Abstract
The purpose of this paper is to discuss the mechanisms of α-[11C]methyl-l-tryptophan (AMT) PET as an in vivo biomarker for detection of epileptogenic cortex. AMT was originally designed as a tracer to measure the serotonin synthesis rate. This tracer was first applied in patients with medically refractory epilepsy in an attempt to detect changes in serotonin synthesis based upon reports of increased serotonergic innervation in cortical specimens obtained following epilepsy surgery. The first group of epilepsy patients undergoing AMT PET scans were patients with tuberous sclerosis complex. Studies of brain tissue subsequent to epilepsy surgery in these patients with tuberous sclerosis complex implicated the kynurenine pathway of tryptophan metabolism as a primary mechanism of increased brain tissue retention of AMT in epileptogenic brain regions, rather than alterations in serotonin synthesis. Kinetic analyses of AMT in brain tumors indicate changes in tryptophan transport and tissue retention in other pools as well. These studies indicate that AMT PET may be a biomarker of immune activation in the epileptogenic process.
Keywords: α-methyl-l-tryptophan; brain tumors; epilepsy; indoleamine; 2,3-dioxygenase; kynurenine; serotonin; tuberous sclerosis complex
The original rationale for applying α-[11C] methyl-l-tryptophan (AMT; abbreviation used in this article for both [11C]-labeled and unlabeled compound) as a PET tracer to identify epileptogenic brain regions in patients with pharmacoresistant epilepsy was based on evidence implicating serotonergic mechanisms in epileptogenesis. The first study applying AMT in patients with epilepsy was in a group of patients with tuberous sclerosis complex (TSC) [1]. This study showed large increases in the uptake of AMT in epileptogenic tubers (see Kumar and coworkers [2]). In contrast to the studies of temporal lobe epilepsy described above, biochemical studies of tissue from TSC patients obtained at surgery that showed that increased AMT uptake in cortical tubers [1] did not represent increased serotonin synthesis, but rather implicated the kynurenine pathway of tryptophan metabolism [3]. In this article, the AMT model for the measurement of serotonin synthesis is described. This is followed by a summary of serotonergic mechanisms in epilepsy. Metabolite and immunohistochemistry studies in surgical specimens from patients with TSC and those with brain tumors are then described, as well as a description of AMT kinetics in brain tumors. Finally, studies of the kynurenine pathway in animal models of epilepsy are presented together with potential new agents for the treatment of epilepsy based on modifying the kynurenine pathway.
α-methyl-l-tryptophan model for measurement of brain serotonin synthesis capacity
α-methyl-l-tryptophan is an analogue of tryptophan (Figure 1), the precursor of the neurotransmitter serotonin. Serotonin is synthesized from the precursor tryptophan [4]. Tryptophan is an essential amino acid that must be derived from the diet, and constitutes only 1% of the total amino acid pool. In the blood, the majority of tryptophan is bound to plasma protein. It is the free plasma tryptophan that is available to be transported into the brain. Tryptophan is transported into the brain via the large neutral amino acid carrier (LAT1), where it competes for transport with the other large neutral amino acids [5,6]. The first, and rate-limiting, step of serotonin synthesis is the formation of 5-hydroxytryptophan catalyzed by the enzyme tryptophan hydroxylase (TPH) (Figure 2). TPH is only 50% saturated with tryptophan, resulting in the dependence of brain serotonin levels on the plasma concentration of free tryptophan as well as the plasma levels of the other large neutral amino acids [7].
Figure 1. The amino acid tryptophan and the carbon-11 labeled analogue α-methyl-l-tryptophan.
Figure 2. Tryptophan metabolism by the serotonin pathway.
The synthesis of serotonin from tryptophan catalyzed by TPH and AADC. Serotonin is stored in synaptic vesicles mediated by VMAT. Following release from the terminal, serotonin diffuses to both synaptic and extrasynaptic serotonin receptors. Serotonin is taken back up into the terminal by the SERT and metabolized by MAO to 5-HIAA. 5-HIAA: 5-hydroxyindole acetic acid; AADC: Aromatic amino acid decarboxylase; MAO: Monoamine oxidase; SERT: Serotonin transporter; TPH: Tryptophan hydroxylase; VMAT: Vesicular monoamine transporter.
In preclinical studies in the development of AMT as a tracer of serotonin synthesis, Diksic and colleagues performed numerous studies of radiolabeled AMT in rats and dogs with the goal of validating AMT as a tracer for the measurement of the rate of serotonin synthesis in vivo in humans with PET [8,9]. Following the administration of labeled AMT in rats, autoradiograms elegantly demonstrated a distribution of high tracer concentration in serotonergic cell bodies in the raphe nuclei. The synthesis of α-methyl-serotonin in the brain following tracer administration was confirmed by high-performance liquid chromatography [8,10]. The α-[3H]methyl-serotonin synthesized in the brain was localized to serotonergic neurons and nerve terminals by combined autoradiography and TPH immunocytochemistry at the electron microscopic level [11]. Furthermore, α-[3H]methyl-serotonin present in nerve terminals was released by K+ induced depolarization, suggesting that this tracer is stored with the releasable pool of serotonin [11]. In addition, AMT, unlike tryptophan, was not incorporated into protein in significant amounts [8,12]. These studies strongly suggested that AMT was a suitable tracer for the measurement of serotonin synthesis in vivo in humans with PET.
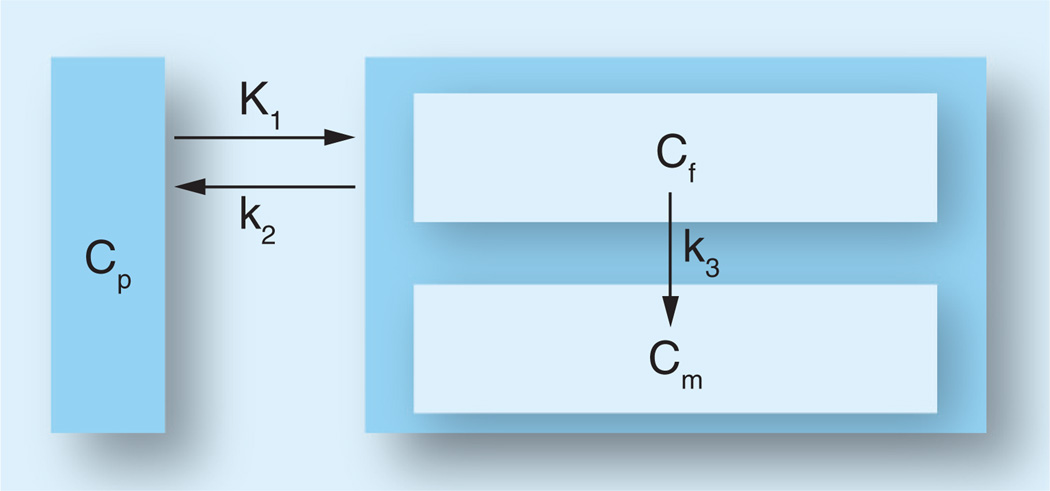
Studies of [14C]AMT in rats [8] and [11C] AMT in dogs [9], baboons [13] and humans [14–16] have suggested that the kinetic behavior of the tracer AMT can be described by a three-compartment model using first-order rate constants (schematically represented in Figure 3). The inflow rate constant K1 (ml/g/min) and the outflow rate constant k2 (1/min) both describe the exchange of AMT between vascular space and the cell cytoplasm (transport across the blood–brain barrier, the interstitial space and the cell membrane), and k3 (1/min) describes the rate of synthesis of α-methyl-serotonin. Since α-methyl-serotonin is not a substrate for the degradative enzyme monoamine oxidase [10], accumulation of α-methyl-serotonin occurs in serotonergic terminals. Shoaf et al. showed that very little AMT is converted to α-[11C]methylserotonin during the scanning time period and suggested that AMT was merely a measure of tryptophan transport [17]. In contrast, Chugani et al. [16] demonstrated that the unidirectional uptake of AMT in different brain regions exhibited the same rank order as that for serotonin content and proposed that the unidirectional uptake rate constant (K-complex) for AMT is a suitable index of serotonin synthesis in the human brain. We have referred to this index as the ‘serotonin synthesis capacity’ (for review see [18]).
Figure 3. Three-compartment model for α-[11C]methyl-l-tryptophan kinetics in the brain, using first-order rate constants.
K1 and k2 describe the exchange of α-[11C] methyl-l-tryptophan between CP and Cf. Furthermore, irreversible enzymatic conversion of α-[11C]methyl-l-tryptophan to its metabolite(s) (in Cm) is characterized by k3. Cf: Cell cytoplasm; Cm: Metabolic pool; CP: Vascular space; K1: Transport rate constant; k2: Outflow rate constant; k3: Metabolic rate constant.
Evidence for altered serotonin mechanisms in epilepsy
We initially chose the tracer AMT to study epilepsy owing to the recognition that serotonin plays a role in epileptic mechanisms based on several lines of evidence from studies in both animal models of epilepsy and humans. For example, in the genetically epilepsy-prone rat model of generalized epilepsy, a decrease is found in brain concentration of serotonin [19] as well as decreased Vmax for [3H]serotonin uptake by synaptosomes and TPH activity [20]. Indeed, pharmacologic treatments that facilitate serotonergic neurotransmission inhibit seizures in many animal models of epilepsy, including the genetically epilepsy-prone rat, maximal electroshock model, pentylenetetrazol administration, kindling, and bicuculline microinjections in the anterior piriform cortex (area tempestas) [20]. Conversely, reduction of brain serotonin concentrations leads to an increase in seizure susceptibility in animal models of epilepsy [21,22] as well as in humans [23,24]. In human brain tissue surgically removed for seizure control, the level of 5-hydroxyindole acetic acid (5-HIAA), which is a breakdown product of serotonin, was found to be higher in actively spiking temporal cortex as compared with normal tissue [25,26]. Finally, Trottier et al. demonstrated with immunohistochemical methods that epileptogenic dysplastic tissue was characterized by serotonergic hyperinnervation in patients with focal cortical dysplasia [27]. These studies provided the background and rationale for applying PET scanning of serotonin synthesis capacity in patients with epilepsy. Subsequently, a number of studies have used PET to image serotonin 5-hydroxytryptamine 1A (5-HT1A) receptors in patients with epilepsy, particularly temporal lobe epilepsy. Toczek et al. reported decreased binding of the PET tracer [18F]trans-4-fluoro-N-2-[4-(2-methoxyphenyl) piperazin-1-yl]ethyl-N-(2-pyridyl) cyclohexane carboxamide to the 5-HT1A receptor in both medial and lateral temporal regions ipsilateral to the epileptic focus as well as in the brainstem of patients with temporal lobe epilepsy [28]. By using PET with [11C] N-[2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl]-N-(2-pyridyl)cyclohexane carboxamide, Savic et al. addressed whether extratemporal abnormalities of 5-HT1A receptors are related to affective symptoms in patients with mesial temporal lobe epilepsy [29]. They found decreased binding not only in the epileptic focus (i.e., the hippocampus and the amygdala) but also in limbic connections such as insula and cingulate, thus suggesting a potential mechanism for affective symptoms in patients with mesial temporal lobe epilepsy. The findings were confirmed in a study by Merlet et al. [30]. These same investigators correlated intracranial stereo-EEG recordings with 5-HT1A-receptor binding, measured by using the PET tracer 2′-methoxyphenyl-(N-2′-pyridinyl)-p-18F-fluoro-benzamidoethylpiperazine in patients with temporal lobe epilepsy [31]. They found that epileptogenic areas as well as areas of seizure propagation showed lower binding than nonepileptogenic areas. Bagdy et al. has reviewed the role of serotonin in epilepsy [32].
In vitro measures of serotonin markers in TSC surgical specimens
As described by Kumar and coworkers [2], AMT PET can identify epileptogenic tuber(s) in almost two thirds of children with tuberous sclerosis and intractable epilepsy [1,33,34]. Due to the validation of AMT as a tracer for serotonin synthesis and the body of work showing that serotonin plays a role in epilepsy, we expected to find very high levels of serotonin and serotonin markers in the surgically resected TSC brain tissue. Resected tissues included epileptogenic tubers with high AMT uptake, nonepileptogenic tubers with low AMT uptake and cortex adjacent to the tubers [1]. The brain specimens were analyzed for the tissue content of tryptophan, serotonin, 5-HIAA, and activity of TPH. Cortical tissue and tubers with low AMT uptake contained measurable amounts of serotonin, 5-HIAA and tryptophan, but only tryptophan was detected in tubers with high AMT uptake. In addition, the activity of TPH, the rate-limiting enzyme in the synthesis of serotonin, was assayed in the tissues. TPH activity was very low in epileptogenic tubers. Thus, although we had evidence of high trapping of AMT in the epileptogenic brain tissue, this did not correspond to elevations in any of the serotonergic markers measured in the tissue.
Evidence for role of tryptophan metabolism by the kynurenine pathway in epilepsy
In addition to being converted into serotonin or incorporated into protein, tryptophan, under some circumstances (e.g., ischemia, immune activation and epilepsy), may be metabolized by tryptophan 2,3-dioxygenase [35] and indoleamine 2,3-dioxygenase (IDO) [36] via the kynurenine pathway in the brain (Figure 4). Several kynurenine pathway metabolites (e.g., quinolinic acid, kynurenine and 3-hydroxykynurenine) are convulsants acting as agonists at NMDA receptors [37–42]. The intravenous injection of quinolinic acid in rats was shown to cause EEG changes but no seizures when the blood–brain barrier was intact [43], while tonic–clonic seizures were observed following administration in rats in which the blood–brain barrier was broken by irradiation. Increased quinolinic acid and kynurenine pathway enzymes have been reported in epilepsy-prone E1 mice [44,45]. Animal models also suggest that seizures associated with measles virus infection are related to tryptophan metabolites of the kynurenine pathway. Lehrmann et al. showed that there was activation of microglia and astrocytes after innoculation with hamster neurotropic measles virus in weanling Balb/C mice, associated with increased levels of the neurotoxic kynurenine metabolites 3-hydroxykynurenine and quinolinic acid in the hippocampus [46]. The authors hypothesized that elevation of 3-hydroxykynurenine and quinolinic acid cause subsequent seizures and neurodegeneration in this model. Therefore, it has been postulated that kynurenine pathway metabolites might play a role in human epileptogenesis [47,48]. On the other hand, kynurenic acid, another metabolite in this pathway and an NMDA antagonist [37], suppresses epileptiform activity in animal models of epilepsy [49]. Wu et al. reported 1.7-fold higher levels of kynurenic acid 1 week after completion of either amygdala or hippocampal kindling [50]. These authors hypothesized that enhanced release of kynurenic acid might be a mechanism related to control of hippocampal excitability during epileptogenesis. The same group showed elevation of kynurenic acid in the pentylenetetrazole, pilocarpine, bicuculline and kainic acid seizure models [51].
Figure 4. Tryptophan metabolism by the kynurenine pathway.
Under normal circumstances, tryptophan metabolites of the kynurenine pathway are 100–1000-times lower than tryptophan concentration in brain [52]. In comparison, the sum of the concentrations of serotonin and its metabolite 5-HIAA is approximately a fifth the concentration of tryptophan in brain [53]. However, IDO induction can result in a ten-fold increase of quinolinic acid in brain [52]. While quinolinic acid concentrations were not increased in the seizure focus as compared with nonfocus brain regions from adults undergoing temporal lobectomy [48], we have found that brain tissue showing increased AMT uptake on PET shows much higher levels of quinolinic acid compared with adjacent brain tissue and tubers not showing increased AMT uptake [16]. In the very same specimens showing no serotonin, 5-HIAA or TPH activity, quinolinic acid, a tryptophan metabolite of the kynurenine pathway, was fivefold higher in the epileptogenic tubers than in the nonepileptogenic tubers and cortex.
These findings indicate that epileptogenic tubers are associated with IDO induction leading to the production of endogenous convulsants and/or anticonvulsants, and provide an intriguing perspective on epileptogenesis in TSC and possibly other epileptic disorders. Our preliminary studies show expression of IDO, not only in microglia/macrophages and astrocytes, but also in balloon cells and in the neuropil [54]. Increased expression of IDO in TSC may be related to perturbed IFN-γ-Jak-signal transducers and activators of transcription (STAT) signaling in TSC [55,56]. Specifically, there is a decrease in IFN-γ levels and an increase in STAT1 level and constitutive phosphorylation of STAT1 in both humans with TSC and Tsc mouse models. pSTAT promotes transcription of a number of IFN-γ-regulated genes through binding to IFN-γ activation sites. IDO is induced by IFN-γ [57,58] through STAT1 binding two IFN-γ activation sites [59]. Thus, a constitutive increase in pSTAT due to TSC mutations would be expected to result in an increase in the transcription of the IDO gene in multiple cell types.
In vivo factors, such as inflammation, may lead to differential effects of TSC1 & TSC2 on IDO expression
Cortical tubers and subependymal glial cell tumors contain large numbers of activated microglia and CD68-positive macrophages, as well as perivascular and parenchymal T lymphocytes [60]. These cells show persistent and complex activation of inflammatory pathways. As discussed above, IDO expression is induced by IFN-γ. In brain, IDO is primarily produced by activated microglia and macrophages. Furthermore, inhibition of PI3K with LY294002 or wortmannin reduced the expression of IFN-γ-dependent IDO in microglia [61]. Although the protein products of the TSC genes hamartin and tuberin perform many functions together as a dimer regulating the PI3K pathway, there is a huge divergence in their molecular regulatory partners. For example, Cdk1 and IKKβ have been shown to phosphorylate hamartin, while Erk, Akt, MK2, AMPK and RSK1 phosphorylate tuberin and GSK3 phosphorylates both (for a review see [62]). Of these, IKKβ may be a key link between IDO and the PI3K pathway and neuroinflammation in TSC. IKKβ, a major downstream kinase in the TNF-α signaling pathway, physically interacts with and phosphorylates hamartin at Ser487 and Ser511, resulting in suppression of hamartin [63]. On the other hand, murine fibroblasts lacking TSC2 IL-12 could not be induced with lipopolysaccharide treatment, and TNF-α was reduced and IL-10 was increased with lipopolysaccharide stimulation in TSC2 silenced cells (using RNA interference) [64]. Thus, the effect of inflammatory cytokines associated with activated microglia and macrophages in epileptogenic tubers [60] on PI3K signaling and IDO induction might differ between patients with TSC1 and TSC2 mutations. Such inflammatory mechanisms might also play a role in differences in AMT uptake among tubers in the same patient.
Kinetic analysis of AMT PET in brain tumors
Despite the known association between brain tumors and seizures, the pathophysiological mechanisms contributing to epileptogenesis in patients with brain tumors remain poorly understood. One of the unexplained aspects of tumor-associated epilepsy is the fact that low-grade tumors are generally more epileptogenic than malignant ones. Accumulation of AMT in brain tumors may occur through a number of different mechanisms, such as alterations at the blood–brain barrier, increased vascularization, increased transport via the large amino acid transporter, and increased metabolism. If the blood–brain barrier is intact, the unidirectional uptake of AMT (K-complex parameter; [15,65]) is proportional to the metabolic rate of tryptophan. If this condition is met, a semi-quantitative approach using static uptake images or the K-complex is useful to estimate the metabolic rate of tryptophan in the brain. Quantification of AMT uptake and metabolism in brain tumors revealed different mechanisms of increased AMT uptake in different tumor types and grades [66]. Increased AMT uptake in astrocytic gliomas was mostly due to high metabolic rates. High AMT uptake due to high volume of tracer distribution was prominent in oligodendrogliomas; this may be attributed to a variety of mechanisms, including disrupted blood–brain barrier, increased tumor vascularization, increased amino acid transport or a combination of these. Increased tryptophan metabolism in low-grade gliomas may have implications for tumor-associated epilepsy. Although increased AMT uptake can occur in nontumorous epileptogenic lesions, such as malformations of cortical development, the general magnitude of increase appears to be much higher in brain tumors: the mean increase of AMT uptake was only 13 ± 6% in our previous series of children with intractable epilepsy not related to tumors [67]. By contrast, the mean increase in brain tumors was almost threefold higher. Thus, AMT PET may aid in the differentiation of brain tumors from nontumorous lesions. This distinction may guide clinical management of patients with epileptogenic lesions.
Immunocytochemical studies on resected tumors demonstrated that tumor cells in six of seven low-grade, well-differentiated tumors expressed IDO, and this immunoreactivity was associated with additional perivascular and neuropil expression in four cases [68]. By contrast, most high-grade tumors either did not express IDO or the expression was confined to the endothelium. Differences in AMT metabolic rates in tumors with widespread versus no or minimal IDO expression support the notion that high metabolic rate of AMT reflects increased metabolism of tryptophan via IDO in primary brain tumors.
The kynurenine pathway is a pharmacological target for the treatment of epilepsy
Interest in tryptophan metabolism via the kynurenine pathway has increased due to the recognition that induction of IDO leads to local tryptophan depletion thus inhibiting cell growth in malignant tumors [69–72]. IDO may also play an important role in antitumoral immune processes by stimulating natural killer cells to express antitumoral killing activity [73,74]. These observations have led to the proposal that pharmacological induction of IDO may be an effective way to slow down tumor cell growth. By contrast, other studies have suggested that enhanced IDO activity in and around tumors may actually exert an immunosuppressive effect by blocking T-lymphocyte proliferation locally, thus diminishing T-cell-mediated tumor rejection [75–78]. Based on this latter mechanism, pharmacological blockade of IDO (e.g., by using the IDO inhibitor 1-methyl-tryptophan) could be clinically useful to reverse local immune suppression [75,77,78]. Several pharmacological approaches to treating epilepsy with agents aimed at the kynurenine pathway in animal models have been reported. Chiarugi et al. studied m-nitrobenzoylalanine, an inhibitor of kynurenine hydroxylase, and o-methoxybenzoylalanine, an inhibitor of kynureninase, and found that both agents increased the concentration of kynurenic acid in hippocampal extracellular space in rats [79]. In addition, they showed that these agents protected against audiogenic seizures in DBA/2 mice. Nemeth et al. administered kynurenine with probenecid to rats with pentylenetetrazol-induced seizures and showed marked inhibition of electrophysiological and behavioral seizure activity [80]. Zhang and colleagues have administered 4-chlorokynurenine, which is converted by astrocytes to the NMDA glycine-site antagonist 7-chlorokynurenic acid, to rats with kainate-induced seizures [81] and in chronic limbic epilepsy [82]. 4-chloro-kynurenine administration in kainate-treated rats delayed seizure onset, reduced total time in seizures and prevented lesions in the piriform cortex in the CA1 region of the hippocampus. Administration of 4-chloro-kynurenine resulted in decreased amplitude and number of population spikes in response to electrical stimulation in the chronic limbic epilepsy rats, but showed no effect on the evoked response in control animals. The same group reported enhanced formation of 7-chloro-kynurenic acid from administered 4-chloro-kynurenine in the pilocarpine seizure model [83]. They hypothesized enhanced conversion was related to astrocytosis induced by pilocarpine treatment.
Future perspective
In the near future, the pharmacological agents under development for treating tumors and epilepsy animal models that target the kynurenine pathway may be applied to epilepsy of many different causes. Furthermore, treatment with these agents might be aided by AMT PET as a biomarker for induction of the kynurenine pathway. In addition, new tracers specific to the serotonin and kynurenine pathways will be useful in understanding specific biochemical changes in epileptic brain. PET tracers labeled with fluorine-18 might aid in more widespread availability of this method to patients.
Executive summary.
-
▪
α-methyl-l-tryptophan is a tryptophan analogue that traces tryptophan transport and metabolism in brain by both the serotonin and kynurenine pathways.
-
▪
Increased uptake of α-methyl-l-tryptophan in epileptic brain regions appears to be primarily, although not exclusively, driven by the kynurenine pathway of tryptophan metabolism.
-
▪
Induction of the kynurenine pathway may be related to immune activation in epileptic cortex and brain lesions such as tubers in patients with tuberous sclerosis complex, and in brain tumors.
-
▪
New pharmacological treatments aimed at the kynurenine pathway, either by enhancing production of neuroprotective metabolites or decreasing production of neurotoxic metabolites, might be guided by imaging tryptophan metabolism with α[11C]methyl-l-tryptophan PET.
-
▪
New tracers specific to tryptophan metabolism by the kynurenine pathway may aid in further understanding the role of this pathway in epileptogenesis.
Footnotes
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Chugani DC, Chugani HT, Muzik O, et al. Imaging epileptogenic tubers in children with tuberous sclerosis complex using α-[11C]methyl-l-tryptophan positron emission tomography. Ann. Neurol. 1998;44:858–866. doi: 10.1002/ana.410440603. [DOI] [PubMed] [Google Scholar]
- 2. Kumar A, Asano E, Chugani HT. α-[11C]- methyl-l-tryptophan PET for tracer localization of epileptogenic brain regions: clinical studies. Biomarkers Med. 2011;5(5):577–584. doi: 10.2217/bmm.11.68. ▪ Describes the clinical application of α-methyl-l-tryptophan in patients with epilepsy.
- 3.Chugani DC, Heyes MP, Kuhn DM, Chugani HT. Evidence that α[11C] methyl-l-tryptophan PET traces tryptophan metabolism via the kynurenine pathway in tuberous sclerosis complex. Soc. Neurosci. Abstracts. 1998;24:1757. [Google Scholar]
- 4.Hamon M, Bourgoin S, Artaud F, El Mestikawy S. The respective roles of tryptophan uptake and tryptophan hydroxylase in the regulation of serotonin synthesis in the central nervous system. J. Physiol. 1981;77:269–279. [PubMed] [Google Scholar]
- 5.Pardridge WM. Kinetics of competitive inhibition of neutral amino acid transport across the blood–brain barrier. J. Neurochem. 1977;28:103–108. doi: 10.1111/j.1471-4159.1977.tb07714.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith QR, Monna S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood–brain barrier. J. Neurochem. 1987;49:1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 7.Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. 1971;173:149–151. doi: 10.1126/science.173.3992.149. [DOI] [PubMed] [Google Scholar]
- 8. Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL. A new method to measure brain serotonin synthesis in vivo, 1: theory and basic data for a biological model. J. Cereb. Blood Flow Metab. 1990;9:1–12. doi: 10.1038/jcbfm.1990.1. ▪ Describes the original description for the use of α-methyl-l-tryptophan to measure serotonin synthesis in vivo.
- 9.Diksic M, Nagahiro S, Chaly T, Sourkes TL, Yamamoto YL, Feindel W. Serotonin synthesis rate measured in living dog brain by positron emission tomography. J. Neurochem. 1991;56:153–162. doi: 10.1111/j.1471-4159.1991.tb02575.x. [DOI] [PubMed] [Google Scholar]
- 10.Missala K, Sourkes TL. Functional cerebral activity of an analogue of serotonin formed in situ. Neurochem. Int. 1988;12:209–214. doi: 10.1016/0197-0186(88)90129-5. [DOI] [PubMed] [Google Scholar]
- 11.Cohen Z, Tsuiki K, Takada A, Beaudet A, Diksic M, Hamel E. In vivo-synthesis radioactively labelled α-methyl serotonin as a selective tracer for visualization of brain serotonin neurons. Synapse. 1995;21:21–28. doi: 10.1002/syn.890210104. [DOI] [PubMed] [Google Scholar]
- 12.Madras BK, Sourkes TL. Metabolism of α-methyl-tryptophan. Biochem. Pharmacol. 1965;14:1499–1506. doi: 10.1016/0006-2952(65)90003-1. [DOI] [PubMed] [Google Scholar]
- 13.Shoaf SE, Schmall B. Pharmacokinetics of α-methyl-l-tryptophan in rhesus monkeys and calculation of the lumped constant for estimating the rate of serotonin synthesis. J. Pharmacol. Exp. Therapeutics. 1996;277:219–224. [PubMed] [Google Scholar]
- 14.Nishizawa S, Benkelfat C, Young SN, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc. Natl Acad. Sci. USA. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muzik O, Chugani DC, Chakraborty P, Mangner T, Chugani HT. Analysis of [11C] α-methyl-tryptophan kinetics for the estimation of serotonin synthesis rate in vivo. J. Cereb. Blood Flow Metab. 1997;17:659–669. doi: 10.1097/00004647-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Chugani DC, Muzik O, Chakraborty P, Mangner T, Chugani HT. Human brain serotonin synthesis capacity measured in vivo with α[11C]methyl-l-tryptophan. Synapse. 1998;28:33–43. doi: 10.1002/(SICI)1098-2396(199801)28:1<33::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Shoaf SE, Carson RE, Hommer D, et al. The suitability of [11C]-α-methyl-l-tryptophan as a tracer for serotonin synthesis: studies with dual administration of [11C] and [14C] labeled tracer. J. Cereb. Blood Flow Metab. 2000;20:244–252. doi: 10.1097/00004647-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 18. Chugani DC, Muzik O. α[11C]methyl-l-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J. Cereb. Blood Flow Metab. 2000;20:2–9. doi: 10.1097/00004647-200001000-00002. ▪▪ Describes the strengths and weaknesses of α-methyl-l-tryptophan as a tracer for both the serotonin and kynurenine pathways.
- 19.Dailey JW, Reigel CE, Mishra PK, Jobe PC. Neurobiology of seizure predisposition in the genetically epilepsy-prone rat. Epilepsy Res. 1989;3:317–320. doi: 10.1016/0920-1211(89)90063-6. [DOI] [PubMed] [Google Scholar]
- 20.Statnick MA, Dailey JW, Jobe PC, Browning RA. Abnormalities in brain serotonin concentration, high-affinity uptake, and tryptophan hydroxylase activity in severe-seizure genetically epilepsy-prone rats. Epilepsia. 1996;37:311–321. doi: 10.1111/j.1528-1157.1996.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 21.Wenger GR, Stitzel RE, Craig CR. The role of biogenic amines in the reserpine-induced alteration of minimal electroshock seizure thresholds in the mouse. Neuropharmacology. 1973;12:693–703. doi: 10.1016/0028-3908(73)90122-6. [DOI] [PubMed] [Google Scholar]
- 22.Lazarova M, Bendotti C, Samanin R. Studies on the role of serotonin in different regions of the rat central nervous system on pentylenetetrazol-induced seizures and the effect of di-n-propylacetate. Arch. Pharmacol. 1983;322:147–152. doi: 10.1007/BF00512388. [DOI] [PubMed] [Google Scholar]
- 23.Maynert EW, Marczynski TJ, Browning RA. The role of the neurotransmitters in the epilepsies. Adv. Neurol. 1975;13:79–147. [PubMed] [Google Scholar]
- 24.Pallister PD. Aggravation of epilepsy by reserpine, associated with possible bleeding and clotting disturbances. Rocky Mt Med. J. 1982;56:45–50. [PubMed] [Google Scholar]
- 25.Louw D, Sutherland GB, Glavin GB, Girvin J. A study of monoamine metabolism in human epilepsy. Can. J Neurol. Sci. 1989;16:394–397. doi: 10.1017/s0317167100029449. [DOI] [PubMed] [Google Scholar]
- 26.Pintor M, Mefford IN, Hutter I, et al. The levels of biogenic amines, their metabolites and tyrosine hydroxylase in the human epileptic temporal cortex. Synapse. 1990;5:152–156. doi: 10.1002/syn.890050210. [DOI] [PubMed] [Google Scholar]
- 27.Trottier S, Evrard B, Vignal JP, Scarabin JM, Chauvel P. The serotonergic innervation of the cerebral cortex in man and its changes in focal cortical dysplasia. Epilepsy Res. 1996;25:79–106. doi: 10.1016/0920-1211(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 28.Toczek MT, Carson RE, Lang L, et al. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003;60:749–756. doi: 10.1212/01.wnl.0000049930.93113.20. [DOI] [PubMed] [Google Scholar]
- 29.Savic I, Lindström P, Gulyás B, Halldin C, Andrée B, Farde L. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004;62(8):1343–1351. doi: 10.1212/01.wnl.0000123696.98166.af. [DOI] [PubMed] [Google Scholar]
- 30.Merlet I, Ryvlin P, Costes N, et al. Statistical parametric mapping of 5-HT1A receptor binding in temporal lobe epilepsy with hippocampal ictal onset on intracranial EEG. Neuroimage. 2004;22:886–896. doi: 10.1016/j.neuroimage.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Merlet I, Ostrowsky K, Costes N, et al. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an [18F]MPPF-PET study. Brain. 2004;127:900–913. doi: 10.1093/brain/awh109. [DOI] [PubMed] [Google Scholar]
- 32.Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J. Neurochem. 2007;100:857–873. doi: 10.1111/j.1471-4159.2006.04277.x. [DOI] [PubMed] [Google Scholar]
- 33.Asano E, Chugani DC, Muzik O, et al. Multimodality imaging for improved detection of epileptogenic foci in tuberous sclerosis complex. Neurology. 2000;54:1976–1984. doi: 10.1212/wnl.54.10.1976. [DOI] [PubMed] [Google Scholar]
- 34.Fedi M, Reutens D, Okazawa H, et al. Localizing value of α-methyl-l-tryptophan PET in intractable epilepsy of neocortical origin. Neurology. 2001;57:1629–1636. doi: 10.1212/wnl.57.9.1629. [DOI] [PubMed] [Google Scholar]
- 35.Haber R, Bessette D, Hulihan-Giblin B, et al. Identification of tryptophan 2,3-dioxygenase RNA in rodent brain. J. Neurochem. 1993;60:1159–1162. doi: 10.1111/j.1471-4159.1993.tb03269.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki F, Kuroiwa T, Takikawa O, Kido R. Human indolylamine 2,3-dioxygenase. Its tissue distribution and characterization of the placental enzyme. Biochem. J. 1985;230:635–638. doi: 10.1042/bj2300635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with endogenous excitant quinolinic acid. Brain Res. 1982;247(1):184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 38.Perkins MN, Stone TW. Pharmacology and regional variation of quinolinic acid-evoked excitations in rat central nervous system. J. Pharmacol. Exp. Ther. 1983;226:551–557. [PubMed] [Google Scholar]
- 39.Vezzani A, Ungerstedt U, French ED, Schwartz R. In vivo brain dialysis of amino acids and simultaneous EEG measurements following intrahippocampal quinolinic acid injections: evidence for a dissociation between neurochemical changes and seizures. J. Neurochem. 1985;45:335–344. doi: 10.1111/j.1471-4159.1985.tb03993.x. [DOI] [PubMed] [Google Scholar]
- 40.Lapin IP. Stimulant and convulsant effects of kynurenines injected into brain ventricles in mice. J. Neural Transm. 1978;32:37–43. doi: 10.1007/BF01262727. [DOI] [PubMed] [Google Scholar]
- 41.Lapin IP. Effect of kynurenine and quinolinic acid on the actions of convulsants in mice. Pharmacol. Biochem. Behav. 1980;13:17–20. [PubMed] [Google Scholar]
- 42.Lapin IP. Convulsant action of intracerebroventricularly administered l-kynurenine sulphate, quinolinic acid and of derivatives of succinic acid, and effects of amino acids, structure–activity relationships. Neuropharmacology. 1982;21(12):1227–1233. doi: 10.1016/0028-3908(82)90125-3. [DOI] [PubMed] [Google Scholar]
- 43.Vezzani A, Stasi M, Wu HQ, Castiglioni M, Weckermann B, Samanin R. Studies on the potential neurotoxic and convulsant effects of increased blood levels of quinolinic acid in rats with altered blood–brain barrier permeability. Exp. Neurol. 1989;106:90–98. doi: 10.1016/0014-4886(89)90149-0. [DOI] [PubMed] [Google Scholar]
- 44.Nakano K, Takahashi S, Mizobuchi M, Kuroda T, Masuda K, Kitoh J. High levels of quinolinic acid in brain of epilepsy-prone E1 mice. Brain Res. 1993;619:195–198. doi: 10.1016/0006-8993(93)91612-v. [DOI] [PubMed] [Google Scholar]
- 45.Eastman CL, Urbanska EM, Chapman AG, Schwarcz R. Differential expression of the astrocytic enzymes 3-hydroxyanthranilic acid oxygenase, kynurenine aminotransferase and glutamine synthetase in seizure-prone and non-epileptic mice. Epilepsy Res. 1994;18:185–194. doi: 10.1016/0920-1211(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 46.Lehrmann E, Guidetti P, Love A, Williamson J, Bertram EH, Schwarcz R. Glial activation precedes seizures and hippocampal neurodegeneration in measles virus-infected mice. Epilepsia. 2008;49(Suppl. 2):13–23. doi: 10.1111/j.1528-1167.2008.01489.x. [DOI] [PubMed] [Google Scholar]
- 47.Feldblum S, Rougier A, Loiseau H, et al. Quinolinic acid phosphoribosyl transferase activity is decreased in epileptic human brain tissue. Epilepsia. 1988;29:523–529. doi: 10.1111/j.1528-1157.1988.tb03756.x. [DOI] [PubMed] [Google Scholar]
- 48.Heyes MP, Wyler AR, Devinsky O, et al. Quinolinic acid concentrations in brain and cerebrospinal fluid of patients with intractable complex partial seizures. Epilepsia. 1990;31:172–177. doi: 10.1111/j.1528-1167.1990.tb06302.x. [DOI] [PubMed] [Google Scholar]
- 49.Scharfman HE, Ofer A. Pretreatment with L-kynurenine, the precursor to the excitatory amino acid antagonist kynurenic acid, suppresses epileptiform activity in combined entorhinal/hippocampal slices. Neurosci. Lett. 1997;224:115–118. doi: 10.1016/s0304-3940(97)13472-3. [DOI] [PubMed] [Google Scholar]
- 50.Wu HQ, Monno A, Schwarcz R, Vezzani A. Electrical kindling is associated with a lasting increase in the extracellular levels of kynurenic acid in the rat hippocampus. Neurosci. Lett. 1995;198:91–94. doi: 10.1016/0304-3940(95)11971-x. [DOI] [PubMed] [Google Scholar]
- 51.Wu HQ, Schwarcz R. Seizure activity causes elevation of endogenous extracellular kynurenic acid in the rat brain. Brain Res. Bull. 1996;39(3):155–162. doi: 10.1016/0361-9230(95)02087-x. [DOI] [PubMed] [Google Scholar]
- 52.Saito K, Nowak TS, Jr, Suyama K, et al. Kynurenine pathway enzymes in brain: responses to ischemic brain injury versus systemic immune activation. J. Neurochem. 1993;61:2061–2070. doi: 10.1111/j.1471-4159.1993.tb07443.x. [DOI] [PubMed] [Google Scholar]
- 53.Hery F, Chouvet G, Kan JP, et al. Daily variations of various parameters of serotonin metabolism in the rat brain. II Circadian variations in serum and cerebral tryptophan levels: lack of correlations with 5-HT turnover. Brain Res. 1977;123:137–145. doi: 10.1016/0006-8993(77)90648-5. [DOI] [PubMed] [Google Scholar]
- 54.Batista C, Chugani DC, Luat A, et al. Differential expression of the kynurenine pathway enzymes in epileptogenic tubers in tuberous sclerosis complex (TSC). Presented at: 64th Annual Meeting of the American Epilepsy Society; 3–7 December 2010; San Antonio, TX, USA. [Google Scholar]
- 55.El-Hashemite N, Zhang H, Walker V, Hoffmeister KM, Kwiatkowski DJ. Perturbed IFN-γ-Jak-Signal transducers and activators of transcription signaling in tuberous sclerosis mouse models: synergistic effects of rapamycin-IFN-γ treatment. J. Cancer Res. 2004;64:3436–3443. doi: 10.1158/0008-5472.CAN-03-3609. [DOI] [PubMed] [Google Scholar]
- 56.El-Hashemite N, Kwiatkowski DJ. Interferon-γ-Jak-Stat signaling in pulmonary lymphangioleio-myomatosis and renal angiomyolipoma. Am. J. Respir. Cell Mol. Biol. 2005;33:227–230. doi: 10.1165/rcmb.2005-0152RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Byrne GI, Lehmann LK, Landry GJ. Induction of tryptophan catabolism is the mechanism for γ-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect. Immun. 1986;53:347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfefferkorn ER, Rebhun S, Eckel M. Characterization of an indoleamine 2,3-dioxygenase induced by γ-interferon in cultured human fibroblasts. J. Interferon Res. 1986;6:267–279. doi: 10.1089/jir.1986.6.267. [DOI] [PubMed] [Google Scholar]
- 59.Chon SY, Hassanain HH, Pine R, Gupta SL. Involvement of two regulatory elements in interferon-γ-regulated expression of human indoleamine 2,3-dioxygenase gene. J. Interferon Cytokine Res. 1995;15:517–526. doi: 10.1089/jir.1995.15.517. [DOI] [PubMed] [Google Scholar]
- 60.Boer K, Jansen F, Nellist M, et al. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res. 2008;78(1):7–21. doi: 10.1016/j.eplepsyres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Yadav MC, Burudi EM, Alirezaei M, et al. IFN-γ-induced IDO and WRS expression in microglia is differentially regulated by IL-4. Glia. 2007;55(13):1385–1396. doi: 10.1002/glia.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosner M, Hanneder M, Siegel N, Valli A, Hengtstschlager M. The mTOR pathway and its role in human genetic diseases. Mutation Res. 2008;658(3):234–246. doi: 10.1016/j.mrrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Lee DF, Kuo HP, Chen CT, et al. IKKβ suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130(3):440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 64.Weichhart T, Costantion G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Chugani DC, Muzik O, Chakraborty P, Mangner T, Chugani HT. Human brain serotonin synthesis capacity measured in vivo with α[11C]methyl-l-tryptophan. Synapse. 1998;28:33–43. doi: 10.1002/(SICI)1098-2396(199801)28:1<33::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 66.Juhász C, Chugani DC, Muzik O, et al. α-methyl-l-tryptophan PET detects epileptogenic cortex in children with intractable epilepsy. Neurology. 2003;60:960–968. doi: 10.1212/01.wnl.0000049468.05050.f2. [DOI] [PubMed] [Google Scholar]
- 67.Juhász C, Chugani DC, Muzik O, et al. In vivo uptake and metabolism of α-[11C] methyl-l-tryptophan in human brain tumors. J. Cereb. Blood Flow Metab. 2006;26(3):345–357. doi: 10.1038/sj.jcbfm.9600199. [DOI] [PubMed] [Google Scholar]
- 68.Batista CE, Juhász C, Muzik O, et al. Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol. Imaging Biol. 2009;11(6):460–466. doi: 10.1007/s11307-009-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ozaki Y, Edelstein MP, Duch DS. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon-γ. Proc. Natl Acad. Sci. USA. 1988;85:1242–1246. doi: 10.1073/pnas.85.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aune TM, Pogue SL. Inhibition of tumor cell growth by interferon-γ is mediated by two distinct mechanisms dependent upon oxygen tension: induction of tryptophan degradation and depletion of intracellular nicotinamide adenine dinucleotide. J. Clin. Invest. 1989;84:863–875. doi: 10.1172/JCI114247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor MW, Feng GS. Relationship between interferon-γ, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 72.Burke F, Knowles RG, East N, Balkwill FR. The role of indoleamine 2,3-dioxygenase in the anti-tumour activity of human interferon-γ in vivo. Int. J. Cancer. 1995;60:115–122. doi: 10.1002/ijc.2910600117. [DOI] [PubMed] [Google Scholar]
- 73.Kai S, Goto S, Tahara K, Sasaki A, Kawano K, Kitano S. Inhibition of indoleamine 2,3-dioxygenase suppresses NK cell activity and accelerates tumor growth. J. Exp. Ther. Oncol. 2003;3:336–345. doi: 10.1111/j.1533-869x.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- 74.Kai S, Goto S, Tahara K, Sasaki A, Tone S, Kitano S. Indoleamine 2,3-dioxygenase is necessary for cytolytic activity of natural killer cells. Scand. J. Immunol. 2004;59:177–182. doi: 10.1111/j.0300-9475.2004.01378.x. [DOI] [PubMed] [Google Scholar]
- 75.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol. Today. 1999;20:469–473. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 76.Friberg M, Jennings R, Alsarraj M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int. J. Cancer. 2002;101:151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 77.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 78.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol. Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 79.Chiarugi A, Carpenedo R, Molina MT, Mattoli L, Pellicciari R, Moroni F. Comparison of the neurochemical and behavioral effects resulting from the inhibition of kynurenine hydroxylase and/or kynureninase. J. Neurochem. 1995;65:1176–1183. doi: 10.1046/j.1471-4159.1995.65031176.x. [DOI] [PubMed] [Google Scholar]
- 80.Nemeth H, Robotka H, Kis Z, et al. Kynurenine administered together with probenecid markedly inhibits pentylenetetrazol-induced seizures. An electrophysiological and behavioural study. Neuropharmacology. 2004;47:916–925. doi: 10.1016/j.neuropharm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 81.Wu HQ, Lee SC, Scharfman HE, Schwarcz R. l-4-chlorokynurenine attenuates kainate-induced seizures and lesions in the rat. Exp. Neurol. 2002;177:222–232. doi: 10.1006/exnr.2002.7971. [DOI] [PubMed] [Google Scholar]
- 82.Zhang DX, Williamson JM, Wu HQ, Schwarcz R, Bertram EH. In situ-produced 7-chlorokynurenate has different effects on evoked responses in rats with limbic epilepsy in comparison to naive controls. Epilepsia. 2005;46(11):1708–1715. doi: 10.1111/j.1528-1167.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 83.Wu HQ, Rassoulpour A, Goodman JH, Scharfman HE, Bertram EH, Schwarcz R. Kynurenate and 7-chlorokynurenate formation in chronically epileptic rats. Epilepsia. 2005;47(7):1010–1016. doi: 10.1111/j.1528-1167.2005.67404.x. [DOI] [PubMed] [Google Scholar]