Abstract
Background and Aims
The combination of polyamine and prostaglandin E2 (PGE2) synthesis inhibitors reduced the risk of colorectal adenoma (CRA) by 70% in patients that received polypectomies. We studied the effects of the combination of difluoromethylornithine (DFMO) and sulindac on biomarkers and investigated factors that might modify efficacy of these drugs.
Methods
We analyzed rectal mucosal levels of polyamines (spermidine, spermine, and putrescine) and PGE2, treatment regimens, and risk of CRA in 267 participants of a phase IIb/III trial of DFMO/sulindac for prevention of CRA recurrence.
Results
In the group that received DFMO/sulindac, the spermidine to spermine ratio (Spd:Spm) in rectal mucosa decreased between baseline and the 12- and 36-month follow-up examinations (0.30, 0.23, and 0.24, respectively; P < 0.001 for both comparisons to baseline). Putrescine levels decreased between baseline and 12 months (0.46 to 0.15 nmol/mg protein; P < 0.001) but rebounded between 12 and 36 months (0.15 to 0.36 nmol/mg protein; P = 0.001). PGE2 levels did not change, though aspirin use was significantly associated with lower baseline levels of PGE2. There were no significant associations between changes in biomarker levels and efficacy. However, drug efficacy was greatest in subjects with low Spd:Spm and high levels of PGE2 at baseline. None of these subjects developed CRA, whereas 39% of the patients that received placebo did develop CRA (P < 0.001). Efficacy was lowest in subjects with high Spd:Spm and low levels of PGE2 at baseline; 28% developed CRA, compared with 36% of the patients given placebo (P = 0.563).
Conclusions
A combination of DFMO and sulindac significantly suppressed production of rectal mucosal polyamines but not PGE2. There was no relationship between changes in biomarker levels and response. However, baseline biomarker levels modified the effect of DFMO/sulindac for CRA prevention.
Keywords: biomarkers, chemoprevention, colorectal cancer
Introduction
We recently reported on the positive results of a randomized trial of difluoromethylornithine (DFMO) plus sulindac (a nonsteroidal anti-inflammatory drug, or NSAID) in subjects with a history of colorectal adenoma (CRA)1. In the postpolypectomy setting, combination DFMO/sulindac was associated with a significant 70% risk reduction in the development of metachronous CRA, and greater than 90% risk reduction in advanced or multiple lesions, compared with double placebo.
DFMO is a suicide inhibitor of ornithine decarboxylase (ODC) that has been shown to decrease colorectal tissue polyamine levels and tumorigenesis2,4. Polyamines are produced as a critical component of cellular metabolism, acting as signaling molecules for cell growth5. The pharmacologic inhibition of polyamine synthesis results in growth arrest and prevention of tumor development in animal models5. Sulindac acts on both prostaglandin E2 (PGE2) and polyamine metabolism through inhibition of cyclooxygenase (COX)2-derived prostanoids6 and by increased cellular polyamine catabolism and export7. The role of prostaglandins in tumorigenesis is well documented5. Modulation of these pathways and their mediators, polyamines and PGE2, is strongly suspected to explain the anti-cancer activity of DFMO and NSAIDs.
In the present analysis, we examined the relationships between polyamine and PGE2 levels in normal rectal mucosa, DFMO/sulindac treatment, and development of metachronous CRA among subjects treated postpolypectomy. We hypothesized that tissue polyamine and PGE2 levels would decrease in response to DFMO/sulindac treatment as biomarkers of drug response. Secondarily, we investigated the relationships between these biomarker levels and DFMO/sulindac drug efficacy. We also explored the potential role of low dose aspirin use in these associations.
Subjects and Methods
Study population
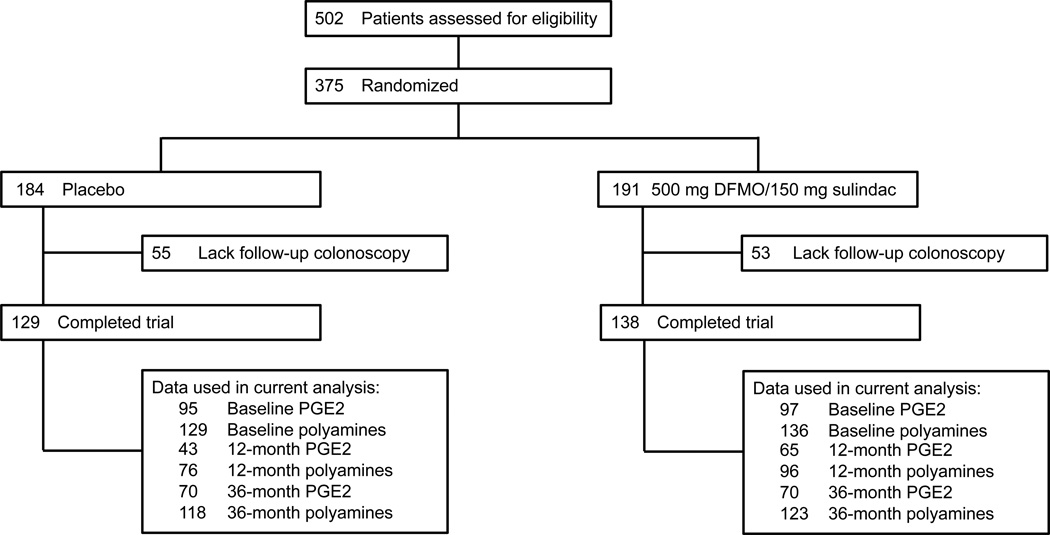
The details of our phase IIb/III clinical trial (NCT00005882, NCT00118365) of combination DFMO/sulindac have been reported1. Briefly, this double-blinded, placebo-controlled, multicenter randomized trial tested the efficacy of combination DFMO/sulindac on preventing metachronous CRA. A total of 375 participants with prior history of CRA were randomized to receive daily DFMO (500 mg) plus sulindac (150 mg) or double placebo for three years (Figure 1). Data on self-reported regular low-dose aspirin use (≤ 81 mg daily or ≤ 325 mg twice weekly) were collected at baseline; patients using full-dose aspirin (325 mg/day) were excluded from the study. The trial was terminated early by the Data and Safety Monitoring Board because efficacy endpoints had been achieved; thus, 267 participants had an end-of-study colonoscopy. Of these 267 individuals, 178 were from the Phase IIb portion of the study and 89 from the Phase III. In the Phase IIb protocol, a 12 month rectal biopsy was required for eligibility but involved a less invasive endoscopy procedure and was not a colonoscopy. This eligibility requirement was dropped for the Phase III component. Thus, subjects in the Phase IIb protocol had a 12-month endoscopy procedure specifically for rectal biopsy (n = 177 performed). The mean time to the end of study colonoscopy for the 267 subjects was 36.0 ± 4.3 months and is designated as '36 months'.
Figure 1. Study schema.
Colon biopsy sample collection
Normal (tumor-free) rectal mucosal biopsies were obtained during three separate endoscopy procedures: at baseline and after approximately 12 (phase IIB participants only) and 36 months (end of study)8. Three of eight cores were placed in separate standard minifuge tubes for polyamine analyses, and the remaining five were placed in similar tubes containing indomethacin in buffer to inhibit in vitro formation of PGE2; all eight were immediately snap frozen in liquid nitrogen.
PGE2 content
PGE2 analysis was performed as described previously9. Briefly, tissue cores that were frozen in indomethacin were thawed, homogenized in an indomethacin buffer, and clarified at low speed. PGE2 content was measured in the supernatant using the Prostaglandin E2 Biotrak enzyme immunoassay (EIA) system (GE Healthcare Life Sciences, Piscataway, NJ). Samples were assayed in duplicate at two dilutions that were ten-fold different in concentration. Nine samples were excluded from the data set due to vial mishandling. Values that fell outside the linear portion of the curve were also excluded: 41 participants (19 placebo, 22 treatment) at baseline, 19 (11 placebo, 8 treatment) at 12 months, and 51 (27 placebo, 24 baseline) at 36 months. Furthermore, values for which the coefficient of variation (CV) between duplicates was > 15% were excluded: 0 participants at baseline, 1 (placebo) at 12 months, and 1 (placebo) at 36 months. Finally, values whose associated standard curves were of low quality were excluded: 37 participants (18 placebo, 19 treatment) at baseline, 46 (24 placebo, 22 treatment) at 12 months, and 50 (31 placebo, 19 treatment) at 36 months. Among those who finished the trial, 192 participants (95 placebo, 97 treatment) had valid PGE2 measurements at baseline, along with 108 (43 placebo, 65 treatment) at 12 months and 140 (70 placebo, treatment) at 36 months. A sensitivity analysis was performed by conducting analyses with and without the excluded values. Including these values introduced noise, as would be expected; the results did not substantially differ, but generally they were less precise and the significance of the associations was diminished. The mean CV for all samples falling within the linear range (excluding all nonlinear samples and respective duplicates) was 11.3%; after excluding measurements with CV > 15%, the mean CV was 5.7%. Measures below the limit of detection were set to the lower-limit value of 0.04 ng/mg protein.
Polyamine content
Polyamine analysis was performed as described previously3. Briefly, frozen tissue cores were homogenized and extracted. Polyamine (spermidine, spermine, and putrescine) content was measured using reverse-phase, ion-paired high performance liquid chromatography. Protein contents were determined using the bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific, Rockford, IL). The same nine samples as for PGE2 were excluded from the data set due to vial mishandling. We used the spermidine-to-spermine ratio (Spd:Spm) in our analyses to minimize the influence of assay variability8. The determinations of day-to-day precision and accuracy of measurements for the polyamines were carried out based on the performance of the standards, which were separately injected at intervals of every 5 study specimens. Every study specimen was spiked with an internal standard to estimate recovery and to assess performance of the chromatography. The CV for the measurement of the internal spiked sample 1,6-diaminohexane was < 10% in all batches conducted in the study and averaged 5.45%. The CV for the measurement of spermine, spermidine, and putrescine in the standard in all runs were below 15% and averaged 5.06%, 2.82%, and 3.07%, respectively. Among those who finished the trial, 265 participants (129 treatment, 136 placebo) had valid polyamine measurements at baseline, along with 172 (76 treatment, 96 placebo) at 12 months and 241 (118 treatment, 123 placebo) at 36 months. Measures below the limit of detection were set to the lower-limit value of 0.01 nmol/mg protein.
Assessment of metachronous CRA
Endpoint colonoscopies were performed approximately 36 months (the mean time was 36.0 ± 4.3 months) after baseline colonoscopy for 267 participants3. The reports for all resected polyps were submitted for central pathology review. A person was classified as having a metachronous CRA if at least one adenomatous polyp or adenocarcinoma was diagnosed during the trial.
Statistical methods
Individuals’ tissue biomarker (PGE2, Spd:Spm, and putrescine) levels were tested for change between each pair of time points (baseline and 12 months, baseline and 36 months, 12 months and 36 months) using non-parametric paired sign-rank tests. Relative risks (RRs) and 95% confidence intervals (CIs) were calculated for the association between DFMO/sulindac treatment and CRA, stratified by baseline biomarker levels and by biomarker response. For baseline biomarker levels, we divided the population into “low” versus “high” groups according to the median of the study population. We defined biomarker response using a cut point of ≥ 30% decrease in biomarker levels between baseline and 36 months, to be consistent with standard clinical trial assessments for solid tumors10. Logistic regression was used to model the presence of metachronous CRA as the outcome variable and predictors including treatment, baseline biomarker group (or biomarker response), and interaction between treatment and baseline biomarker group (or biomarker response). Similarly, logistic regression was used to model the presence of metachronous CRA as the outcome variable and predictors including treatment, aspirin use, and interaction between treatment and aspirin use. Likelihood ratio tests were applied to test for interactions. Adjustment for age and sex did not substantially change the models, so we present unadjusted models only. Fisher’s Exact Tests were used to assess differential drug efficacy when considering two different biomarkers simultaneously. All statistical analyses were performed using Stata 11.0 (StataCorp, College Station, Texas), all reported P values are two-sided, and no adjustments were made for multiple comparisons.
Results
Baseline characteristics
The treatment arms were well-balanced on sex (76% male), age (mean 61 y), ethnicity (83% white), and self-reported regular low-dose aspirin use (39%; Table 1). There were no significant differences in any baseline characteristics between the participants who did and did not complete the trial. Additionally, there were no substantial differences in baseline measures of PGE2, Spd:Spm, or putrescine by treatment group. Aspirin users had significantly lower median PGE2 levels at baseline than non aspirin users (0.21 versus 0.42 ng/mg protein, P < 0.001; Table 2). We detected no significant differences in baseline Spd:Spm or putrescine levels according to aspirin use.
Table 1.
Baseline characteristics of clinical trial participants, by treatment group
| All randomized participants (n =375) |
Subjects who completed the trial (n = 267) |
|||
|---|---|---|---|---|
| Placebo n = 184 |
DFMO/sulindac n = 191 |
Placebo n = 129 |
DFMO/sulindac n = 138 |
|
| Male sex, n (%) | 138 (75.0) | 147 (77.0) | 94 (72.9) | 108 (78.3) |
| Age (y), mean ± SD | 61.4 ± 8.2 | 60.6 ± 8.6 | 61.4 ± 8.0 | 60.2 ± 8.5 |
| White ethnicity/race, n (%) | 157 (85.3) | 155 (81.2) | 109 (84.5) | 115 (83.3) |
| Aspirin*, n (%) | 69 (37.5) | 77 (40.3) | 50 (38.8) | 53 (38.4) |
| PGE2 (ng/mg protein), mean ± SD† PGE2 (ng/mg protein), median † |
0.67 ± 0.94 0.38 |
0.81 ± 1.32 0.33 |
0.53 ± 0.59 0.38 |
0.66 ± 0.99 0.27 |
| Spd:Spm, mean ± SD‡ Spd:Spm, median ‡ |
0.32 ± 0.09 0.29 |
0.33 ± 0.11 0.30 |
0.32 ± 0.10 0.30 |
0.32 ± 0.11 0.30 |
| Putrescine (nmol/mg protein), mean ± SD ‡ Putrescine (nmol/mg protein), median ‡ |
0.63 ± 0.55 0.50 |
0.70 ± 0.82 0.46 |
0.64 ± 0.54 0.50 |
0.69 ± 0.83 0.46 |
Abbreviations: DFMO, difluoromethylornithine; SD, standard deviation; PGE2, prostaglandin E2, Spd:Spm, spermidine-to-spermine ratio
Self-reported regular aspirin use at baseline
Sample size for PGE2 was 134 (placebo) and 135 (treatment) among all randomized participants; 95 (placebo) and 97 (treatment) among those who finished the trial
Sample size for polyamines was 184 (placebo) and 188 (treatment) among all randomized participants; 129 (placebo) and 136 (treatment) among those who finished the trial
Table 2.
Biomarker levels at baseline, by self-reported aspirin use (n = 267)
| Non-aspirin users |
Aspirin users |
P* |
|
|---|---|---|---|
| PGE2 (ng/mg protein), mean ± SD† | 0.73 ± 0.89 | 0.36 ± 0.61 | |
| PGE2 (ng/mg protein), median† | 0.42 | 0.21 | < 0.001 |
| Spd:Spm, mean ± SD‡ | 0.32 ± 0.11 | 0.33 ± 0.11 | |
| Spd:Spm, median‡ | 0.30 | 0.30 | 0.757 |
| Putrescine (ng/mg protein), mean ± SD‡ | 0.64 ± 0.72 | 0.72 ± 0.68 | |
| Putrescine (ng/mg protein), median‡ | 0.47 | 0.51 | 0.158 |
Abbreviations: PGE2, prostaglandin E2, SD, standard deviation; Spd:Spm, spermidine-to-spermine ratio
Rank-sum P value for difference in median biomarker levels between aspirin groups
Sample size for PGE2 was 122 (non-aspirin users) and 70 (aspirin users)
Sample size for polyamines was 163 (non-aspirin users) and 102 (aspirin users)
Effect of DFMO/sulindac on biomarker levels
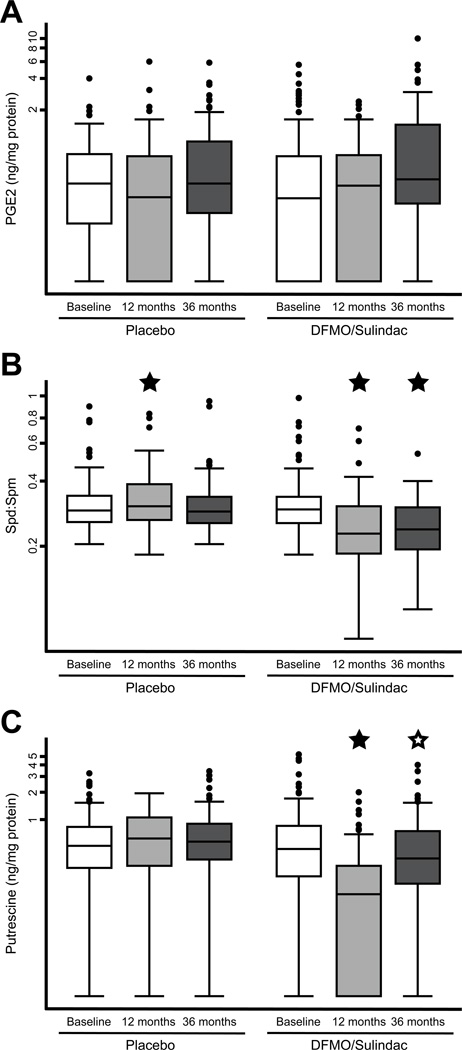
The mean (± SD) time between baseline and the 12-month rectal biopsy procedure was 13.0 ± 1.6 months, and the mean (± SD) time to the 36-month procedure was 36.0 ± 4.3 months. The mean time to the 12-month procedure in the placebo and treatment arms was 12.8 and 13.2 months, respectively (P = 0.254), and the mean time to the 36-month procedure in the two groups was 35.7 and 36.3 months, respectively (P = 0.304). We detected no change in median PGE2 levels between any pair of time points in either group (all P > 0.05; Figure 2A).
Figure 2. Biomarker levels over time, by treatment group.
Box plots are shown at three time points for normal rectal mucosal levels of (A) prostaglandin E2 (PGE2), (B) spermidine-to-spermine ratio (Spd:Spm), and (C) putrescine. Y-axes are drawn on a logarithmic scale because the distributions of these variables are right-skewed. A closed star indicates a significant difference in the median between paired values from baseline, and an open star indicates a significant difference in the median between paired values from 12 months.
Median Spd:Spm levels decreased significantly in the DFMO/sulindac group between baseline and 12 months (0.30 to 0.23, P < 0.001) and between baseline and 36 months (0.30 to 0.24, P < 0.001), but there was no significant change between 12 and 36 months (0.23 to 0.24, P = 0.638; Figure 2B). In the placebo group, Spd:Spm levels increased slightly between baseline and 12 months (0.30 to 0.31, P = 0.012), but there was no significant change between baseline and 36 months (0.30 to 0.29, P = 0.459) nor between 12 and 36 months (0.31 to 0.29, P = 0.368).
Median putrescine levels decreased significantly in the DFMO/sulindac group between baseline and 12 months (0.46 to 0.15, P < 0.001), but they increased between 12 and 36 months (0.15 to 0.36, P = 0.001; Figure 2C). The apparent rebound did not fully restore putrescine levels to baseline concentrations, but there was no significant difference in putrescine levels between baseline and 36 months (0.46 to 0.36, P = 0.108). In the placebo group, putrescine levels increased slightly between baseline and 12 months (0.50 to 0.61, P = 0.041), but there was no significant change between baseline and 36 months (0.50 to 0.55, P = 0.359) nor between 12 and 36 months (0.61 to 0.55, P = 0.634).
Effect of baseline biomarker levels on the association between treatment and metachronous CRA
We stratified the analysis of the association between DFMO/sulindac treatment and metachronous CRA by baseline biomarker levels. We found no significant differences in the effect of treatment on metachronous CRA according to individual baseline biomarker levels (all P > 0.05; Table 3). However, our results suggest possible effect modification by baseline Spd:Spm ratio (P = 0.087), whereby participants with low baseline Spd:Spm achieved a marginally significant 2.5-fold greater reduction in risk with DFMO/sulindac (RR = 0.17, 95% CI = 0.07 to 0.41) than those with high baseline Spd:Spm (RR = 0.42, 95% CI = 0.23 to 0.77). Participants with high baseline PGE2 had a similar, non significant 2.5 fold greater benefit from treatment (RR = 0.17, 95% CI = 0.05 to 0.54) than those with low baseline PGE2 (RR = 0.50, 95% CI = 0.28 to 0.91; P = 0.132). When we restricted these analyses to subjects who received their follow up colonoscopy between 33 and 39 months, our findings did not substantially change, though the interaction between treatment and baseline Spd:Spm levels on CRA became more significant (P = 0.040). Baseline polyamine and PGE2 levels were not correlated (ρ = 0.034, P = 0.639), suggesting that any effect modification of treatment response by each of these biomarkers likely act independently of each other.
Table 3.
Treatment efficacy in preventing metachronous colorectal adenoma, stratified by biomarker level at baseline
| Biomarker level at baseline* |
||||
|---|---|---|---|---|
| Low |
High |
P† |
||
| PGE2 | ||||
| Placebo, events/total (%) | 19/42 (45.2) | 21/53 (39.6) | ||
| DFMO/sulindac, events/total (%) | 12/53 (22.6) | 3/44 (6.82) | ||
| RR (95% CI) | 0.50 (0.28 – 0.91) | 0.17 (0.05 – 0.54) | 0.132 | |
| Spd:Spm | ||||
| Placebo, events/total (%) | 31/68 (45.6) | 24/61 (39.3) | ||
| DFMO/sulindac, events/total (%) | 5/64 (7.81) | 12/72 (16.7) | ||
| RR (95% CI) | 0.17 (0.07 – 0.41) | 0.42 (0.23 – 0.77) | 0.087 | |
| Putrescine | ||||
| Placebo, events/total (%) | 24/62 (38.7) | 31/67 (46.3) | ||
| DFMO/sulindac, events/total (%) | 7/70 (10.0) | 10/66 (15.2) | ||
| RR (95% CI) | 0.26 (0.12 – 0.56) | 0.33 (0.18 – 0.61) | 0.796 | |
Abbreviations: DFMO, difluoromethylornithine; PGE2, prostaglandin E2; RR, relative risk; CI, confidence interval; Spd:Spm, spermidine-to-spermine ratio
Low versus high determined by the median biomarker level: low is below the median; high is above the median
P value for the likelihood ratio test of interaction between treatment group and low versus high biomarker level at baseline
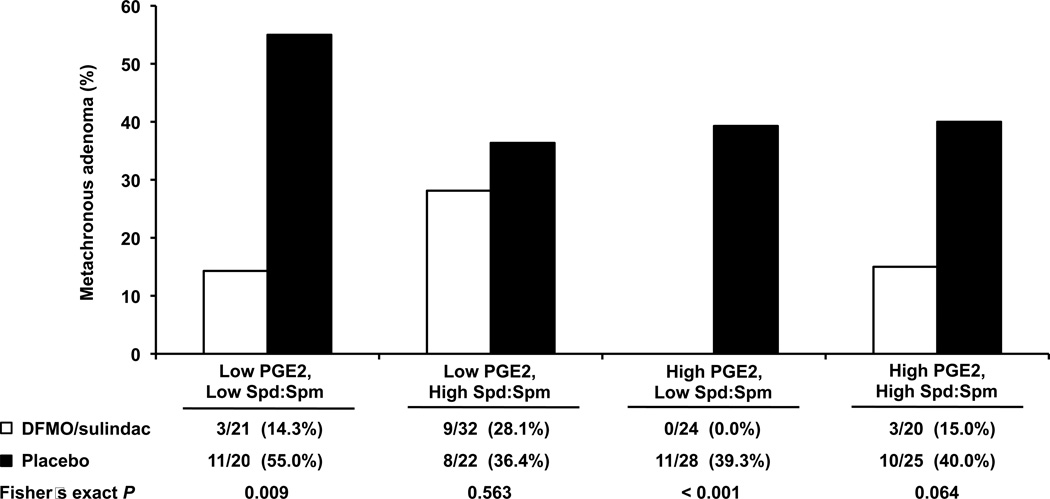
When considering these two biomarkers simultaneously, we observed an important combined effect of baseline PGE2 and Spd:Spm on treatment efficacy. Among participants with both low Spd:Spm and high PGE2, zero of 24 (0%) individuals developed CRA on treatment, compared with 11 of 28 (39%) in the placebo group (P < 0.001; Figure 3). Among those with low PGE2 and low Spd:Spm, 3 of 21 (14%) treated individuals developed CRA, versus 11 of 20 (55%) in the placebo group (P = 0.009). Among those with high PGE2 and high Spd:Spm, 3 of 20 (15%) treated individuals developed CRA, versus 10 of 25 (40%) in the placebo group (P = 0.100). Finally, among those with low PGE2 and high Spd:Spm, 9 of 32 (28%) treated individuals developed CRA, versus 8 of 22 (36%) in the placebo group (P = 0.563). Though limited by small numbers, these results suggest that baseline biomarker levels, in combination, are important determinants of DFMO/sulindac efficacy.
Figure 3. DFMO/sulindac efficacy, by biomarker levels.
Participants were categorized according to their baseline levels of prostaglandin E2 (PGE2) and the spermidine-to-spermine ratio (Spd:Spm). Low versus high cut points were defined by the median biomarker levels in the study population. The table below the figure includes the number (and percent) of participants with metachronous adenoma in each stratum. A Fisher’s exact test was conducted to test for a significant difference between the two groups in the proportion of individuals with metachronous adenoma.
Effect of changing biomarker levels on the association between treatment and metachronous adenoma
We also stratified the analysis of the association between DFMO/sulindac treatment and metachronous CRA by biomarker response. We divided the study population into two groups based on the level of biomarker response, which was defined as a decrease in biomarker level ≥30% between baseline and 36 months. For all three biomarkers (PGE2, Spd:Spm, and putrescine), we detected no significant interactions between biomarker response and DFMO/sulindac treatment on metachronous CRA (all P > 0.1; Table 4). Participants with ≥30% decrease in Spd:Spm may have achieved a greater benefit from DFMO/sulindac treatment (RR = 0.23, 95% CI = 0.11 to 0.49) than those lacking Spd:Spm response (RR = 0.49, 95% CI = 0.25 to 0.96). However, tests for interaction between Spd:Spm response and treatment on CRA were not significant (P = 0.202), even after restricting the analysis to subjects with a final colonoscopy between 33 and 39 months (P = 0.482).
Table 4.
Treatment efficacy in preventing metachronous colorectal adenoma, stratified by level of biomarker response (percent change in biomarker level between baseline and 36 months)
| Biomarker decreased by ≥ 30% (Responders) |
Biomarker increased, or decreased by < 30% (Non-responders) |
P* | ||
|---|---|---|---|---|
| PGE2 | ||||
| Placebo, events/total (%) | 4/17 (23.5) | 15/32 (46.9) | ||
| DFMO/sulindac, events/total (%) | 1/11 (9.09) | 8/35 (22.9) | ||
| RR (95% CI) | 0.39 (0.05 – 3.02) | 0.49 (0.24 – 0.99) | 0.980 | |
| Spd:Spm | ||||
| Placebo, events/total (%) | 17/41 (41.5) | 33/77 (42.9) | ||
| DFMO/sulindac, events/total (%) | 8/83 (9.64) | 8/38 (21.1) | ||
| RR (95% CI) | 0.23 (0.11 – 0.49) | 0.49 (0.25 – 0.96) | 0.202 | |
| Putrescine | ||||
| Placebo, events/total (%) | 22/46 (47.8) | 28/72 (38.9) | ||
| DFMO/sulindac, events/total (%) | 9/61 (14.8) | 7/60 (11.7) | ||
| RR (95% CI) | 0.31 (0.16 – 0.61) | 0.30 (0.14 – 0.64) | 0.886 | |
Abbreviations: DFMO, difluoromethylornithine; PGE2, prostaglandin E2; RR, relative risk; CI, confidence interval; Spd:Spm, spermidine-to-spermine ratio
P value for the likelihood ratio test of interaction between treatment group and biomarker response
Potential effect modification by aspirin
Given the recognized inhibitory action of aspirin on rectal mucosal PGE2 levels (see Table 2 and Krishnan et al.11) and suspected role in polyamine catabolism12, we investigated whether potential effect modification by self-reported low-dose aspirin use at baseline could account for differences in treatment response by subgroups in Figure 3. We detected no interaction between aspirin and DFMO/sulindac treatment on metachronous CRA (P = 0.443). Furthermore, we detected no significant interactions between aspirin and DFMO/sulindac on biomarker response (defined as ≥30% decrease in biomarker level between baseline and 36 months) for PGE2 (P = 0.582), Spd:Spm (P = 0.140), nor putrescine (P = 0.868). To explore this question further, we conducted a multivariate analysis by adjusting for aspirin use. We found no evidence that aspirin use confounds the relationships between biomarker levels and drug efficacy. Thus, despite the significant association observed between aspirin and baseline PGE2 levels, aspirin use at baseline does not appear to explain the differential drug efficacy by biomarker levels shown in Figure 3. However, this analysis is limited by its small sample size, as well as imprecise measures of aspirin exposure, and cannot rule out the potential for effect modification of DFMO/sulindac by aspirin use.
Discussion
In this study, we examined the effect of combination DFMO/sulindac treatment on rectal mucosal concentrations of polyamines and PGE2 as putative drug response biomarkers. Sulindac, an NSAID and nonselective COX inhibitor, has known inhibitory action on PGE2 as well as effects on polyamine transport5. DFMO inhibits ODC with demonstrated effects on tissue polyamine levels, particularly putrescine and spermidine13. We also explored the potential impact of self-reported low-dose aspirin use on biomarker levels, as aspirin is a commonly used NSAID with similar properties to sulindac.
Consistent with previous studies showing that DFMO treatment depletes putrescine and lowers Spd:Spm in rectal mucosa3,13, we found a significant effect of DFMO/sulindac on rectal mucosal polyamines. In the treatment group, the decline in Spd:Spm was achieved by 12 months, with no evidence of additional change between 12 and 36 months. Putrescine levels showed a similar behavior to Spd:Spm, with change achieved by 12 months. However, putrescine subsequently increased between 12 and 36 months; consequently, putrescine levels at 36 months were no longer significantly different from those at baseline. A similar pattern was observed in a prior phase IIb biomarker trial investigating tissue polyamine responses in CRA patients after random assignment to 0.075 g/m2 DFMO, 0.2 g/m2 DFMO, 0.4 g/m2 DFMO, or placebo3. In the two lower-dose DFMO groups (approximately 150 mg/day and 400 mg/day – i.e., lower than the 500 mg/day dose used in the present study), putrescine inhibition was observed at the earlier time point (6 months), but not the later time point (12 months). In contrast, putrescine levels remained suppressed at both 6 and 12 months in the highest DFMO group (approximately 800 mg/day). Effects of DFMO on putrescine levels at 36 months were not previously assessed. Our findings here in the 3-year trial strongly validate the results of the prior dose de-escalation study and support the selection of 500 mg/day as the lowest possible dose to achieve an effect on polyamine endpoints at the tissue level. Taken together, these findings support the potential for an adaptive tissue response to prolonged ODC suppression, resulting in increased cellular uptake of diet- or bacteria-derived putrescine from the colonic lumen13,14. Polyamines are an important factor in colonic mucosa renewal15; thus, prolonged inhibition of ODC with DFMO and increased export by sulindac may lead to compensatory uptake of luminal polyamines in the normal rectal mucosa.
In contrast with the polyamines, we found no measurable effect of treatment on PGE2 concentrations in rectal mucosa. Although we observed a clear difference in PGE2 levels at baseline between aspirin users and non-users that is consistent with PGE2 as a "drug-effect surrogate biomarker”11, we found no evidence of an effect of DFMO/sulindac on PGE2 at 12 nor 36 months. The reason for the lack of treatment effect on tissue PGE2 levels is unknown and should be interpreted with caution given the sample size. The lack of evidence for a strong effect of treatment on PGE2 could alternatively suggest that treatment-associated reductions in colorectal tissue polyamine contents, as shown in this report, might be more important than previously appreciated for CRA prevention with combination DFMO/sulindac, as reported earlier1. As noted, sulindac has been demonstrated to activate polyamine catabolism and export7, possibly acting to complement the effects of DFMO to reduce tissue polyamine pools. This hypothesis needs to be tested in future clinical trials.
When we evaluated the role of biomarker change and response to intervention, we found no effect of biomarker response (i.e., percent change over time) on DFMO/sulindac efficacy for any of the three biomarkers investigated. We had hypothesized that individuals who achieved benefit from treatment would demonstrate greater reductions in their polyamine or PGE2 levels. It is notable that we observed a non significant greater benefit for participants who exhibited a ≥ 30% decrease in Spd:Spm (i.e., responders) compared with non-responders.
To further explore a potential mechanistic role for PGE2 and polyamines in DFMO/sulindac treatment for CRA prevention, we stratified the association between treatment and metachronous CRA by baseline biomarker levels. We investigated whether or not baseline status of the biomarker could act as an indicator of drug response. Though we lacked sufficient power to detect statistically significant differences, we found evidence suggesting that baseline PGE2 and Spd:Spm levels may modify responsiveness to DFMO/sulindac for CRA prevention. Our findings suggest that subjects with low baseline Spd:Spm were more responsive to the chemopreventive effects of DFMO/sulindac than individuals with high baseline Spd:Spm. Interestingly, the most responsive subgroup to treatment were subjects who entered the study with both high PGE2 and low Spd:Spm in their rectal mucosal biopsy. This subgroup experienced zero recurrences, compared with 39% observed in the corresponding placebo subgroup. Strikingly, the subgroup with the exact opposite characteristics, low PGE2 and high Spd:Spm, showed no significant treatment benefit. These findings suggest that high Spd:Spm levels in tissues, which may differ among individuals as a consequence of genetic variability (e.g., ODC polymorphisms) and/or exposures to dietary or gut flora polyamines, render subjects less pharmacologically responsive to DFMO/sulindac for CRA prevention. Further, the magnitude of risk reduction for CRA appears to be reduced in individuals who do not express a COX dependent, pro-PGE2 background in the target tissue. Importantly, among individuals with low baseline Spd:Spm levels, we observed significant risk reduction with DFMO/sulindac even in the absence of high baseline PGE2, though this subgroup experienced a few “breakthrough” CRAs. Possible COX2 negativity of these few CRAs should be considered. While highly speculative, we believe that these results show an important role for polyamine metabolism in CRA risk and suggest that the combination of DFMO/sulindac is most effective when the NSAID target (COX2) is present and the polyamine levels are in a treatable range (low). In a separate analysis, we found significant metachronous CRA risk reduction ascribed to DFMO/sulindac in patients consuming low-to-moderate levels of dietary polyamines, but there was no benefit among individuals in the highest quartile of polyamine intake (Raj KP et al., unpublished results; Abstract #279; Gastrointestinal Cancers Symposium; Orlando, Florida; Jan.22 24, 2010). Taken together, these data also suggest that the ability to overcome high polyamine background levels (e.g., dietary modification to reduce polyamine intakes or other drug targets in the pathway) may lead to even greater efficacy of drug combinations directed at the polyamine/COX2 or polyamine/”other” pathways. Although these results derive from subgroup analyses, they strongly suggest a role for baseline PGE2 and polyamine status as potential drug-response predictors and warrant further investigation.
There are a number of limitations in the current study. A strong overall effect of DFMO/sulindac treatment on the development of CRA and resulting small sample size from the early termination of the trial yielded inadequate statistical power. Thus, we were limited in our ability to fully study the relationships between biomarker levels and CRA development as originally planned. Additional limitations are inherent to the measurement of rectal mucosal levels of PGE2 and polyamines, which are prone to measurement error, and, as intermediate markers, they may not accurately reflect biological effects at the CRA level. This latter issue may be particularly true for normal rectal colonocytes, which, unlike colonocytes taken from neoplastic tissues, express low levels of the DFMO and sulindac drug targets, ODC16 and COX217, respectively. Further, greater dependence of normal rectal mucosa on exogenously derived luminal polyamines18 may explain the significant increase in putrescine between 12 and 36 months and our inability to associate biomarker change in the surrogate tissue with response to intervention.
In conclusion, future studies should consider the measurement of surrogate tissue sources of Spd:Spm and PGE2 as potential baseline predictors of drug responsiveness. Investigation of urinary measures, as suggested by Kawakita et al.19, may prove more feasible in monitoring response to DFMO/NSAIDs in planned future trials than the collection of rectal mucosal biopsy specimens. Finally, although the number of metachronous CRAs in the DFMO/sulindac treatment group was low, additional study of the factors contributing to these “breakthrough” adenomas is needed.
ACKNOWLEDGEMENTS
We thank Dave Stringer and Melissa May, who were instrumental in the acquisition and review of the raw biomarker data, respectively.
Grant Support: This work was supported in part by NCI contract NO CN 85182 (FLM) and grants CA59024 (FLM), RO 1 CA88078 (FLM, CEM), P30 CA62230 (FLM), CA23074 (EWG), and CA95060 (EWG).
Abbreviations
- CI
confidence interval
- COX
cyclooxygenase
- CRA
colorectal adenoma
- DFMO
difluoromethylornithine
- ODC
ornithine decarboxylase
- RR
relative risk
- Spd:Spm
spermidine-to-spermine ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Involvement of each Author:
PAT – analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis
BCW – analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis
JAZ – critical revision of the manuscript for important intellectual content
WPC – technical support of data for clinical trial
CEM – study concept and design; critical revision of manuscript for important intellectual content
BJL – important intellectual content
FLM – study concept and design; acquisition of data; obtained funding
EWG – study concept and design; acquisition of data; obtained funding
References
- 1.Meyskens FL, Jr, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyskens FL, Jr, Emerson SS, Pelot D, et al. Dose de-escalation chemoprevention trial of alpha-difluoromethylornithine in patients with colon polyps. J Natl Cancer Inst. 1994;86:1122–1130. doi: 10.1093/jnci/86.15.1122. [DOI] [PubMed] [Google Scholar]
- 3.Meyskens FL, Jr, Gerner EW, Emerson S, et al. Effect of alpha-difluoromethylornithine on rectal mucosal levels of polyamines in a randomized, double-blinded trial for colon cancer prevention. J Natl Cancer Inst. 1998;90:1212–1218. doi: 10.1093/jnci/90.16.1212. [DOI] [PubMed] [Google Scholar]
- 4.Thomas T, Thomas TJ. Polyamine metabolism and cancer. J Cell Mol Med. 2003;7:113–126. doi: 10.1111/j.1582-4934.2003.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerner EW, Meyskens FL., Jr Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin Cancer Res. 2009;15:758–761. doi: 10.1158/1078-0432.CCR-08-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerner EW, Meyskens FL, Jr, Goldschmid S, et al. Rationale for, design of, a clinical trial targeting polyamine metabolism for colon cancer chemoprevention. Amino Acids. 2007;33:189–195. doi: 10.1007/s00726-007-0515-2. [DOI] [PubMed] [Google Scholar]
- 7.Babbar N, Ignatenko NA, Casero RA, Jr, et al. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem. 2003;278:47762–47775. doi: 10.1074/jbc.M307265200. [DOI] [PubMed] [Google Scholar]
- 8.Hixson LJ, Emerson SS, Shassetz LR, et al. Sources of variability in estimating ornithine decarboxylase activity and polyamine contents in human colorectal mucosa. Cancer Epidemiol Biomarkers Prev. 1994;3:317–323. [PubMed] [Google Scholar]
- 9.Finley PR, Bogert CL, Alberts DS, et al. Measurement of prostaglandin E2 in rectal mucosa in human subjects: a method study. Cancer Epidemiol Biomarkers Prev. 1995;4:239–244. [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan K, Ruffin MT, Normolle D, et al. Colonic mucosal prostaglandin E2 and cyclooxygenase expression before and after low aspirin doses in subjects at high risk or at normal risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:447–453. [PubMed] [Google Scholar]
- 12.Babbar N, Gerner EW, Casero RA., Jr Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochem J. 2006;394:317–324. doi: 10.1042/BJ20051298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 14.Alhonen-Hongisto L, Seppanen P, Janne J. Intracellular putrescine and spermidine deprivation induces increased uptake of the natural polyamines and methylglyoxal bis(guanylhydrazone) Biochem J. 1980;192:941–945. doi: 10.1042/bj1920941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace HM. Polyamines in human health. Proc Nutr Soc. 1996;55:419–431. doi: 10.1079/pns19960039. [DOI] [PubMed] [Google Scholar]
- 16.Elitsur Y, Gesell M, Luk GD. ODC activity and polyamine levels in isolated human colonocytes. Life Sci. 1993;53:945–952. doi: 10.1016/0024-3205(93)90447-b. [DOI] [PubMed] [Google Scholar]
- 17.Eberhart CE, Coffey RJ, Radhika A, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 18.Blachier F, Mariotti F, Huneau JF, et al. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids. 2007;33:547–562. doi: 10.1007/s00726-006-0477-9. [DOI] [PubMed] [Google Scholar]
- 19.Kawakita M, Hiramatsu K. Diacetylated derivatives of spermine and spermidine as novel promising tumor markers. J Biochem. 2006;139:315–322. doi: 10.1093/jb/mvj068. [DOI] [PubMed] [Google Scholar]