The influence of the discrepancy between the Oncologic Drugs Advisory Committee (ODAC) and European Medicines Agency positions on bevacizumab prescribing practice for metastatic breast cancer in Austria during January 2006 to June 2011 was investigated. The Austrian bevacizumab prescribing practice was found to be significantly influenced by the ODAC statement issued in July 2010.
Keywords: Bevacizumab, Breast cancer, EMA, FDA, Oncologic Drugs Advisory Committee, Prescription Practice
Abstract
Background.
Results of trial E2100 led to the accelerated approval of bevacizumab as first-line therapy for patients with metastatic breast cancer (MBC) in the U.S. in February 2008. Based on results from subsequent trials, the U.S. Food and Drug Administration Oncologic Drugs Advisory Committee (ODAC) issued a statement proposing to withdraw the license for bevacizumab in July 2010, whereas bevacizumab approval for MBC was not withdrawn in Europe.
In this nationwide survey, we investigated the influence of the discrepancy between the ODAC and European Medicines Agency (EMA) positions on the prescription practice of bevacizumab for MBC in Austria during the period January 2006 to June 2011.
Methods.
The absolute number of bevacizumab administrations for MBC patients per month in all Austrian hospitals within the mentioned time frame was retrieved from a comprehensive national database. Bevacizumab prescription numbers for other malignancies were retrieved in order to rule out that a change in bevacizumab prescribing practice might reflect general changes in Austrian health care policy.
Results.
A steady increase in bevacizumab use was seen from January 2006 to June 2010 (42 versus 1,357 administrations per month) for MBC. Thereafter, a significant decline in bevacizumab prescriptions for MBC became evident, with numbers dropping to 842 in March 2011 and 662 in June 2011. Bevacizumab prescriptions showed only minor variations in control cohorts.
Conclusions.
The Austrian bevacizumab prescribing practice in MBC patients was significantly influenced by the ODAC statement issued in July 2010, whereas the EMA position was accepted to a lesser degree.
Introduction
Tumor growth depends on malignant neoangiogenesis, which is mainly driven by vascular endothelial growth factor (VEGF) [1]. Therefore, blocking VEGF or its receptors is a rational biological treatment approach.
Bevacizumab is a 149-kDa humanized monoclonal antibody targeting VEGF-A [2]. Today, the drug is approved for the treatment of advanced adenocarcinoma of the lung, advanced renal cell cancer, advanced colorectal cancer, recurrent glioblastoma (U.S.), and advanced breast cancer (Europe).
The first randomized phase III trial of bevacizumab in breast cancer patients yielded negative results. Heavily pretreated patients with metastatic breast cancer (MBC) were randomized to bevacizumab in combination with capecitabine or capecitabine alone. The progression-free survival (PFS) interval, which was defined as the primary study endpoint, did not differ between the two treatment groups [3]. Resistance to VEGF inhibition resulting from redundant angiogenic pathways in heavily pretreated patients is believed to have caused those negative results [4]. Therefore, further development of bevacizumab was conducted in the first-line setting.
In trial E2100, patients with human epidermal growth factor receptor 2–negative MBC were randomly assigned to weekly paclitaxel with or without bevacizumab (10 mg/kg administered every 2 weeks) [5]. Here, the addition of bevacizumab led to a longer median PFS time, which was defined as the primary study endpoint, to a clinically relevant extent (5.9 months versus 11.8 months; hazard ratio [HR] for progression, 0.60; p < .001). The response rate was superior in the combination group as well (36.9% versus 21.2%; p < .001), yet no benefit in terms of the overall survival (OS) time was observed. Based upon results of trial E2100, the European Medicines Agency approved the combination of paclitaxel and bevacizumab for this indication in March 2007. Accelerated approval of bevacizumab as first-line therapy for MBC was granted in the U.S. by the Food and Drug Administration (FDA) in February 2008; for permanent approval, however, confirmatory results of further trials were demanded [6].
In this study, we investigated whether or not the ODAC statement issued in July 2010 impacted bevacizumab prescribing practice in a representative European country while the European license was still retained.
The Avastin® and Docetaxel (AVADO) trial was a prospective, randomized, placebo-controlled phase III study. Patients with chemotherapy-naïve MBC were randomized to docetaxel plus placebo, docetaxel plus low-dose bevacizumab (7.5 mg/kg body weight every 3 weeks), or docetaxel plus standard-dose bevacizumab (15 mg/kg body weight every 3 weeks). For statistical analyses, both bevacizumab arms were compared separately with the control arm. The median PFS interval (the primary study endpoint) in the standard-dose group was 10.1 months, versus 8.2 months in the control arm (HR for progression, 0.77; 95% confidence interval [CI], 0.64–0.93; p = .006). In the low-dose group, no significant effect was seen (9 months versus 8.2 months; HR, 0.86; 95% CI, 0.72–1.04; p = .12) [7]. Again, the OS time was not longer with the addition of bevacizumab to taxane-based chemotherapy.
The third first-line study, RIBBON-1 (randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer), featured a different design. Based on the investigator's choice, patients were allocated to a cohort with anthracyclines or taxanes as the chemotherapy backbone or a second group with capecitabine-based chemotherapy. In both cohorts, randomization to bevacizumab or placebo was performed. In the anthracycline or taxane group, the addition of bevacizumab led to a longer median PFS interval (the primary study endpoint), 9.2 months versus 8 months (HR, 0.64; 95% CI, 0.52–0.8; p < .0001), similar to the results of the AVADO trial [8]. A more pronounced benefit was observed in the capecitabine cohort (PFS time, 5.7 months versus 8.6 months; HR, 0.69; 95% CI, 0.56–0.84; p = .0002). In neither cohort was a significantly longer OS time found.
Based up the results of the E2100, AVADO, and RIBBON-1 trials, the combinations of paclitaxel plus bevacizumab (March 2007) as well as docetaxel plus bevacizumab (September 2009) and capecitabine plus bevacizumab (June 2011) were licensed by the European Commission (EC); approval for the docetaxel combination, however, was revoked again in March 2011 [9]. In the U.S., the relatively small benefit of bevacizumab plus chemotherapy over chemotherapy alone in the AVADO trial and in the anthracycline or taxane cohort of the RIBBON-1 trial as well as the potential side effects prompted the FDA Oncologic Drugs Advisory Committee (ODAC) to recommend complete withdrawal of the bevacizumab license for breast cancer.
In this study, we investigated whether or not the ODAC statement issued in July 2010 impacted bevacizumab prescribing practice in a representative European country while the European license was still retained [10]. Austria is a member of the European Union (EU), has a population of ∼8,400,000, and has a well-developed health care system, which provides access to all approved drugs to all citizens by a compulsory social security health insurance system. In addition, Austria, together with Spain and Switzerland, is leading in the uptake of novel anticancer drugs and very quickly responds to innovations in the field of oncology [11].
Patients and Methods
For this analysis, we retrieved the absolute number of bevacizumab administrations to breast cancer patients in all Austrian acute care hospitals from January 2006 to June 2011 from a comprehensive national health care database, which records, in a compulsory and standardized way, all bevacizumab administrations for accounting purposes [12]. Bevacizumab prescription numbers for breast cancer as well as glioblastoma, colorectal cancer, lung cancer, ovarian cancer, and renal cell cancer were assessed per month over the mentioned time period. We analyzed whether or not the ODAC statement issued in July 2010 had any significant influence on bevacizumab prescribing practice for advanced breast cancer in Austria. To this end, we compared the numbers of bevacizumab administrations in the year before the ODAC statement (July 2009 to June 2010) with the numbers in the year after the ODAC statement (July 2010 to June 2011). Because of the favorable results of trial E2100, the European license for bevacizumab in combination with paclitaxel was retained and later on expanded to include combination with capecitabine.
The numbers of bevacizumab administrations for other malignancies served as controls in order to rule out the possibility that any changes in bevacizumab prescriptions for breast cancer patients reflected general changes in Austrian health care policy.
Statistical Assessment
The effect of the ODAC statement on bevacizumab administrations was assessed with a segmented line regression model. That is, both the monthly figures of the year before the ODAC statement (July 2009 to June 2010) and those of the year thereafter (July 2010 to June 2011) were regressed on time, and we tested for any change in the corresponding regression slopes. A statistical significance level of 5% was applied. Multiple testing for six different malignancies was addressed with a Bonferroni–Holm correction.
Results
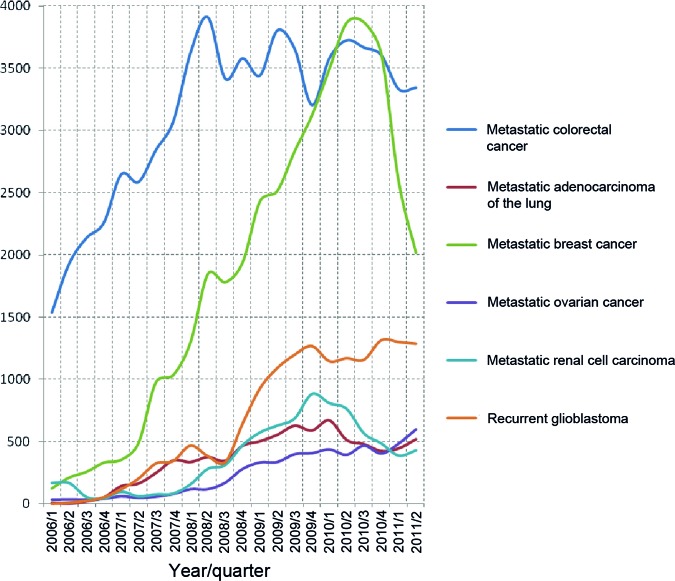
Between January 2006 and June 2011, in total, 145,969 bevacizumab administrations were recorded in Austria (metastatic colorectal cancer, 68,881; MBC, 40,977; recurrent glioblastoma, 14,744; metastatic renal cell carcinoma, 8,215; metastatic lung adenocarcinoma, 7,833; metastatic ovarian cancer, 5,318). Monthly bevacizumab administrations in patients with MBC increased steadily from January 2006 to June 2010. In June 2010, the number peaked at 1,357. Starting in July 2010, a decline in the bevacizumab prescription number was observed. In that month, the ODAC statement was issued recommending the withdrawal of the license for bevacizumab for breast cancer. In March 2011 (the time point of the EC's decision to withdraw the combination of bevacizumab with docetaxel from the label), a total of 842 bevacizumab administrations for MBC was counted, equaling 62% of the June 2010 level. Thereafter, a further decline in prescription number was observed. In June 2011, 662 administrations of bevacizumab in patients with MBC were counted in Austria, equaling 49% of the June 2010 number (see supplemental online Table 1).
With the exception of renal cell cancer, bevacizumab prescriptions per month remained stable or showed only slight variations in the control groups (recurrent glioblastoma, metastatic colorectal cancer, lung cancer, ovarian cancer, and renal cell cancer) (see supplemental online Table 1). Therefore, it is unlikely that the observed changes in bevacizumab use for MBC were caused by any general change in Austrian health care policy.
Only for renal cell cancer was a notable reduction in bevacizumab prescriptions observed as well (May 2010, time point of pazopanib approval within the EU, n = 250; June 2011, last observed data point, n = 169 [68%]). In contrast, for recurrent glioblastoma, bevacizumab administrations increased steadily over the observation period, and a slight increase was also observed for ovarian cancer. For adenocarcinoma of the lung as well as metastatic colorectal cancer, bevacizumab prescriptions remained stable after an initial increase over most of the observation period (Fig. 1).
Figure 1.
Numbers of bevacizumab administrations in January 2006 to June 2011 for different malignancies in Austrian acute care hospitals.
The difference in the monthly bevacizumab prescription number 1 year before and 1 year after the ODAC statement was statistically significant for MBC (p < .0001, segmented line regression model and Bonferroni–Holm correction), but not for any of the other five malignancies (p > .05 each, segmented line regression model and Bonferroni–Holm correction).
Discussion
Bevacizumab showed evidence of antineoplastic activity in two prospective randomized trials (the E2100 and RIBBON-1 [capecitabine cohort] trials) [5, 8]. In the AVADO trial as well as in the anthracycline or taxane cohort of the RIBBON-1 trial, the relative benefit of bevacizumab added to chemotherapy was far less pronounced, casting doubts on the value of VEGF inhibition in MBC patients. Also, bevacizumab is associated with high costs and potential side effects, such as hemorrhages, wound-healing complications, gastrointestinal perforation, and hypertension. Furthermore, critics of the use of bevacizumab for MBC repeatedly noted that bevacizumab did not alter the OS outcome in either the individual trials—with the limitation that they were not powered for survival analysis—or in a recent meta-analysis [13]. The OS time, however, may well be an elusive end point in MBC: crossover, postprogression treatment, and causes of mortality unrelated to cancer obscure the effect of treatment on OS outcomes [14].
We consider these results quite intriguing because they clearly show the considerable impact of U.S. drug licensing authorities on an international prescribing practice and predominance over the European recommendations for the first time.
Still, results in the AVADO trial and in the anthracycline or taxane cohort of the RIBBON-1 trial, potential side effects, as well as the apparent lack of a survival benefit prompted the FDA ODAC to recommend withdrawal of the license for bevacizumab for MBC in July 2010.
Although European approval initially remained unchanged, the ODAC statement caused a heated discussion concerning the role of bevacizumab in breast cancer treatment throughout the U.S. and Europe. Therefore, we aimed to investigate whether or not this debate might have influenced the prescribing practice in Europe.
As shown in this study, the absolute number of bevacizumab administrations to patients with MBC increased steadily from January 2006 to June 2010 in all Austrian acute care hospitals. However, directly after the ODAC statement was issued, a decline in the number of prescriptions was observed, with the number dropping by ∼40%, to 842 administrations, in March 2011. In March 2011, the EC removed the combination of bevacizumab and docetaxel for the first-line treatment of MBC patients from the label. Although the approvals for bevacizumab and paclitaxel were retained, a further drop in bevacizumab prescriptions was seen until June 2011 (n = 662). Whether this decline reflects the ongoing North American discussion or the EC's decision remains a matter of speculation, and no final conclusion can be drawn from our data. It is worthwhile mentioning, however, that, in June 2011, the EC decided to expand the approval to the combination of bevacizumab and capecitabine because of the favorable results of the RIBBON-1 trial.
To rule out any potential connections between bevacizumab prescription numbers and changes in general Austrian health care politics, we included bevacizumab prescription numbers for other malignancies, such as glioblastoma, colorectal cancer, lung cancer, ovarian cancer, and renal cell cancer as controls. With the notable exception of renal cell cancer, prescription numbers showed a constant increase or remained stable over the observation period. For renal cell cancer, an initial increase in bevacizumab use was followed by a decrease during the year 2010, potentially reflecting the approval of pazopanib and other tyrosine kinase inhibitors as additional first-line treatment options [15]. Therefore, we were able to rule out changes in the bevacizumab prescription practice for MBC being caused by factors other than the ODAC statement. This is further underlined by the fact that no major controlled clinical trials with regard to bevacizumab-based treatment for MBC were published in the last phase of the observation period, excluding major scientific evidence, aside from the one mentioned above, to explain the described phenomenon. Whether or not recent reports on the activity of bevacizumab in the preoperative setting will also affect prescription practice is awaited [16, 17].
Conclusions
Our data suggest that the ODAC statement issued in July 2010 had a major impact on the bevacizumab prescribing practice for MBC in Austria, although until March 2011, no change in the license of bevacizumab for MBC was implemented within the EU. This notable change also took place despite rapidly increasing experience with bevacizumab over previous years, without the occurrence of any previously undetected acute or long-term side effects and without any new background financial pressure from the health care system. Given the usually rapid uptake of innovations in the field of oncology, the change in prescription behavior in Austria is even more impressive. This prescription behavior seems to reflect a major influence of the discussion of the topic in important peer-reviewed medical journals [18]. We consider these results quite intriguing because they clearly show the considerable impact of U.S. drug licensing authorities on an international prescribing practice and predominance over the European recommendations for the first time. Interestingly, and in line with our findings, a recent report showed a discrepancy between the Italian Medicine Agency's approval status for bevacizumab and the clinical use of this drug for metastatic colorectal cancer in Lomardy, Italy [19]. Implementation of an international drug evaluation committee with stringent criteria for drug licensing recommendations should therefore be considered.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Matthias Preusser, Rupert Bartsch, Gerhard Fülöp
Provision of study material or patients: Matthias Preusser, Rupert Bartsch, Gerhard Fülöp
Collection and/or assembly of data: Matthias Preusser, Rupert Bartsch, Gerhard Fülöp, Anna Sophia Berghoff, Harald Heinzl
Data analysis and interpretation: Matthias Preusser, Rupert Bartsch, Gerhard Fülöp, Anna Sophia Berghoff, Harald Heinzl, Guenther G. Steger, Richard Greil, Christoph C. Zielinski
Manuscript writing: Matthias Preusser, Rupert Bartsch, Gerhard Fülöp, Anna Sophia Berghoff, Harald Heinzl, Guenther G. Steger, Richard Greil, Christoph C. Zielinski
Final approval of manuscript: Matthias Preusser, Rupert Bartsch, Gerhard Fülöp, Anna Sophia Berghoff, Harald Heinzl, Guenther G. Steger, Richard Greil, Christoph C. Zielinski
Section Editors' Note:
As part of an on-going engagement, we encourage the submission of articles that promote the discussion of European-related issues, framed within the context of the global oncology community. This month's European Perspective examines the influence of American guidelines on Austrian prescription practices with regard to the use of bevacizumab in the first line treatment of metastatic breast cancer (MBC). Bevacizumab received accelerated approval by both the FDA and the EMA, based on the original E2100 randomised trials (paclitaxel +/- bevacizumab), but the relatively small benefit of bevacizumab plus chemotherapy over chemotherapy alone in the subsequent phase III trials AVADO and RIBBON, coupled with potential side effects, prompted ODAC to recommend the withdrawal of the bevacizumab licence in MBC. The EMA has, however, maintained the licence for paclitaxel plus bevacizumab and capecitabine plus bevacizumab combinations in Europe, although approval for docetaxel plus bevacizumab combination was rescinded in March 2011.
In this context, Preusser et al. have critically investigated the prescription patterns of bevacizumab in Austria, a representative European country significantly dedicated to innovation in oncology. The intriguing results indicate that despite an active license in Europe, Austrian cancer healthcare providers have been influenced by the ODAC statement, with a validated decline in bevacizumab use in MBC from the time of the ODAC statement (July 2010) and subsequent further decline following the EMA withdrawal of the docetaxel plus bevacizumab combination (March 2011). Thus, the debate both in Europe and worldwide on the ODAC statement is reflected in a change in prescription practice in a representative European country. It would be interesting to investigate if the trend observed in Austria is reflected in other European countries. The recent publication in this journal of the practice of prescribing bevacizumab in the Lombardy region of Italy [1] adds to this important cancer healthcare debate. Both the Italian study and, in particular, the Preusser et al. publication provide very relevant data showing how regulatory issues and informed scientific debate can influence drug prescription practices. Further data of this kind, particularly in the context of the potential influence of bevacizumab in the preoperative setting, will help both to inform the debate and to help guide clinical decision making in this disease.
Reference
1. Bonifazi M, Rossi M, Moja L et al. Bevacizumab in clinical practice: Prescribing appropriateness relative to national indications and safety. The Oncologist 2012;17:117-124.
References
- 1.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 2.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 4.Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute. FDA Approval for Bevacizumab. [accessed December 11, 2011]. Available at http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab.
- 7.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 8.Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 9.Roche Global. Media Release: European Commission Continues to Support Avastin in Combination with Paclitaxel as an Effective Treatment Option for Women with Metastatic Breast Cancer. [accessed December 10, 2011]. Available at http://www.roche.com/media/media_releases/med-cor-2011-03–02.htm.
- 10.U.S. Food and Drug Administration. Food and Drug Administration Oncologic Drugs Advisory Committee: Avastin (Bevacizumab)/Genentech, Inc. [accessed December 11, 2011]. Available at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM221998.pdf.
- 11.Wilking N, Jönsson B. Stockholm, Sweden: Karolinska Institutet; 2005. [accessed January 10, 2012]. A Pan-European Comparison Regarding Patient Access to Cancer Drugs. Available at http://ki.se/content/1/c4/33/52/Cancer_Report.pdf. [Google Scholar]
- 12.Gesundheit Österreich GmbH. [accessed December 10, 2011]. Available at http://www.goeg.at.
- 13.O'Shaughnessy J, Miles D, Gray R, et al. A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC) [abstract 1005] J Clin Oncol. 2010;28(suppl 15):115S. [Google Scholar]
- 14.Saad ED, Katz A, Hoff PM, et al. Progression-free survival as surrogate and as true end point: Insights from the breast and colorectal cancer literature. Ann Oncol. 2010;21:7–12. doi: 10.1093/annonc/mdp523. [DOI] [PubMed] [Google Scholar]
- 15.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 16.Bear HD, Tang G, Rastogi P, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012;366:310–320. doi: 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Minckwitz G, Eidtmann H, Rezai M, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 18.D'Agostino RB., Sr Changing end points in breast-cancer drug approval—the Avastin story. N Engl J Med. 2011;365:e2. doi: 10.1056/NEJMp1106984. [DOI] [PubMed] [Google Scholar]
- 19.Bonifazi M, Rossi M, Moja L, et al. Bevacizumab in clinical practice: Prescribing appropriateness relative to national indications and safety. The Oncologist. 2012;17:117–124. doi: 10.1634/theoncologist.2011-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.