Abstract
During normal aging, men experience a significant decline in testosterone levels and a compensatory elevation in levels of gonadotropin luteinizing hormone (LH). Both low testosterone and elevated LH have been identified as significant risk factors for the development of Alzheimer's disease (AD) in men. It is unclear whether changes in testosterone or LH primarily underlie the relationship with AD, and therefore may be a more suitable therapeutic target. To examine this issue, we compared levels of β-amyloid (Aβ) immunoreactivity in male 3xTg-AD mice under varying experimental conditions associated with relatively low or high levels of testosterone and/or LH. In gonadally intact mice, Aβ accumulation increased after treatment with the gonadotropin releasing hormone agonist leuprolide, which inhibits the hypothalamic-pituitary-gonadal (HPG) axis and reduces both testosterone and LH levels. In gonadectomized (GDX) mice with low testosterone and high LH, we also observed increased Aβ levels. Treatment of GDX mice with testosterone significantly reduced Aβ levels. In contrast, leuprolide did not significantly decrease Aβ levels and moreover, inhibited the Aβ-lowering effect of testosterone. Evaluation of hippocampal-dependent behavior revealed parallel findings, with performance in GDX mice improved by testosterone but not leuprolide. These data suggest that Aβ-lowering actions of testosterone are mediated directly by androgen pathways rather than indirectly via regulation of LH and the HPG axis. These findings support the clinical evaluation of androgen therapy in the prevention and perhaps treatment of AD in hypogonadal men.
Keywords: Alzheimer’s disease, β-amyloid, androgen, leuprolide, luteinizing hormone, testosterone
1. Introduction
The normal, age-related depletion of testosterone in men is an established risk factor for the development of Alzheimer’s disease (AD) (Pike et al., 2009; Raber, 2004; Rosario and Pike, 2008). Testosterone and its androgen metabolites have been found to exert several beneficial actions in brain that may modulate vulnerability to AD and other neurodegenerative diseases, including promotion of synaptic plasticity (Leranth et al., 2003), neuroprotection (Ahlbom et al., 2001; Hammond et al., 2001; Pike, 2001; Ramsden et al., 2003b; Zhang et al., 2004), and inhibition of tau phosphorylation in a heat shock model (Papasozomenos, 1997). Of particular relevance to AD, androgens also reduce levels of β-amyloid (Aβ), a protein widely theorized to contribute to AD pathogenesis (Hardy and Selkoe, 2002). In humans (Almeida and Papadopoulos, 2003; Gandy et al., 2001; Gillett et al., 2003; Rosario et al., 2011) and wild-type rodents (Ramsden et al., 2003a; Rosario et al., 2009; Wahjoepramono et al., 2008) androgen levels are inversely correlated with Aβ levels. In transgenic mouse models of AD, experimental depletion of endogenous testosterone accelerates neuropathology (McAllister et al., 2010; Rosario, 2006), which can be prevented by androgen treatment (Rosario, 2006). These androgen actions are likely carried out through androgen receptors (McAllister et al., 2010; Yao et al., 2008), which are located in several brain regions relevant to AD (Kerr et al., 1995; Simerly et al., 1990).
Age-related testosterone depletion in men is accompanied by increased levels of luteinizing hormone (LH) (Kaufman et al., 1990; Tenover et al., 1987). Testosterone levels in men are regulated by the hypothalamic-pituitary-gonadal (HPG) axis (Sam and Frohman, 2008). In response to low circulating levels of testosterone, the hypothalamus secretes gonadotropin-releasing hormone (GnRH), which acts on gonadotropes in the anterior pituitary to promote the secretion of the gonadotropin LH. In turn, LH stimulates Leydig cells in the testes to produce and secrete testosterone, which negatively feeds back on the hypothalamus and anterior pituitary (Finkelstein et al., 1991; Marshall, 2001; Sam and Frohman, 2008). During normal aging in men, testosterone production declines as a result of both Leydig cell atrophy and dysfunction of the HPG axis. Pulsatile release of LH shows alterations beginning in middle age (Warner et al., 1985), but an overall increase in circulating LH levels is observed during advanced aging in men (Morley et al., 1997; Morley, 2001).
Like low testosterone, the age-related increase in LH has been identified as a risk factor for AD in men (Bowen et al., 2000; Hogervorst et al., 2003; Hogervorst et al., 2004; Short et al., 2001). Several lines of evidence are consistent with the possibility that elevated LH may contribute to AD pathogenesis in men (Barron et al., 2006; Casadesus et al., 2007; Meethal et al., 2005). For example, exposure of cultured cells to LH can alter processing of the Aβ precursor protein towards increased production of Aβ (Bowen et al., 2004). In rodent models, Aβ levels can be decreased by the GnRH analogue leuprolide acetate (Bowen et al., 2004; Casadesus et al., 2006b), which reduces both testosterone and LH levels following long-term treatment. Similarly, knockout of the LH receptor is associated with reduction of AD-like neuropathology in a transgenic mouse model of AD (Lin et al., 2010).
Since testosterone and LH levels are linked through HPG feedback loops, understanding whether AD risk is largely associated with low testosterone, elevated LH, or both has been a challenge. For example, aging men with AD have been found to have both lower androgen levels and higher LH levels in comparison to age-matched controls (Bowen et al., 2000; Hogervorst et al., 2001; Hogervorst et al., 2003; Short et al., 2001). In animal models, gonadectomy (GDX), a common approach to examine the effects of endogenous androgen depletion, results in not only depletion of androgens but also elevation of LH (Lindzey et al., 1998). In the current study, we addressed this important issue by experimentally manipulating testosterone and LH to achieve conditions in which the two hormones are differentially elevated or depleted. We accomplished this by assessing individual and combined effects of long-term treatments with testosterone and/or leuprolide acetate in GDX or sham GDX male 3xTg-AD mice. The data suggest that testosterone but not LH is associated with regulation of Aβ levels and behavioral performance in a mouse model of AD.
2. Results
2.1 Experiment 1: Leuprolide acetate accelerates β-amyloid accumulation
As an initial step in assessing the contributions of testosterone and LH in regulating Aβ accumulation, we utilized 3xTg-AD mice which have been reported as having normal reproductive functions (Oddo et al., 2003) and normal circulating levels of testosterone (Rosario et al., 2006), estrogen and progesterone (Carroll et al., 2007; Carroll and Pike, 2008; Carroll et al., 2010b)), comparable to WT mice. We established two conditions that are both associated with low testosterone: one with low LH, and one with high LH. The first group consisted of gonadally intact male 3xTg-AD mice treated for 4 mo with leuprolide acetate (LA), a GnRH agonist that, with chronic exposure, strongly attenuates HPG function thereby causing depletion of both LH and T (Bowen et al., 2004; Okada et al., 1996). The second group was gonadectomized (GDX), a procedure that removes the primary source of endogenous testosterone but results in a compensatory increase of over 9-fold in LH via HPG feedback (Gharib et al., 1986). The efficacy of these experimental manipulations in affecting androgen and HPG status was functionally assessed after 4 mo using both a tissue bioassay and a behavioral test. First, we measured seminal vesicle weight, an established biomarker of androgen levels (Ayata et al., 1987; Ayata et al., 1988; Yamane et al., 1986; Zanato et al., 1994). As expected, both GDX and sham GDX 3xTg-AD mice treated with LA (sham GDX+LA) exhibited significant decreases in seminal vesicle weight in comparison to vehicle-treated sham GDX animals (Table 1). We also examined bedding preference as a behavioral assay of androgen status action in a subset of mice to assure the neural efficacy of treatments. Male GDX mice showed a significant reduction in time spent with female bedding as compared to the gonadally intact male mice, which prefer female bedding. In the sham GDX+LA mice, time spent with the female bedding was also significantly reduced (Table 1), indicating low testosterone in the brain and confirming the efficacy of LA treatment.
Table 1.
Effects of leuprolide on androgen bioassays.
| Condition | Body wt (g) | Seminal vesicle wt/ Body wt (g) |
% time w/ female bedding |
|---|---|---|---|
| Sham | 30.0 ± 1.4 | 3.9 × 10−3 ± 0.2 × 10−3 | 65.7 ± 7.1 |
| GDX | 29.4 ± 1.4 | 0.8 × 10−3 ± 0.2 × 10−3* | 32.0 ± 11.2* |
| Sham + leuprolide | 34.3 ± 1.7* | 1.9 × 10−3 ± 0.3 × 10−3* | 30.9 ± 7.9* |
P < 0.05 relative to Sham
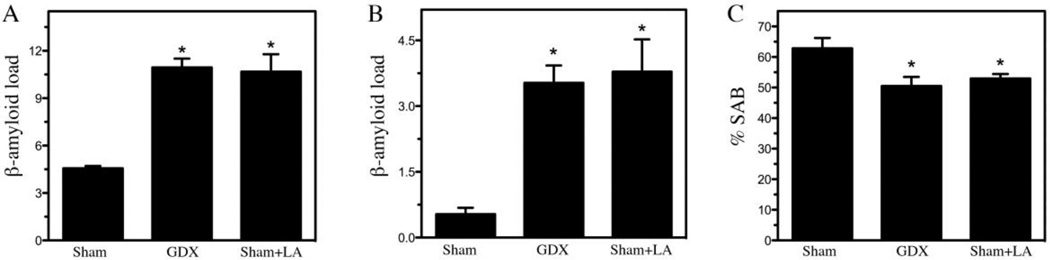
Next, brain levels of Aβ were examined under the two conditions, sham GDX+LA (low testosterone, low LH) and GDX (low testosterone, high LH). In comparison to the sham GDX reference group, both the sham GDX+LA and GDX groups showed similar, statistically significant increases in Aβ load in subiculcum and in hippocampus CA1 (Fig. 1 A, B). Behaviorally, performance in hippocampal-dependent SAB was significantly impaired in the sham GDX+LA and GDX groups relative to the control group of sham GDX mice (Fig. 1C).
Figure 1.
Comparison of the effects of GnRH agonist leuprolide acetate (LA) and gonadectomy (GDX) on Aβ accumulation and spontaneous alternation behavior in male 3xTg-AD mice. After a 4 mo treatment period, levels of Aβ load in subiculum (A) and CA1 region of hippocampus (B) of male 3xTg-AD mice were quantified in the following treatment groups: Sham GDX, GDX, and Sham + LA. Data show mean Aβ load values (± SEM). (C) Prior to sacrifice, male 3xTgAD mice in all groups were assesses for spontaneous alternation behavior (SAB) in the Y-maze. Data show mean alternation percentage (±SEM). * Denotes p < 0.05 in comparison to Sham GDX (Sham) group.
2.2 Experiment 2: Effects of testosterone and leuprolide acetate in gonadectomized mice
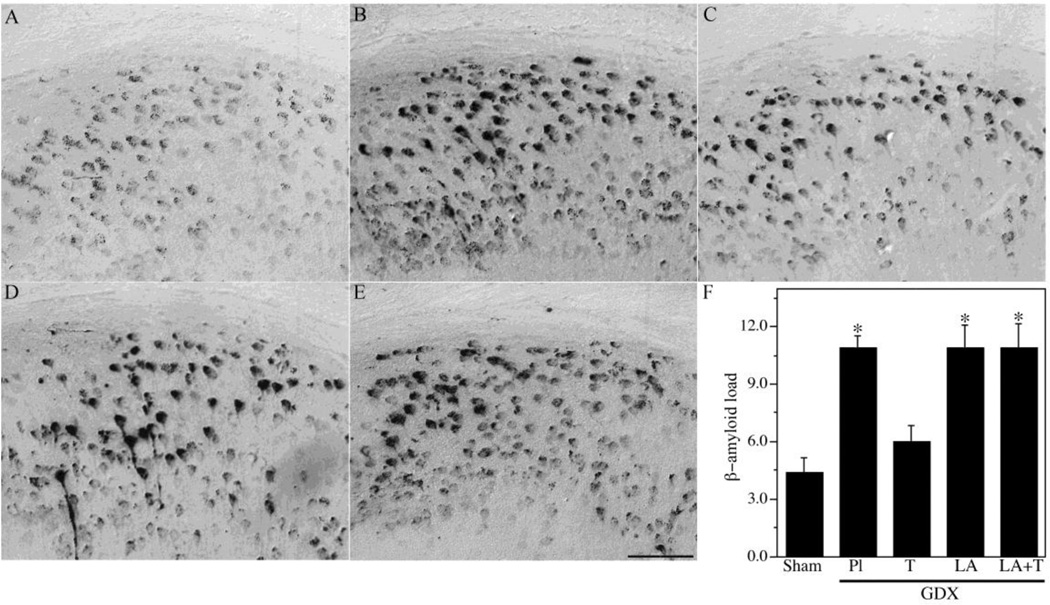
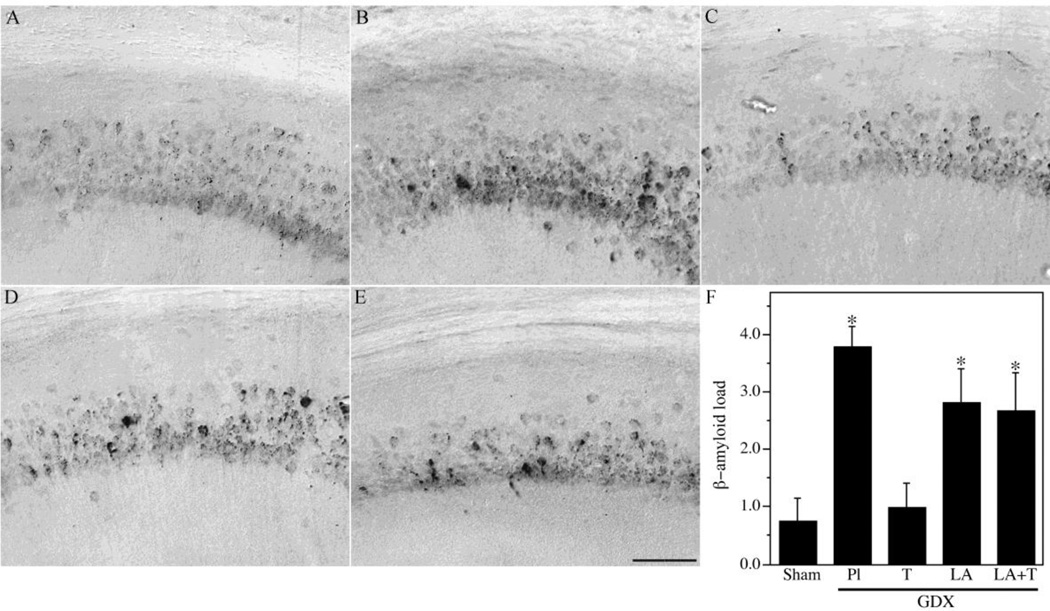
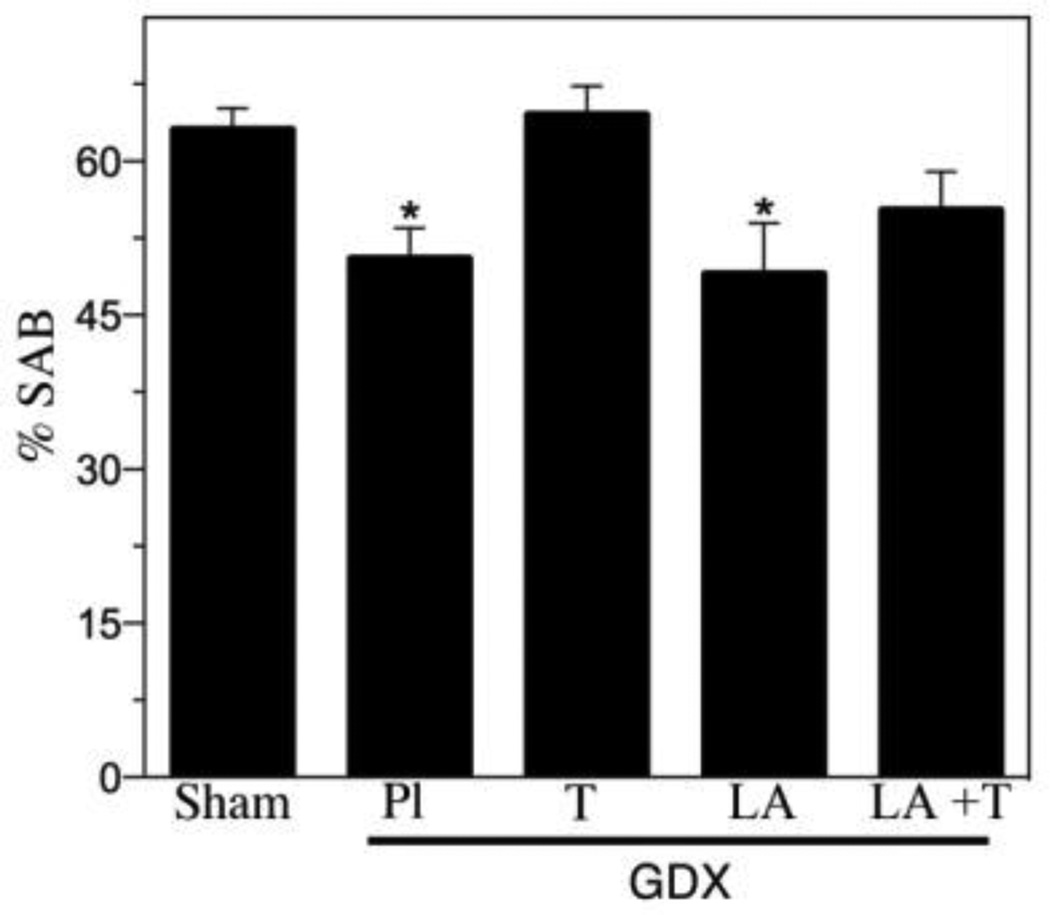
To further investigate the relationships between LH and testosterone in the regulation of Aβ, we analyzed additional treatment groups associated with different combinations of LH and testosterone. We utilized a GDX group (low testosterone, high LH) as well as GDX mice treated for four months with testosterone (GDX+T; high testosterone, low LH), LA (GDX+LA; low testosterone, low LH), or testosterone and LA (GDX+T+LA; high testosterone, low LH). Efficacy of testosterone treatments was confirmed by analysis of seminal vesicle weights (Table 2). Consistent with our previous observations, GDX significantly increased Aβ load across brain regions. Testosterone treatment in GDX mice prevented the increased Aβ accumulation in subiculum (Fig. 2) and hippocampus CA1 (Fig. 3), demonstrating Aβ load values near those observed in the sham GDX group. In contrast, the GDX+LA group exhibited Aβ load values that were significantly higher than the sham GDX group and not significantly different from the GDX group (Figs. 2–3). Interestingly, the combination of LA and testosterone treatments yielded elevated Aβ levels similar to those in the GDX and GDX+LA groups but significantly higher than those in the sham GDX and GDX+T groups (Fig. 2–3). As in the first experiment, behavior closely mirrored Aβ load. The GDX group exhibited significantly impaired SAB relative to the sham GDX, a deficit that was rescued in the GDX+T but not the GDX+LA group. SAB values in the GDX+T+LA group showed a statistically non-significant trend of impaired performance.
Table 2.
Effects of experimental manipulations on an androgen bioassay.
| Condition | Body wt (g) | Seminal vesicle wt/ Body wt (g) |
|---|---|---|
| Sham | 31.2 ± 1.6 | 3.3 × 10−3 ± 0.3 × 10−3 |
| GDX | 28.1 ± 1.4 | 0.8 × 10−3 ± 0.4 × 10−3* |
| GDX + T | 35.5 ± 1.2* | 2.8 × 10−3 ± 0.4 × 10−3 |
| GDX + leuprolide | 27.3 ± 1.4 | 0.6 × 10−3 ± 0.4 × 10−3* |
| GDX + leuprolide + T | 37.5 ± 1.4* | 3.0 × 10−3 ± 0.5 × 10−3 |
P < 0.05 relative to Sham
Figure 2.
Hormone regulation of Aβ in subiculum of male 3xTg-AD mice. Representative high magnification photomicrographs show Aβ immunoreactivity in male 3xTg-AD mice in the following treatment groups: Sham GDX (A), GDX+PL (B), GDX+T (C), GDX+Leu (D), and GDX+Leu + T (E). Scale bar = 100µm. (F) Levels of Aβ immunoreactive load in subiculum were quantified across groups. Data show mean Aβ load values (± SEM). * Denotes p < 0.05 in comparison to Sham GDX (Sham) group.
Figure 3.
Hormone regulation of Aβ in hippocampus CA1 of male 3xTg-AD mice. Representative high magnification photomicrographs show Aβ immunoreactivity in male 3xTg-AD mice in the following treatment groups: Sham GDX (A), GDX+PL (B), GDX+T (C), GDX+Leu (D), and GDX+Leu + T (E). Scale bar = 100µm. (F) Levels of Aβ immunoreactive load in CA1 of hippocampus were quantified across groups. Data show mean Aβ load values (± SEM). * Denotes p < 0.05 in comparison to Sham GDX (Sham) group.
3. Discussion
Both low levels of androgens and elevated levels of LH have been implicated as risk factors for AD (Bowen et al., 2000; Rosario et al., 2011; Rosario, 2004; Short et al., 2001). Previous work examining these risk factors have been unable to clearly determine the relative roles of these hormones in regulating AD pathology since conditions exhibiting low levels of androgens also have elevated levels of LH. For example, because of the negative feedback regulating androgen and gonadotropin levels, GDX not only reduces endogenous androgen levels but also increases LH. At the same time, reducing LH levels with a GnRH agonist such as LA also reduces androgen levels. Our goal in this study was to differentiate the effects of these hormones to better understand the individual roles of T and LH in the progression of AD-related pathology. Consistent with our previous work (Rosario et al., 2006), we observed a significant increase in Aβ pathology and worsened memory impairment following GDX-induced androgen depletion which was prevented with T treatment. However, LA treatment, which lowers LH levels in both sham GDX and GDX mice, had no benefit on Aβ levels or working memory and even blocked the Aβ–lowering actions of androgens. Thus, these findings suggest that androgens, not LH, are the primary hormones in the HPG axis involved in regulation of Aβ in male 3xTg-AD mice.
Our findings support previous work by our group and others showing the protective and beneficial actions of androgens in regulating Aβ and AD pathology (Gillett et al., 2003; McAllister et al., 2010; Ramsden et al., 2003a; Rosario et al., 2011; Rosario, 2006). For example, studies in both human and rodent models have found that age-related androgen depletion significantly correlates with increases in soluble Aβ (Gillett et al., 2003; Rosario et al., 2011). Experimental androgen depletion in both rats and 3xTg-AD mice has been shown to regulate levels of Aβ accumulation and deposition (Ramsden et al., 2003a; Rosario et al., 2006). In these models Aβ levels are significantly increased following GDX, an effect prevented by DHT or T treatment (Ramsden et al., 2003a; Rosario et al., 2006). One mechanism by which androgens reduce Aβ levels is by positively regulating the neural expression of neprilysin, an Aβ-catabolizing enzyme (McAllister et al., 2010; Yao et al., 2008). Our prior work demonstrated that androgen regulation of neprilysin in neurons is androgen receptor (AR)-dependent and that androgen regulation of Aβ is dependent upon both neprilysin activity and the presence of functional AR (Yao et al., 2008). Taken together, these data support the hypothesis that regulation of Aβ in males is directly androgen-mediated and does not involve indirect hormone affects through negative feedback or androgen metabolites.
Despite findings of Aβ reduction associated with LA treatment and other strategies to reduce LH (Casadesus et al., 2006a; Casadesus et al., 2007), we did not observe significant changes in Aβ levels following LA treatment in male 3xTg-AD mice. Since our data demonstrate beneficial actions of androgens and because LA both reduces androgen levels and attenuated androgen effects in this model, our findings argue against LA for the purpose of improving neural health and/or reducing risk of AD. Clinical data are consistent with the possibility that LA can have deleterious effects, perhaps as a result of reducing endogenous androgen levels. For example, LA delivered as part of androgen deprivation therapy (usually includes an androgen receptor antagonist) in men with prostate cancer resulted in a reduction in androgen and estrogen levels and a corresponding increase in Aβ levels (Almeida and Papadopoulos, 2003; Gandy et al., 2001). Androgen deprivation therapy with LA has also been associated with impaired cognitive function and memory that can be reversed after discontinuation of treatment (Cherrier et al., 2009; Green, 2002; Salminen et al., 2004). The mechanisms underlying these effects are unclear, but may involve not only LA suppression of HPG axis regulation of testosterone production but also altered activation of LH receptors (Meethal et al., 2005). In addition, LA may reduce neurosteroidogenesis. Gonadotropins have been shown to regulate levels of a key protein in neurosteroidogenesis, steroidogenic acute regulatory protein, which can alter normal feedback regulation of neural sex steroid production (Meethal et al., 2009).
There are limitations of this study, particularly in regards to relevance to clinical issues. First, surgical castration is not comparable to the natural, steady decline in androgens men experience as a consequence of normal aging. In addition, our hormone depletion and the timing and concentrations of our hormone replacement paradigms alter the HPG feedback axis differently than that observed during normal aging and AD. It is important to note however, that not only did LA fail to reduce Aβ in male mice, it also prevented the Aβ-lowering actions of androgens, thereby suggesting the possibility that LA disrupted androgen signaling and/or HPG function. Second, we cannot exclude the possibility that the effects of LA and gonadotropins on regulation of Aβ may be qualitatively different in females. The early work by Bowen and colleagues demonstrating reduced levels of Aβ following LA treatment was conducted in female mice (Bowen et al., 2004). More recent studies also demonstrate benefits of LA and/or LH reduction in female rodents. For example, LA was shown to improve hippocampal-dependent spatial memory in ovariectomized mice through an estrogen receptor-independent mechanism (Bryan et al., 2010). Also, treatment of ovariectomized rats with Antide (a gonadotrophin releasing hormone receptor antagonist) enhanced spatial memory in a neurotoxin-induced model of AD (Ziegler and Thornton, 2010). Conversely, it has been demonstrated that administration of an LH homologue to ovariectomized rats given estradiol increased soluble levels of Aβ and worsened spatial memory (Berry et al., 2008). Lastly, measurements of serum hormone levels were not available in this study to confirm that GDX, T and LA conditions exerted the expected effects on circulating or brain hormone levels of T, LH and even E2 and P4. However, hormone status was indirectly confirmed using both a well-established biological assay of seminal vesicle weight and a behavioral assay. These assays have previously been shown to be dependent on circulating T levels in rodents (Almenara et al., 2001; Bakker et al., 2002; Bodo and Rissman, 2007; Fawell and Higgins, 1984; Zanato et al., 1994). Further, LA was delivered at a monthly dosage equivalent to (Casadesus et al., 2006b) or higher than (Bowen et al., 2004) prior reports in female mice in which LA was associated with reduced Aβ. Additional work is needed to determine the extent to which LA and gonadotropins differentially regulate Aβ in male versus female brains. The possibility of sex-specific effects is consistent with the growing body of evidence of significant sex differences in regulation of pathology in AD mouse models (Carroll et al., 2010a; Oliveira et al., 2011).
In summary, our data provide additional evidence that androgens are significant negative regulators of Aβ in male brain but question the role of the gonadotropin LH in this process. Because normal male aging is characterized by HPG axis dysfunction involving reduced testosterone and elevated LH, either or both hormones may be significant, direct contributors to a constellation of symptoms associated with hypogonadism, including increased risk of AD. Testosterone-based androgen therapy rather than LA is typically used in men to treat the clinical manifestations of age-related hypogonadism, including sexual dysfunction, osteoporosis, sarcopenia, cognitive impairment, mood and overall quality of life (Bhasin et al., 2006; Bhasin et al., 2007; Kaufman and Vermeulen, 2005). One notable difference between these two approaches is that whereas testosterone treatment elevates androgen levels and reduces LH, LA treatment reduces both hormones. Given the abundant clinical and experimental evidence that testosterone improves neuron viability, synaptic plasticity, and cognition and reduces indices of AD pathology (Janowsky, 2006a; Janowsky, 2006b; Leranth et al., 2004; Papasozomenos, 1997; Pike et al., 2009; Raber et al., 2002; Raber, 2008; Rosario and Pike, 2008), androgen therapy appears to have more potential benefits than LA, a position supported by the present results.
4. Materials and Methods
4.1 Animals and experimental treatments
Male 3xTg-AD mice harboring APPSwe, presenilin 1 PS1M146V and tauP301L mutations (Oddo et al., 2003) were maintained under a 12h light/12h dark schedule and had ad libitum access to food and water. At age 3 mo, all mice were either sham gonadectomized (GDX) or GDX under pentobarbital (50 mg/kg) anesthesia, then were immediately administered a subcutaneous slow-release, 60-day drug delivery pellet (Innovative Research of America, Sarasota, FL) followed by a second pellet after 60 days such that treatments were maintained continuously for 4 months. In Experiment 1, gonadally intact, sham GDX groups (N= 8/group) were treated with pellets containing either placebo or leuprolide acetate (LA, 1 mg). In Experiment 2, mice were GDX (N= 8/group) and subsequently treated with placebo, testosterone (T; first pellet 10 mg 60-day, second pellet 15 mg 90-day), LA (1 mg, 60-day) or both T and LA. Pellets were administered in this manner to ensure continual delivery throughout the 4-month testing period at a dose of 5mg/30 days for T and 0.5mg/30 days for LA. Although hormone delivery via subcutaneous pellets can have limitations (Strom et al., 2008), previous studies from our lab have found this approach suitable for long-term delivery of hormones in 3xTg-AD mice (Carroll et al., 2007; Carroll et al.; Rosario, 2006). Within three days of sacrifice, animals were behaviorally evaluated. After 4 months of hormone treatment, mice were anesthetized with 100 mg/kg pentobarbital and transcardially perfused with PBS. Seminal vesicles were dissected, blotted, and weighed as a bioassay of androgen levels. Brains were dissected, immersion fixed for 48 h at 4°C in 4% paraformaldehyde, then stored in PBS/0.1% sodium azide at 4°C until processed for immunohistochemistry. Animal studies were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and under an institutionally approved animal protocol.
4.2 Behavioral assessments
Stimulus bedding preference test
Male mice were assessed behaviorally for androgen activity by measuring their preference for female stimulus bedding using a protocol adapted from a previously described procedure (Bakker et al., 2002; Bodo and Rissman, 2007). Mice were habituated to the testing room at 18:00 h, the beginning of the lights off period. At 19:00 h, mice were placed into a small, glass cage (8” × 20” ×10”) containing three Petri dishes (10 cm diameter) filled with approximately equal amounts of male-soiled bedding, female-soiled bedding, and neutral, unsoiled bedding. Soiled bedding was provided by mice not involved in the experiment and was uniformly collected from one chosen cage of sexually active breeder male mice and one cage of sexually active breeder female mice. Male- and female-soiled beddings were retrieved from cages housing sexually active male and female mice, respectively. As previously described (Bakker et al., 2002), male-soiled bedding came from cages housing only male breeders on a brief breeding hiatus. Female-soiled bedding came from cages housing only sexually active female mice with a recent history of conception. After a 1 week period of breeding in which one male and one female were housed together, mice were returned to their respective individual cages and bedding was collected within 24 hr. Neutral bedding consisted of unused bedding squares (Ancare Corp., Bellmore, NY). Male mice were placed in the testing chamber for 5 minutes and the amounts of time spent with male, female and neutral beddings were recorded by an experimenter blinded to treatment conditions. Preference for female stimulus bedding was calculated as the proportion of time spent with the female bedding to the total time spent with any bedding.
4.3 Spontaneous alternation behavior
Spontaneous alternation behavior in the Y-maze (SAB), a hippocampal-dependent task of working memory and visual attention, was assessed as previously described (Carroll et al., 2007; Carroll and Pike, 2008; Carroll et al., 2010b; Rosario, 2006). In brief, each mouse was allowed 8 minutes to freely explore a Y-maze while an experimenter blinded to condition recorded the total numbers of arm choices and alternations. An arm choice was defined as both front and hind paws fully entering the arm. The maze was cleaned with 70% ethanol between animals to minimize odor cues. SAB score was calculated as the proportion of alternations (an arm choice differing from the previous 2 choices) to the total number of alternation opportunities (total arm entries - 2).
4.4 Immunohistochemistry
Fixed 3xTg-AD brain tissue was processed for immunohistochemistry using the avidin:biotinylated enzyme complex approach, as previously described (Carroll et al., 2007;Carroll et al.; Rosario, 2006). Fixed brains were sectioned exhaustively in the horizontal plane (40 µm) using a vibratome then incubated with sequential rinses in 1.5% H2O2 to quench endogenous peroxidase activity and 99% formic acid as an antigen unmasking step to increase Aβ immunoreactivity (Cummings, 2002). Next, sections were rinsed in 0.1 M Tris-buffered saline (TBS) and incubated in TBS containing 0.1% Triton-X100 and 2% normal serum to block nonspecific binding. Pretreated sections were incubated overnight in primary anti-Aβ antibody (1:300 dilution of antibody 71-5800; Zymed, San Francisco, CA) in TBS buffer with 0.1% Triton-X100 and 2% normal serum. After rinsing in TBS, sections were sequentially incubated with biotinylated anti-rabbit antibody and ABC complex (Vector Elite ABC kit; Vector, Burlingame, CA). Immunohistochemical reaction was visualized with diaminobenzidine (Vector). Immunolabeled sections were dehydrated by rinses in a graded series of ethanol solutions then cover-slipped in permanent mounting medium. To control for inter-experiment variability, tissue sections were immunostained in a few large experiments that were balanced by treatment group.
4.5 Immunohistochemical quantification
Quantification of Aβ immunohistochemistry was performed using an immunoreactive load technique previously described (Carroll et al., 2007; Rosario, 2006). Briefly, high magnification fields (420 µm × 330 µm) from Aβ-immunolabeled sections were digitized and stored on computer using a video capture system (B/W CCD camera coupled to an Olympus BX40 microscope). Using NIH Image 1.61 software, gray scale images were thresholded at a predetermined, constant level creating a binary separation between positive and negative immunoreactivity. The percentage of pixels positively immunolabeled is defined as load. Load values were determined in subiculum and hippocampus CA1 by averaging values obtained from a representative sampling of 2–3 non-overlapping fields from each of 5 separate sections per brain.
4.6 Statistical analysis
Raw data were statistically analyzed by oneway ANOVA using StatView. Significant main effects were further analyzed with between group comparisons using Fisher LSD test. Effects with P < 0.05 were considered statistically significant. Data are presented as means ± SEM.
Figure 4.
Hormone regulation of working memory behavior. Hippocampal-dependent working memory performance in male 3xTgAD mice was measured using spontaneous alternation behavior in a Y-maze in the following treatment groups: Sham GDX, GDX+PL, GDX+T, GDX+Leu, and GDX+Leu + T. Data show % alternations (± SEM). * Denotes p < 0.05 in comparison to Sham GDX (Sham) group.
Highlights.
GnRH agonist leuprolide increased β-amyloid levels in male 3xTg-AD mice
Treatment of gonadectomized mice with testosterone not leuprolide reduced β-amyloid
Leuprolide inhibited beneficial effects of testosterone in gonadectomized mice
These findings support androgen therapy use to prevent Alzheimer’s in hypogonadal men
Acknowledgments
This study was supported by funding from the National Institute on Aging (AG23739 and AG34103).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors disclose no conflicts of interest.
References
- Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Research. 2001;892:255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- Almeida TA, Papadopoulos N. Progression model of prostate cancer. Methods Mol Biol. 2003;222:211–222. doi: 10.1385/1-59259-328-3:211. [DOI] [PubMed] [Google Scholar]
- Almenara A, Escalante G, Gazzo E, Gonzales GF. Transillumination to evaluate spermatogenesis: effect of testosterone enanthate in adult male rats. Arch Androl. 2001;46:21–27. doi: 10.1080/01485010150211119. [DOI] [PubMed] [Google Scholar]
- Ayata M, Yamane T, Okamoto S, Kitamura Y, Matsumoto K. Effect of long term androgen removal on androgen-induced proliferation of seminal vesicle cells in adult mice. J Steroid Biochem. 1987;28:399–403. doi: 10.1016/0022-4731(87)91057-0. [DOI] [PubMed] [Google Scholar]
- Ayata M, Yamane T, Li W, Terada N, Kitamura Y, Matsumoto K. Proliferative response of seminal vesicle cells to androgen in mice castrated neonatally and pretreated with estrogen or androgen at adulthood. Endocrinol Jpn. 1988;35:511–515. doi: 10.1507/endocrj1954.35.511. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron AM, Verdile G, Martins RN. The role of gonadotropins in Alzheimer's disease: potential neurodegenerative mechanisms. Endocrine. 2006;29:257–269. doi: 10.1385/ENDO:29:2:257. [DOI] [PubMed] [Google Scholar]
- Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-beta levels in female rats. Horm Behav. 2008;54:143–152. doi: 10.1016/j.yhbeh.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Parker RA, Sattler F, Haubrich R, Alston B, Umbleja T, Shikuma CM Team., A.C.T.G.P.A.S. Effects of testosterone supplementation on whole body and regional fat mass and distribution in human immunodeficiency virus-infected men with abdominal obesity. J Clin Endocrinol Metab. 2007;92:1049–1057. doi: 10.1210/jc.2006-2060. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Isley JP, Atkinson RL. An association of elevated serum gonadotropin concentrations and Alzheimer disease? J Neuroendocrinol. 2000;12:351–354. doi: 10.1046/j.1365-2826.2000.00461.x. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J Biol Chem. 2004;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- Bryan KJ, Mudd JC, Richardson SL, Chang J, Lee HG, Zhu X, Smith MA, Casadesus G. Down-regulation of serum gonadotropins is as effective as estrogen replacement at improving menopause-associated cognitive deficits. J Neurochem. 2010;112:870–881. doi: 10.1111/j.1471-4159.2009.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008 doi: 10.1210/en.2007-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Kreimer S, Villamagna A, Gentzschein E, Stanczyk FZ, Pike CJ. Sex differences in beta-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure. Brain Res. 2010a;1366:233–245. doi: 10.1016/j.brainres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Villamagna A, Pike CJ. Continuous and cyclic progesterone differentially interact with estradiol in the regulation of Alzheimer-like pathology in female 3xTransgenic-Alzheimer's disease mice. Endocrinology. 2010b;151:2713–2722. doi: 10.1210/en.2009-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesus G, Puig ER, Webber KM, Atwood CS, Escuer MC, Bowen RL, Perry G, Smith MA. Targeting gonadotropins: an alternative option for Alzheimer disease treatment. J Biomed Biotechnol. 2006a;2006:39508. doi: 10.1155/JBB/2006/39508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta. 2006b;1762:447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, Smith MA. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007 doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18:237–247. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, Manson A, Kim R, Sheu P, Anderson A. Optimization of techniques for the maximal detection and quantification of Alzheimer's-related neuropathology with digital imaging. Neurobiol Aging. 2002;23:161–170. doi: 10.1016/s0197-4580(01)00316-5. [DOI] [PubMed] [Google Scholar]
- Fawell SE, Higgins SJ. Androgen regulation of specific mRNAs, endoplasmic reticulum and Golgi-system. Mol Cell Endocrinol. 1984;37:15–27. doi: 10.1016/0303-7207(84)90124-2. [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Whitcomb RW, O'Dea LS, Longcope C, Schoenfeld DA, Crowley WF., Jr Sex steroid control of gonadotropin secretion in the human male. I. Effects of testosterone administration in normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab. 1991;73:609–620. doi: 10.1210/jcem-73-3-609. [DOI] [PubMed] [Google Scholar]
- Gandy S, Almeida OP, Fonte J, Lim D, Waterrus A, Spry N, Flicker L, Martins RN. Chemical andropause and amyloid-beta peptide. JAMA. 2001;285:2195–2196. doi: 10.1001/jama.285.17.2195-a. [DOI] [PubMed] [Google Scholar]
- Gharib SD, Bowers SM, Need LR, Chin WW. Regulation of rat luteinizing hormone subunit messenger ribonucleic acids by gonadal steroid hormones. J Clin Invest. 1986;77:582–589. doi: 10.1172/JCI112340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett MJ, Martins RN, Clarnette RM, Chubb SA, Bruce DG, Yeap BB. Relationship between testosterone, sex hormone binding globulin and plasma amyloid beta peptide 40 in older men with subjective memory loss or dementia. J Alzheimers Dis. 2003;5:267–269. doi: 10.3233/jad-2003-5401. [DOI] [PubMed] [Google Scholar]
- Green HJ. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing analogues and cyproteronw acetate: a randomized controlled trial. BJU International. 2002;90:427–432. doi: 10.1046/j.1464-410x.2002.02917.x. [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. Journal of Neurochemistry. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Barnetson L, Combrinck M, Smith AD. Serum total testosterone is lower in men with Alzheimer's disease. Neuroendocrinol Lett. 2001;22:163–168. [PubMed] [Google Scholar]
- Hogervorst E, Combrinck M, Smith AD. Testosterone and gonadotropin levels in men with dementia. Neuroendocrinol Lett. 2003;24:203–208. [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer's disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. Thinking with your gonads: testosterone and cognition. Trends Cogn Sci. 2006a;10:77–82. doi: 10.1016/j.tics.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006b;138:1015–1020. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Deslypere JP, Giri M, Vermeulen A. Neuroendocrine regulation of pulsatile luteinizing hormone secretion in elderly men. J Steroid Biochem Mol Biol. 1990;37:421–430. doi: 10.1016/0960-0760(90)90493-5. [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005 doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J.Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Li X, Yuan F, Lin L, Cook CL, Rao Ch V, Lei Z. Genetic ablation of luteinizing hormone receptor improves the amyloid pathology in a mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2010;69:253–261. doi: 10.1097/NEN.0b013e3181d072cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindzey J, Wetsel WC, Couse JF, Stoker T, Cooper R, Korach KS. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-alpha knockout mice. Endocrinology. 1998;139:4092–4101. doi: 10.1210/endo.139.10.6253. [DOI] [PubMed] [Google Scholar]
- Marshall JC. Endocrinology. Vol. Philadelphia: Saunders; 2001. Regulation of gonadotropin synthesis and secretion. [Google Scholar]
- McAllister C, Long J, Bowers A, Walker A, Cao P, Honda S, Harada N, Staufenbiel M, Shen Y, Li R. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci. 2010;30:7326–7334. doi: 10.1523/JNEUROSCI.1180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meethal SV, Smith MA, Bowen RL, Atwood CS. The gonadotropin connection in Alzheimer's disease. Endocrine. 2005;26:317–326. doi: 10.1385/ENDO:26:3:317. [DOI] [PubMed] [Google Scholar]
- Meethal SV, Liu T, Chan HW, Ginsburg E, Wilson AC, Gray DN, Bowen RL, Vonderhaar BK, Atwood CS. Identification of a regulatory loop for the synthesis of neurosteroids: a steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic-pituitary-gonadal axis receptors. J Neurochem. 2009;110:1014–1027. doi: 10.1111/j.1471-4159.2009.06192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Perry HM, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism: Clinical and Experimental. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- Morley JE. Androgens and aging. Maturitas. 2001;38:61–71. doi: 10.1016/s0378-5122(00)00192-4. discussion 71–. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Okada H, Doken Y, Ogawa Y. Persistent suppression of the pituitary-gonadal system in female rats by three-month depot injectable microspheres of leuprorelin acetate. J Pharm Sci. 1996;85:1044–1048. doi: 10.1021/js960123a. [DOI] [PubMed] [Google Scholar]
- Oliveira SM, Ribeiro CA, Cardoso I, Saraiva MJ. Gender-dependent transthyretin modulation of brain amyloid-beta levels: evidence from a mouse model of Alzheimer's disease. J Alzheimers Dis. 2011;27:429–439. doi: 10.3233/JAD-2011-110488. [DOI] [PubMed] [Google Scholar]
- Papasozomenos SC. The heat shock-induced hyperphosphorylation of tau is estrogen-independent and prevented by androgens: implications for Alzheimer disease. Proc Natl Acad Sci U S A. 1997;94:6612–6617. doi: 10.1073/pnas.94.13.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Research. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. The Journal of Neuroscience : the Official Journal of the Society For Neuroscience. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J. Androgens, apoE, and Alzheimer's disease. Sci Aging Knowledge Environ. 2004;2004:re2. doi: 10.1126/sageke.2004.11.re2. [DOI] [PubMed] [Google Scholar]
- Raber J. AR, apoE, and cognitive function. Horm Behav. 2008;53:706–715. doi: 10.1016/j.yhbeh.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, Pike CJ. Androgens modulate beta-amyloid levels in male rat brain. J Neurochem. 2003a;87:1052–1055. doi: 10.1046/j.1471-4159.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003b;122:573–578. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer's disease. J Neurosci. 2006;26:13384–13389. doi: 10.1523/JNEUROSCI.2514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Pike CJ. Androgen regulation of beta-amyloid protein and the risk of Alzheimer's disease. Brain Res Rev. 2008;57:444–453. doi: 10.1016/j.brainresrev.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Beckett TL, Carroll JC, Paul Murphy M, Stanczyk FZ, Pike CJ. Age-related changes in serum and brain levels of androgens in male Brown Norway rats. Neuroreport. 2009;20:1534–1537. doi: 10.1097/WNR.0b013e328331f968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging. 2011;32:604–613. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer's disease. J Neurosci. 2006;26:13384–13389. doi: 10.1523/JNEUROSCI.2514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. Jama. 2004;292:1431–1432. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- Salminen EK, Portin RI, Koskinen A, Helenius H, Nurmi M. Associations between serum testosterone fall and cognitive function in prostate cancer patients. Clin Cancer Res. 2004;10:7575–7582. doi: 10.1158/1078-0432.CCR-04-0750. [DOI] [PubMed] [Google Scholar]
- Sam S, Frohman LA. Normal physiology of hypothalamic pituitary regulation. Endocrinol Metab Clin North Am. 2008;37:1–22. vii. doi: 10.1016/j.ecl.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Short RA, Bowen RL, O'Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc. 2001;76:906–909. doi: 10.4065/76.9.906. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. The Journal of Comparative Neurology. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Strom JO, Theodorsson A, Theodorsson E. Substantial discrepancies in 17beta-oestradiol concentrations obtained with three different commercial direct radioimmunoassay kits in rat sera. Scand J Clin Lab Invest. 2008;68:806–813. doi: 10.1080/00365510802254638. [DOI] [PubMed] [Google Scholar]
- Tenover JS, Matsumoto AM, Plymate SR, Bremner WJ. The effects of aging in normal men on bioavailable testosterone and luteinizing hormone secretion: response to clomiphene citrate. J Clin Endocrinol Metab. 1987;65:1118–1126. doi: 10.1210/jcem-65-6-1118. [DOI] [PubMed] [Google Scholar]
- Wahjoepramono EJ, Wijaya LK, Taddei K, Martins G, Howard M, de Ruyck K, Bates K, Dhaliwal SS, Verdile G, Carruthers M, Martins RN. Distinct effects of testosterone on plasma and cerebrospinal fluid amyloid-beta levels. J Alzheimers Dis. 2008;15:129–137. doi: 10.3233/jad-2008-15111. [DOI] [PubMed] [Google Scholar]
- Warner BA, Dufau ML, Santen RJ. Effects of aging and illness on the pituitary testicular axis in men: qualitative as well as quantitative changes in luteinizing hormone. J Clin Endocrinol Metab. 1985;60:263–268. doi: 10.1210/jcem-60-2-263. [DOI] [PubMed] [Google Scholar]
- Yamane T, Kitamura Y, Terada N, Matsumoto K. Proliferative response of seminal vesicle cells to androgen and estrogen in neonatally castrated mice. J Steroid Biochem. 1986;24:703–708. doi: 10.1016/0022-4731(86)90846-0. [DOI] [PubMed] [Google Scholar]
- Yao M, Nguyen TV, Rosario ER, Ramsden M, Pike CJ. Androgens regulate neprilysin expression: role in reducing beta-amyloid levels. J Neurochem. 2008;105:2477–2488. doi: 10.1111/j.1471-4159.2008.05341.x. [DOI] [PubMed] [Google Scholar]
- Zanato VF, Martins MP, Anselmo-Franci JA, Petenusci SO, Lamano-Carvalho TL. Sexual development of male Wistar rats. Braz J Med Biol Res. 1994;27:1273–1280. [PubMed] [Google Scholar]
- Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1-42 toxicity through heat shock protein 70. The Journal of Neuroscience : the Official Journal of the Society For Neuroscience. 2004;24:5315–5321. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SG, Thornton JE. Low luteinizing hormone enhances spatial memory and has protective effects on memory loss in rats. Horm Behav. 2010;58:705–713. doi: 10.1016/j.yhbeh.2010.07.002. [DOI] [PubMed] [Google Scholar]