer incidence when participants used 5ARIs; however, the medication was also found to be associated with an absolute increase in high-grade prostate cancer. Because of this concern, the U.S. Food and Drug Administration has not approved 5ARIs as a chemopreventive agent for prostate cancer.
Globally, a large number of men with BPH are treated with 5ARIs. Thus, even a small magnitude of benefit or hazard has important clinical implications. A large population-based study may help to clarify the controversy. The results from a post hoc analysis of the data in the REDUCE study confirmed the value of dutasteride treatment for reducing the risk of prostate cancer in Japanese men [7]. We were interested in exploring this issue further, so we conducted the current study using data from the National Health Insurance (NHI) system of Taiwan. We analyzed the risk for prostate cancer, as well as other individual cancers and overall cancer risk.
Methods
This study used data retrieved from Taiwan's National Health Research Institute, which is responsible for managing the NHI Research Database (NHIRD). The NHIRD contains all reimbursement claim records from 1996 to 2009 for 1 million randomly selected representative insurance holders. Details of this population-based database have been published previously [8].
In this nested case-control study, we first identified patients who had been newly diagnosed with BPH (ICD-9-CM: 600.xx) and followed up between 1996 and 2009; these patients were included in our exposure cohort. We then excluded from this group patients who had been newly diagnosed with cancer (ICD-9-CM 140–208) before January 1, 2007. We selected patients who were newly diagnosed with cancer between 2007 and 2009 as our study sample and used the date of cancer diagnosis as the patient's index date. To create the comparison group, each cancer case was matched with three randomly selected NHIRD patients without cancer, with frequency matching for age, BPH year, index year, and month.
Medication use was defined as a patient having received a drug prescription during the 2 years before his index date. Medication was classified into four groups: none (control), dutasteride, finasteride, and both.
Statistical analysis
We used the χ-square test to compare the distributions of demographic characteristics and medication use between the cancer and noncancer groups. We used a multivariate logistic regression model to calculate the odds ratio (OR), namely the ratio of cancer risk for medication users to that for nonusers. Multivariate logistic regression was also used to estimate the 95% confidence interval (CI) and specific cancer risks. We furthermore estimated the cancer risk for different dosages of dutasteride and finasteride.
All analyses were performed using the SAS statistical package, version 9.1 (SAS Institute, Cary, NC). The significance level was set at p < .05.
Results
The distributions for demographic variables and medication use for the cancer and noncancer groups are shown in Table 1. Among the 1,489 patients with cancer, most were between 65 and 84 years of age (71.0%). The mean age was 72.5 ± 9.3 years for the cancer group and 72.6 ± 9.5 years for the noncancer group. No statistically significant difference emerged for medication use between patients with cancer (2.2% for dutasteride, 2.6% for finasteride) versus the noncancer control group (1.6% for dutasteride, 1.8% for finasteride).
Table 1.
Demographics and medication use of noncancer and cancer groups
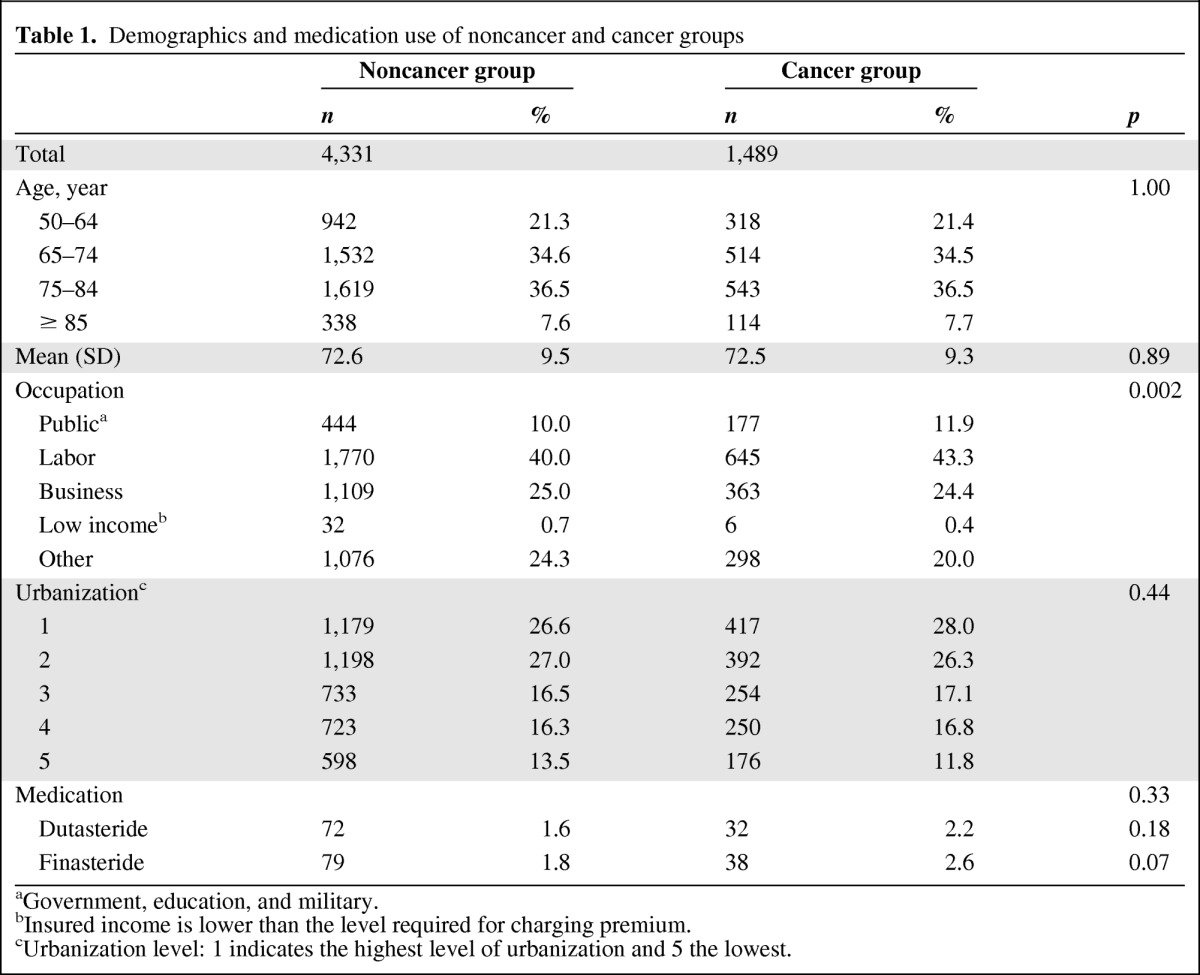
aGovernment, education, and military.
bInsured income is lower than the level required for charging premium.
cUrbanization level: 1 indicates the highest level of urbanization and 5 the lowest.
Overall, compared with patients who did not use 5ARIs, patients with BPH who were using finasteride displayed a marginal but significant increase in overall cancer risk (OR = 1.51, 95% CI: 1.00–2.28; Table 2). The cancer-specific analyses showed that finasteride users had a 1.90-fold increased risk (95% CI: 1.00–3.59) for prostate cancer. For patients who used both medications, the OR was 5.40 (95% CI: 1.17–25.0) for colorectal cancer, indicating that the combined use of the two medications was associated with a substantially increased risk. Dutasteride use was associated with a significant increase in risk for kidney cancer (OR = 9.68, 95% CI: 1.17–80.0; Table 2).
Table 2.
Adjusted odds ratio (OR) and 95% confidence interval (CI) for cancer type by medication
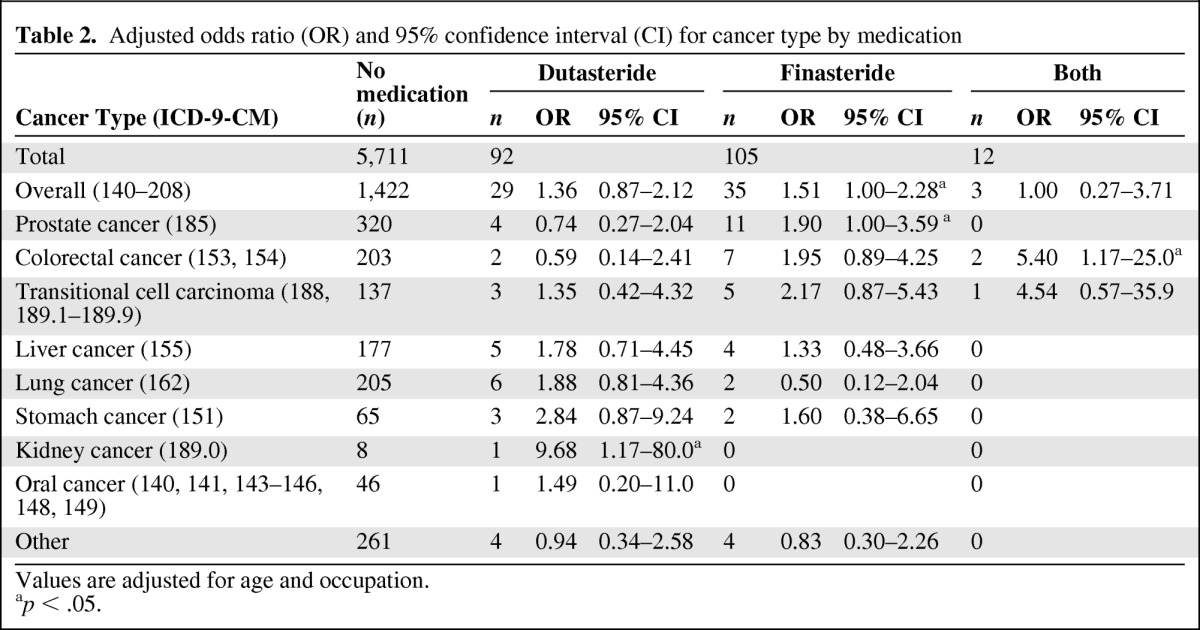
Values are adjusted for age and occupation.
ap < .05.
Furthermore, dosage analysis showed that lower rather than higher doses of finasteride were associated with higher overall cancer risk (OR = 1.96, 95% CI: 1.19–3.22) and prostate cancer risk (OR = 2.20, 95% CI: 1.04–4.65) relative to nonmedication users (Table 3).
Table 3.
Adjusted odds ratio (OR) and 95% confidence interval (CI) of cancer in patients with BPH by medication dosage
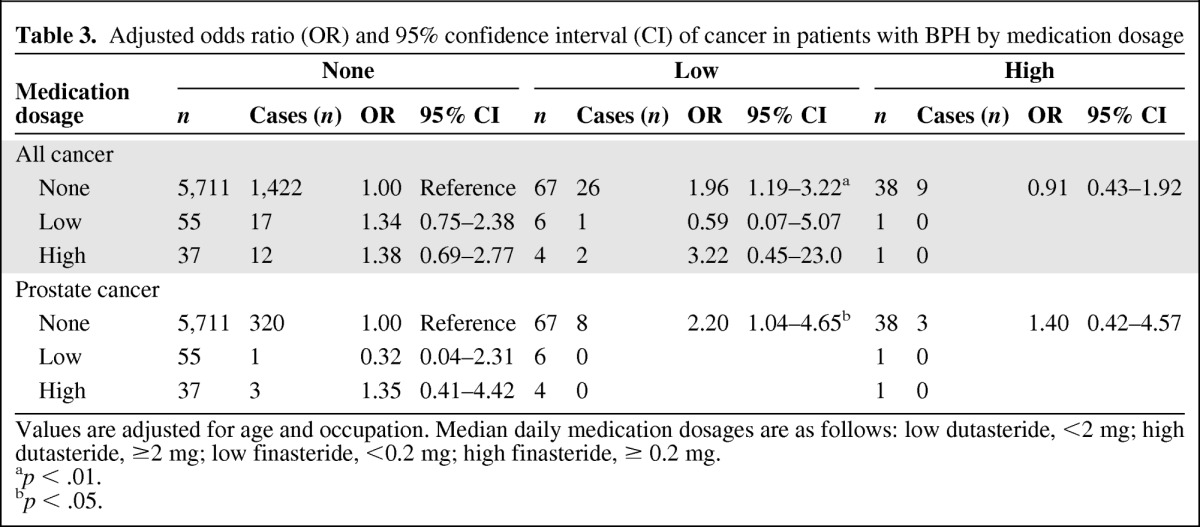
Values are adjusted for age and occupation. Median daily medication dosages are as follows: low dutasteride, <2 mg; high dutasteride, ≥2 mg; low finasteride, <0.2 mg; high finasteride, ≥ 0.2 mg.
ap < .01.
bp < .05.
Discussion
The results of the adjusted analysis from this population-based nested case-control study indicated that finasteride users had a marginal but significant increase in the risk of prostate and overall cancer. Dutasteride use was not related to prostate cancer risk, but it was associated with a significant increase in kidney cancer risk. Dosage analysis showed that lower doses of finasteride were associated with higher prostate and overall cancer risks.
Since 1982, cancer has been the leading cause of death in Taiwan. The age-adjusted incidence rate has increased steadily, and in 2007 it reached 270 new cases per 100,000 population [9]. This trend differs from the U.S. Surveillance Epidemiology and End Results data, which showed that the overall cancer incidence rates in the U.S. decreased by 0.7% per year between 1999 and 2006 [10]. Prostate cancer was the fifth most common cancer in Taiwan in 2008, with the incidence rate increasing dramatically by 15.9% between 2002 and 2006 [9]. As in other Asian countries, in Taiwan the adoption of globalized lifestyles and factors related to diet and the environment have contributed to an increase in cancer rates [11].
Taiwan's NHI program provides comprehensive health coverage to all citizens. The NHIRD contains ambulatory service records, hospital service records, and prescription claims data. The database allows researchers to select both patients and matched comparison patients who are representative of the underlying populations. We recently used the database to conduct a cohort study to evaluate the risk of malignancy for patients with end-stage renal disease. That article has been published and presents some interesting findings [12]. The current study used the same data source but with a different research design (nested case-control study) to investigate whether the use of 5ARIs was associated with an increased risk of prostate or other cancers.
Substantial evidence from previous research suggests that androgens influence the development of prostate cancer [13–16]. Studies of BPH treatment incidentally found preliminary but inconclusive evidence that the use of 5ARIs may reduce the risk of prostate cancer [17, 18]. The PCPT was the first large-scale primary chemoprevention trial conducted with men at risk for prostate cancer. Its findings suggested that finasteride effectively prevented or delayed the occurrence of prostate cancer; however, finasteride use was also found to be associated with a significant increase in the risk of high-grade prostate cancer (Gleason score 7–10) [5]. The REDUCE study later focused on dutasteride and obtained similar results. The incidence of prostate cancer was significantly reduced by the use of dutasteride, but users of this drug were at a significantly greater risk for developing high-grade cancer (Gleason score 8–10) compared with the placebo-controlled group [6]. The REDUCE researchers speculated that this scenario was due to the more frequent early detection of low-grade tumors among the placebo group. A secondary analysis of REDUCE data focused on the outcomes of BPH and found that dutasteride was associated with a decreased risk of BPH progression [19].
The 5ARIs are known to exert a prolonged adverse effect on sexual function, with erectile dysfunction or diminished libido being reported by a subset of men [20]. Extensive research has attempted to evaluate the risks and chemopreventive benefits of 5ARIs, but to date consensus is lacking on which drug is the most promising chemopreventive agent [4, 21, 22]. In addition, physicians may not readily accept the use of chemopreventive agents for prostate cancer [23].
The current study did not find a chemopreventive effect for either finasteride or dutasteride regarding prostate cancer. On the contrary, we observed more prostate cancer cases among finasteride users, with a marginal statistical significance. Unfortunately, the limitations of the data available in the NHIRD meant that we were unable to differentiate between cases of low- versus high-grade cancer. A prior Taiwanese study assessed the pathologic features of prostate cancer and found higher percentages of high-grade cancer (Gleason score 7 or higher): 90.6% and 72.9% for the largest tumor and second largest tumor, respectively [24]. The PCPT analyzed data from cases in the U.S., with the majority of patients being white. For the PCPT cancer cases, a Gleason score of 7 or higher was noted in 47.8% and 29.4% of the finasteride and placebo groups, respectively [5]. The discrepancy between our results and those of the PCPT may possibly be explained by a higher percentage of patients in our study who had Gleason scores of 7–10. If this was the case, the statistical results would have underemphasized the chemopreventive effect of finasteride on low-grade prostate cancer, while highlighting the possible risk of finasteride use in the development of high-grade prostate cancer. We also found prostate cancer to be the most common cancer among the finasteride users, accounting for 31.4% (11/35) of all cancer cases in this group. In contrast, only 22.5% (320/1,422) of patients with cancer who were not treated with 5ARI (control group) had prostate cancer. The obvious difference in the prostate cancer risk between the two groups paralleled the difference in overall cancer risk between the two groups.
The other unexpected finding among our patients with BPH was a significantly higher risk for kidney cancer in the dutasteride users. The kidneys express 5α-reductase type I [25], and an as-yet undetermined mechanism may account for the relationship between dutasteride use and kidney cancer risk. However, when we visually inspected the original data, we could identify only eight cases of kidney cancer in the no-5ARI group and only one case in the dutasteride group. When the number of cases is so small, even minimal effects influence the statistical results dramatically, leading to potentially spurious conclusions. In addition, we could not find any descriptions or discussions in the literature on plausible mechanisms for a cause-and-effect relationship between dutasteride use and kidney cancer. We thus concluded that the statistical difference between our study and control groups for kidney cancer incidence was likely to be a spurious finding.
The same problem was evident in our findings for the incidence of colorectal cancer among the 5ARI users. Both finasteride and dutasteride appeared to be associated with a significant increase in risk of colorectal cancer. However, these results were statistically unreliable because of the extremely small sample size (n = 12), with a minimal number of colorectal cancer cases (n = 2; Table 2).
Another finding of the current research was that lower doses (<0.2 mg per day) rather than higher doses of finasteride were related to the risk of prostate cancer and all other cancers (Table 3). This finding suggests that the possible chemopreventive effect of finasteride requires the medication to be administered in higher doses to counterbalance the risk factor associated with lower doses.
Strengths of the current study included its use of population-based data and NHIRD records rather than self-reported drug use. However, certain limitations should be mentioned. First, important data were missing from the NHIRD, including the histologic grade of prostate cancer and detailed demographic information such as smoking habits, alcohol consumption, body mass index, socioeconomic status, and family history of cancer. These are major risk factors for multiple cancers and may be indirectly associated with 5ARI use. However, because the NHIRD covers a highly representative sample of Taiwan's general population and the reimbursement policy is universally the same, it is unlikely that these factors would have affected the prescription of 5ARIs.
Second, the evidence derived from a nested case-control study is generally lower in quality than that from randomized trials because a nested case-control study design is subject to many biases related to adjustments for confounding variables. Despite our meticulous study design with adequate control of confounding factors, a key limitation is that bias could remain if unmeasured or unknown confounders were present.
Third, the diagnoses recorded in NHI claims primarily serve the purpose of administrative billing and do not undergo verification for scientific purposes. We were unable to contact the patients directly to enquire on their use of 5ARIs because all beneficiaries listed on the NHIRD are protected by anonymity. Furthermore, our analysis was unable to consider prescriptions for 5ARIs issued before 1996. This omission could have led to an underestimation of the cumulative dosage and may have weakened the observed association. However, data on the prescription of 5ARIs and cancer diagnosis were reliable.
In conclusion, this population-based nested case-control study did not find a chemopreventive role of 5ARIs for prostate cancer. On the contrary, our results suggested that low doses of finasteride may actually increase the risk of prostate cancer. Possible underlying mechanisms for such an association have yet to be investigated and identified. Further large population-based studies or large-scale randomized clinical trials to confirm our findings are mandatory before any definite conclusions can be drawn.
See the accompanying commentary on pages 888–890 of this issue.
Acknowledgments
This work was supported by the China Medical University Hospital (DMR-100-110 and DMR-101-080), the Taiwan Department of Health Clinical Trial and Research Center for Excellence (DOH101-TD-B-111-004), and the Taiwan Department of Health Cancer Research Center for Excellence (DOH101-TD-C-111-005).
Author Contributions
Conception/Design: Ji-An Liang, Chia-Hung Kao, Li-Min Sun, Ming-Chia Lin, Shih-Ni Chang, Fung-Chang Sung, Chih-Hsin Muo
Provision of study material or patients: Ji-An Liang, Chia-Hung Kao, Li-Min Sun, Ming-Chia Lin, Shih-Ni Chang, Fung-Chang Sung, Chih-Hsin Muo
Collection and/or assembly of data: Ji-An Liang, Chia-Hung Kao, Li-Min Sun, Ming-Chia Lin, Shih-Ni Chang, Fung-Chang Sung, Chih-Hsin Muo
Data analysis and interpretation: Ji-An Liang, Chia-Hung Kao, Li-Min Sun, Ming-Chia Lin, Shih-Ni Chang, Fung-Chang Sung, Chih-Hsin Muo
Manuscript writing: Ji-An Liang, Chia-Hung Kao, Li-Min Sun, Ming-Chia Lin, Shih-Ni Chang, Fung-Chang Sung, Chih-Hsin Muo
Final approval of manuscript: Chia-Hung Kao, Li-Min Sun, Ming-Chia Lin, Shih-Ni Chang, Fung-Chang Sung, Chih-Hsin Muo, Ji-An Liang
References
- 1.Pritchard CC, Nelson PS. Gene expression profiling in the developing prostate. Differentiation. 2008;76:624–640. doi: 10.1111/j.1432-0436.2008.00274.x. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PS, Clegg N, Arnold H, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci U S A. 2002;99:11890–11895. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6:S31–S39. [PMC free article] [PubMed] [Google Scholar]
- 4.Theoret MR, Ning YM, Zhang JJ, et al. The risks and benefits of 5α-reductase inhibitors for prostate-cancer prevention. N Engl J Med. 2011;365:97–99. doi: 10.1056/NEJMp1106783. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 6.Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 7.Akaza H, Kanetake H, Tsukamoto T, et al. Efficacy and safety of dutasteride on prostate cancer risk reduction in Asian men: the results from the REDUCE study. Jpn J Clin Oncol. 2011;41:417–423. doi: 10.1093/jjco/hyq221. [DOI] [PubMed] [Google Scholar]
- 8.Lu JF, Hsiao WC. Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff (Millwood) 2003;22:77–88. doi: 10.1377/hlthaff.22.3.77. [DOI] [PubMed] [Google Scholar]
- 9.Cancer Statistics Annual Report. Taiwan Cancer Registry. [Accessed November 16, 2011]. Available at http://tcr.cph.ntu.edu.tw/main.php?Page=N2.
- 10.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sim HG, Cheng CW. Changing demography of prostate cancer in Asia. Eur J Cancer. 2005;41:834–845. doi: 10.1016/j.ejca.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 12.Liang JA, Sun LM, Yeh JJ, et al. The association between malignancy and end-stage renal disease in Taiwan. Jpn J Clin Oncol. 2011;41:752–757. doi: 10.1093/jjco/hyr051. [DOI] [PubMed] [Google Scholar]
- 13.Ross RK, Bernstein L, Lobo RA, et al. 5-Alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet. 1992;339:887–889. doi: 10.1016/0140-6736(92)90927-u. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Stampfer MJ, Krithivas K, et al. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci U S A. 1997;94:3320–3323. doi: 10.1073/pnas.94.7.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsing AW, Reichardt JK, Stanczyk FZ. Hormones and prostate cancer: current perspectives and future directions. Prostate. 2002;52:213–235. doi: 10.1002/pros.10108. [DOI] [PubMed] [Google Scholar]
- 16.Rittmaster RS. 5alpha-reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab. 2008;22:389–402. doi: 10.1016/j.beem.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Andriole GL, Guess HA, Epstein JI, et al. Treatment with finasteride preserves usefulness of prostate specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo controlled clinical trial. Urology. 1998;52:195–202. doi: 10.1016/s0090-4295(98)00184-8. [DOI] [PubMed] [Google Scholar]
- 18.Andriole GL, Bautista M, Crawford D, et al. Prostate cancer (CAP) detection in the medical therapy of prostatic symptoms (MTOPS) trial. Abstract presented at: American Urological Association Annual Meeting; April 26–May 1 2003; Chicago, IL. [Google Scholar]
- 19.Roehrborn CG, Nickel JC, Andriole GL, et al. Dutasteride improves outcomes of benign prostatic hyperplasia when evaluated for prostate cancer risk reduction: secondary analysis of the REduction by DUtasteride of prostate Cancer Events (REDUCE) trial. Urology. 2011;78:641–646. doi: 10.1016/j.urology.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 20.Traish AM, Hassani J, Guay AT, et al. Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8:872–884. doi: 10.1111/j.1743-6109.2010.02157.x. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton RJ, Freedland SJ. 5-α reductase inhibitors and prostate cancer prevention: where do we turn now? BMC Med. 2011;9:105. doi: 10.1186/1741-7015-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parekh DJ. Prostate cancer prevention with 5 alpha-reductase inhibitors. Recent Results Cancer Res. 2011;188:109–114. doi: 10.1007/978-3-642-10858-7_9. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton RJ, Kahwati LC, Kinsinger LS. Knowledge and use of finasteride for the prevention of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:2164–2171. doi: 10.1158/1055-9965.EPI-10-0082. [DOI] [PubMed] [Google Scholar]
- 24.Chuang AY, Chang SJ, Horng CF, et al. Study of prostate cancer pathologic features in Chinese populations. Urology. 2007;69:915–920. doi: 10.1016/j.urology.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 25.Gormley GJ. Finasteride: a clinical review. Biomed Pharmacother. 1995;49:319–324. doi: 10.1016/0753-3322(96)82658-8. [DOI] [PubMed] [Google Scholar]


