Abstract
Nitrogen adsorption/desorption isotherms are used to investigate the Brunauer, Emmett, and Teller (BET) surface area and Barrett-Joyner-Halenda (BJH) pore size distribution of physically modified, thermally annealed, and octadecanethiol functionalized np-Au monoliths. We present the full adsorption-desorption isotherms for N2 gas on np-Au, and observe type IV isotherms and type H1 hysteresis loops. The evolution of the np-Au under various thermal annealing treatments was examined using scanning electron microscopy (SEM). The images of both the exterior and interior of the thermally annealed np-Au show that the porosity of all free standing np-Au structures decreases as the heat treatment temperature increases. The modification of the np-Au surface with a self-assembled monolayer (SAM) of C18-SH (coverage of 2.94 × 1014 molecules cm−2 based from the decomposition of the C18-SH using thermogravimetric analysis (TGA)), was found to reduce the strength of the interaction of nitrogen gas with the np-Au surface, as reflected by a decrease in the ‘C’ parameter of the BET equation. From cyclic voltammetry studies, we found that the surface area of the np-Au monoliths annealed at elevated temperatures followed the same trend with annealing temperature as found in the BET surface area study and SEM morphology characterization. The study highlights the ability to control free-standing nanoporous gold monoliths with high surface area, and well-defined, tunable pore morphology.
Introduction
In recent years, nanoporous gold has attracted researchers across multiple disciplines partly by the prospect for its potential applications in electrochemical sensors,1 biosensors,2,3 heat exchange,4 catalysis,5 supported synthesis,6 and actuation.7,8 The unique architecture of np-Au, consisting of a nanometre scale pore network and ligament framework, by which we mean its bicontinuous structure of metallic ligaments and channels on the order of 10–100 nm in size depending on the processing parameters, provides a high surface-to-volume ratio, tunable porosity, chemical stability and biocompatibility. Many recent studies have focused on physical modification,9,10 thermal annealing,11 or functionalization of the np-Au framework.12–17
In order to take advantage of the high surface to volume ratio of np-Au in various applications, it is important to better characterize its surface area. Surface areas of np-Au materials are commonly reported as BET surface areas, SBET, obtained by applying the Brunauer, Emmett, and Teller (BET) theory to analyze nitrogen adsorption/desorption isotherms measured at 77 K. The physical adsorption of inert gases (usually nitrogen, argon or krypton) is the most often used method for obtaining information on the surface areas and morphological properties of porous solids. With the aid of user-friendly commercial equipment with built-in data processing, nitrogen adsorption isotherm measurement and analysis at 77 K is commonly applied for the investigation of new materials. These instruments measure the amount of N2 molecules adsorbed onto the sample using gravimetric, volumetric, or dynamic flow-through methods.18 To determine surface area, the experimental isotherm must be fit using a model, most prevalently that developed by Brunauer, Emmett, and Teller.19 The model for the BET isotherm uses the binding energy between the adsorbate and the surface for the first monolayer taken as equal to the isosteric heat of adsorption, and a second binding energy for adsorption of all subsequent mono-layers that is taken as equal to the molar heat of condensation.
Many studies on np-Au employ machined millimetre-sized ingots, wires, or foils of the initial precursor alloy. Regardless of the end product, the nanoporous gold exhibits a high surface-to-area ratio that is key for using the material in catalysis, molecular separations, etc.20 Understanding the morphological characteristics and surface area should be the starting point before adapting np-Au in various applications. In addition to the total surface area, knowledge of pore size distributions and morphological features of np-Au could aid fundamental studies of mechanical and surface properties, and of surface functionalization via thiol-conjugate chemistry for biological applications.
The most commonly reported method employed for controlling the morphology of nanoporous metals is via coarsening of ligaments and pores by thermal or acid post-treatments.3,5,8,9,21–24 Such preparations are relatively convenient and highly reproducible either via exposing the sample to high temperatures or to an acid environment over a chosen period of time.25–29 Attempts to coarsen the pore and ligament sizes of np-Au were conducted by Sieradzki et al.28 Based on their study, thermal treatment of the np-Au produced a uniformly coarsened surface. Subsequently, Mabuchi et al. compared the effects of thermal treatment and acid treatment in coarsening of the pore and ligament sizes of np-Au sheets.27 They observed that thermal treatment resulted in a uniformly coarsened porous surface, while hydrochloric acid treatment of np-Au produced a new surface morphology.27 Others have shown that prolonged dealloying in nitric acid led to ligament coarsening similar to the coarsening observed during thermal treatment.25 The varied outcomes of these reported treatments may be due to different gold migration routes or diffusion rates arising as the sample attempts to reduce the surface energy by rearrangement of gold atoms in order to form nanoporous structures with minimal surface energy.30 Nevertheless, these simple treatments can drastically alter the morphology of the nanoporous gold and change the surface area.
In this paper, the pore sizes and surface areas of bare and modified np-Au are evaluated using nitrogen adsorption/desorption isotherms and then the SBET and BJH pore size distribution analysis. The use of np-Au monoliths provides a sufficient mass of sample to carry out the isotherm measurements and BET analysis. The evolution of the np-Au samples under various thermal annealing treatments was examined using scanning electron microscopy (SEM) and cyclic voltammetric analysis. We also have surface modified np-Au with a self-assembled monolayer of C18-SH and found that this has a significant impact on the isotherm behavior. Herein, we also demonstrate the application of thermogravimetric analysis (TGA) to estimate the surface coverage of a self-assembled monolayer inside an np-Au monolith. The behavior of samples of np-Au that have been mashed into smaller fragments is compared with that of the intact monoliths. The samples are subject to pore size analysis by the BJH method, and the pore sizes obtained are compared with those seen in the SEM images. The results of the BJH analysis indicate that the behavior of the adsorptive N2 within the pores is subject to pore network and pore blockage effects.
Experimental section
Preparation of nanoporous gold plates
Preparation of nanoporous gold plates (monoliths) was performed according to literature procedures, with a few modifications.3,31 In brief, 10 carat white gold sheets (4.0 inch × 2.0 inch × 0.0098 inch, L × W × H, respectively) were purchased from Hoover and Strong (Richmond, Virginia, USA). The stated atomic composition of this commercial alloy is 41.8% Au, 5.0% Ag, 30.0–35.0% Cu, 8.0–9.0% Zn and 15.0–20.0% Ni. The 10 carat sheet was cut into pieces of size of 2.0 mm × 2.0 mm × 0.25 mm, L × W × H, respectively, and placed in a concentrated nitric acid bath for 48 h (the acid solution was refreshed at the 24 h time point). Trace metal grade nitric acid and sulfuric acid were purchased from Fisher Scientific (Pittsburgh, Philadelphia, USA). The sample size was specifically chosen for convenience of handling during incubation in protein solutions32 and for fitting into a standard BET sample holder (Beckman Coulter SA-3100 Gas Adsorption Surface Area and pore size Analyzer, Beckman Coulter, Inc., California, USA). After the nitric acid de-alloying treatment, the sample was rinsed thoroughly with Milli-Q water (18.2 MU, Millipore Corporation, Boston, USA) to neutral pH, followed by rinsing with HPLC grade ethanol (Sigma Aldrich, St. Louis, Missouri, USA). The microstructure of np-Au was characterized using scanning electron microscopy using a JEOL JSM-6320F field emission SEM (JEOL USA, Inc., California, USA).
Solvents and compounds for SAM formation
1-octadecanethiol (referred to as C18-SH) with a purity of more than 97.0% was purchase from Sigma-Aldrich (St. Louis, Missouri, USA). HPLC grade ethanol solvent was purchased from Sigma-Aldrich (St. Louis, Missouri, USA). All chemicals and reagents were used as received. Modification of np-Au with a SAM of C18-SH was accomplished by immersing the np-Au monolith in a solution of C18-SH in ethanol (20 mM) for a period of 48 h, followed by rinsing and repeated immersion and removal from pure solvent.
Thermal annealing of free-standing nanoporous structures
The freshly dealloyed np-Au pieces were washed and neutralized with ultrapure water, followed by rinsing with ethanol and soaking for 3 h prior to drying. A simple house vacuum system was used to dry the np-Au in a desiccator for 24 h. A commercial Barnstead/Thermolyne furnace (Batavia, Illinois, USA) was preheated to the desired temperature of 100 °C, 200 °C, 300 °C or 400 °C, prior to the introduction of the np-Au (2.0 mm × 2.0 mm × 0.25 mm) samples.
Surface area and pore size analysis of np-Au
The surface structure parameters were calculated from the N2 adsorption/desorption isotherms, which were obtained using an automated surface area analyzer (Beckman Coulter SA-3100 Gas Adsorption Surface Area and Pore Size Analyzer, Beckman Coulter, Inc. California, USA). The instrument has a stated resolution of >0.01 m2 g−1. A standard BET sample holder (3cc RapiTube, model number 7215 006B, Beckman Coulter, Inc. California, USA) was used to hold the nanoporous gold samples. The sample holder was loaded with 20 pieces of np-Au (2.0 mm × 2.0 mm × 0.25 mm) and the total mass of Au present during the analysis was typically 0.15–0.26 g. The samples were first outgassed at 50 °C for 60 min under vacuum to a final pressure of 0.28 Pa and then the isotherm measured over the relative pressure range of (PS/PO) from 0.01 to 0.991 and back. The BET specific surface area (SBET) and the volume of monolayer coverage were determined using the Brunauer-Emmett-Teller (BET) equation. The np-Au surface area was calculated from both the BET and Langmuir models, and the pore volume was calculated. The pore volume versus diameter distribution was calculated by analyzing both the adsorption and desorption branches of the isotherm using the Barrett-Joyner-Halenda method.19,33 The interior and exterior surface morphologies of the np-Au were observed using scanning electron microscopy.
Electrochemical estimation of np-Au surface area
The effective electrode surface areas of the un-annealed and thermally annealed np-Au monoliths were determined using cyclic voltammetry (CV) carried out using a PARSTAT 2273 Advanced Electrochemical System (Princeton Applied Research, Tennessee, USA). All potentials were measured against a saturated Ag|AgCl reference electrode (CH Instruments, Inc., Texas, USA), and the counter electrode was a platinum wire of diameter 0.76 mm that was coiled into a spring shape of length 15.0 mm. All scans were started at the negative end of the potential range of −0.2 V to 1.8 V (vs. Ag|AgCl). The same np-Au monoliths as those used for BET investigations were fashioned into electrodes by wrapping them a few times with a fine diameter gold wire (d = 0.127 mm, Electron Microscopy Sciences, Pennsylvania, USA) from which they would simply hang for immersion into the electrochemical cell. The surface redox behavior of the np-Au substrates was characterized using CV in 0.5 M sulfuric acid. The charge passed during reduction of gold oxide was calculated by integration of the gold oxide stripping peak. The specific charge equivalent of 450 µC cm−2 was used for converting the charge passed during the gold oxide reduction to a total surface area, which was then divided by the mass of the np-Au electrode in order to obtain a specific surface area per unit mass that could be compared with the results of the BET measurements.34 A series of scan rates were applied, ranging from 0.1–100 mV s−1. At the lowest scan rate of 0.1 mV sec−1, the CV took 666.7 min to be acquired.
Thermogravimetric analysis
Thermogravimetric analysis (TGA) was performed using a Q500 Thermogravimetric Analyzer (TA Instruments, Delaware, USA). The mass of each np-Au sample with a typical dimension of 8.0 mm × 8.0 mm × 0.25 mm was approximately 0.08 to 0.1 g. The carrier gas used was nitrogen at a flow rate of 40.0 mL min−1. The np-Au samples were placed in a platinum weighing boat and heated from room temperature to 600 °C at a ramping rate of 5.0 °C min−1. The experiments were repeated multiple times to confirm the reproducibility and authenticity of the generated data.
Result and discussion
Thermal annealing of nanoporous gold
Dealloying in nitric acid is one of the most convenient and reliable strategies used to prepare np-Au.3,6,32 The process of dealloying has been described in terms of a percolation model, in which the less noble component of an alloy dissolves into solution by selective leaching. Percolation allows the solution to move into the bulk of the alloy as the less noble atoms are oxidized and diffuse out leaving the nobler atoms (Au in this case) able to diffuse and aggregate into new structures.35 This process results in an openly nanoporous, bicontinuous structure, following the rearrangement of the gold atoms into interconnected ligaments as the silver atoms are selectively removed.9,21,32
Recent studies focusing on porosity formation have been reported by Erlebacher et al., in which an elegant kinetic Monte Carlo model was used to describe the formation of nanoporosity. 4,9,21,36 In this model, the nanoporosity formation can be explained by a process that is achieved via the suggested spinodal decomposition (or atomistic model) driven rearrangement of the gold atoms from the alloy/electrolyte interface to form two-dimensional clusters at the surface of the alloy, as silver is dissolved. Through this phase separation process at the solid-liquid interface, new underlying alloy is constantly exposed to the electrolyte for further dealloying and the formation of the nanoscale ligaments as this process works its way into the alloy material.
Using field ion microscopy or scanning probe microscopy, atomic resolution imaging of gold atoms can be achieved, thus allowing for reliable measurement of the surface diffusion constant.37,38 Such a study is feasible under wide range of temperatures, and the movement of gold atoms, nanoscale pit decay, and clustering of atoms on gold surfaces can be monitored. In 1995, Blanckenhagen et al., took advantage of the direct surface modification and imaging capability of atomic force microscopy (AFM) to investigate surface self-diffusion on a polycrystalline gold surface.39 His study encompassed the measurement and determination of the diffusion constant in the temperature range from 300 K (26.9 °C) to 773 K (426.9 °C). Based on his AFM measurements, a diffusion constant (Ds) of Ds = 1.2 × 10−18 m2 s−1 was obtained at room temperature. Similarly, Ds values of 1.9 × 10−15 m2 s−1 and 1.2 × 10−14 m2 s−1 were obtained at 300.0 °C and 500.0 °C, respectively.
Due to its high surface area-to-volume ratio, many have been inspired to use np-Au for various applications in sensing15 and in catalysis.3,40 Modifying the np-Au pore size, ligament diameter or overall morphology can be an important requirement for applications, such as surface-enhanced Raman spectroscopy, catalysis, and hosting of biomolecules.41 We follow the most frequently used method to modify the morphology of np-Au by using thermal annealing to coarsen the pore diameter and ligament dimensions. The annealed np-Au was then subject to the BET analysis, in an effort to understand the variations in the surface area, pore volume, and pore size distribution of the annealed np-Au.
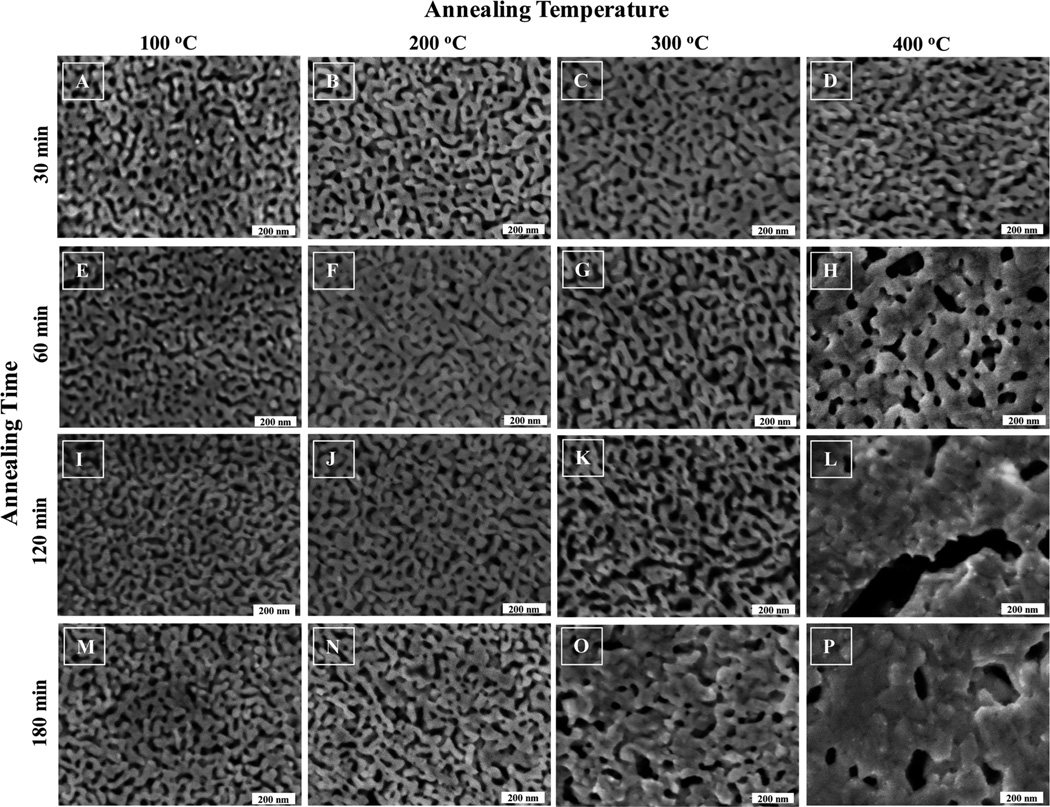
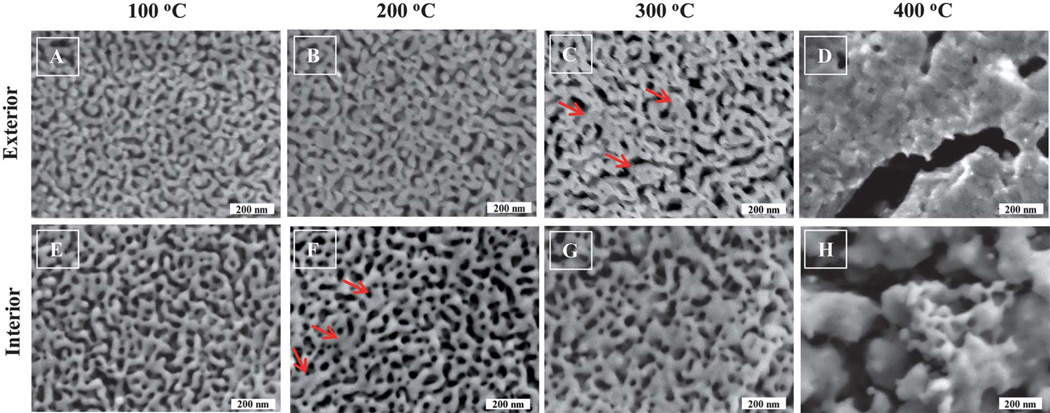
Fig. 1 shows the SEM images of the dealloyed np-Au that was subjected to various annealing temperatures and times. The pores do not present themselves as regularly cylindrical features and instead display a range of irregularly shaped exits on the np-Au exterior. The range of dimension of these openings visually falls approximately in the range 50–200 nm. According to the classification scheme proposed in 1960 by Dubinin, the pores present in these np-Au samples fall in the upper end of the mesopore (2–50 nm) to small macropore (greater than 50 nm) range.33,42 The presence of micropores (<2 nm) is not anticipated. Panel 1 from Fig. 1, is np-Au annealed at 100 °C using a commercial furnace at the various annealing times as listed. From visual observation, no apparent or obvious morphology differences, in term of pore size or ligament diameter, relative to the un-annealed sample are observed. Similar observations are evident for np-Au prepared using the same annealing times of 30, 60, 120 and 180 min at 200 °C. At higher annealing temperatures, such as 300 °C and 400 °C, the appearance of the np-Au morphology changes from those prepared at lower temperatures. This observation is similar to those reported elsewhere, for annealing of thin (<1 µm) np-Au films.29 The images of the exterior of the thermally annealed np-Au show that the porosity of all free standing np-Au structures decreases as the heat treatment temperature increases. At more elevated temperatures, np-Au displays the evolution of large clusters of gold enclosing pores that vary in size with increasing temperature.
Fig. 1.
SEM images of the exterior of thermally annealed nanoporous gold. Nitric acid dealloyed np-Au plates were subjected to the indicated annealing temperatures and times. Gradual and small morphology changes for np-Au were observed for annealing temperatures of 100 °C and 200 °C. More noticeable changes were observed at 300 °C. Drastic changes in np-Au morphology were apparent for np-Au annealed at 400 °C. The first observation of np-Au fusion/nucleation begins at 400 °Cat 60 min annealing time. The SEM image for un-annealed np-Au was very similar to A above, and is not shown.
When comparing the thermally annealed np-Au below 200 °C at the stated time points, the changes in surface area are not obvious from visual inspection based on SEM images. Since surface area is a crucial parameter for optimizing the use of np-Au for many potential applications in emerging fields of technology including catalysis, sensing, optics, and electronics, further study of the surface area properties of the above mentioned np-Au samples was pursued. Surface areas are commonly reported as BET surface areas obtained by applying the theory of Brunauer, Emmett, and Teller to nitrogen adsorption isotherms measured at 77 K.19 Nitrogen at 77 K is considered to be a standard adsorbent for surface area and pore size analysis. This is a standard procedure that allows for comparisons among different materials and with benchmark materials from the literature.
The changes in surface area of these thermally annealed np-Au samples can be seen clearly from the surface area and pore size analysis. Based on visual observation, the np-Au annealed at 300 °C displayed substantial changes in pore size and ligament morphology, when compared to np-Au treated at 100 °C or 200 °C. For detailed BET analysis, we elected to investigate the np-Au annealed for 2 h at various temperatures. The 2 h annealing time selection was chosen because the 1 h annealing does not show much change in morphology, and at the 3 h annealing time, ligament fusion was observed, suggesting much lower surface areas.
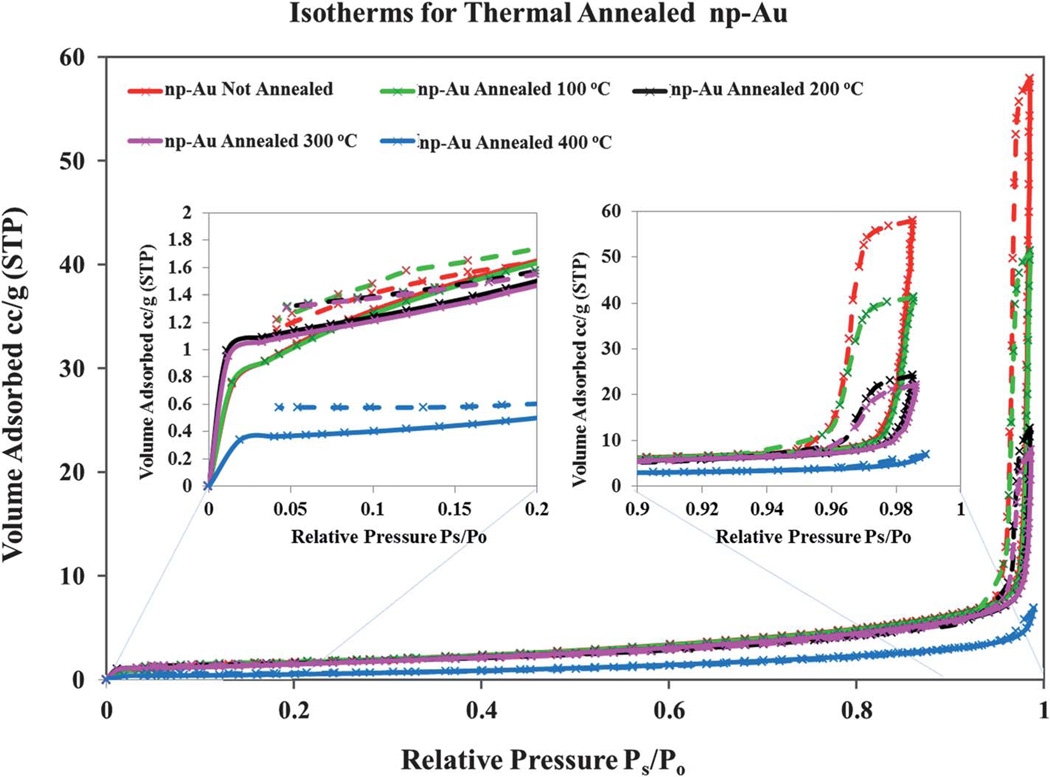
The isotherms shown in Fig. 2 for np-Au that was not annealed and for np-Au annealed at a series of temperature show class IV behavior, as expected for a mesoporous material. In these isotherms, a sharp “knee” point near Ps/Po around 0.01–0.02 was observed (see inset of Fig. 2). In theory, the sharp “knee” point of the isotherm provides a measure of the monolayer capacity, from which the surface area of the adsorbent can be calculated.33,42 The initial part of the curve (at low Ps/Po) is attributed to monolayer-multilayer adsorption on the internal surfaces of the material. The steep increase in the isotherm slope at high Ps/Po above 0.95, can be explained as due to capillary condensation within the pores followed by saturation as the pores become filled with liquid.33,43 Due to the pore size being at the upper mesopore to low macropore range, the capillary condensation and steep rise in the isotherm occurs at relatively high values of Ps/Po, as expected on the basis of the Kelvin equation. The sharpness of the transition in the present case is attributed to a fairly narrow pore size (dp) distribution, between about 50 nm–200 nm, as based on SEM analysis (see Fig. 1). The relative pressure at which the transition occurs depends on the thermal treatment to which the np-Au has been subjected as this alters the pore size. The isotherms rise slightly later for the annealed samples, which should have larger pores overall; this observation is also consistent with the Kelvin equation relating pressure at which condensation occurs to the core diameters of the pores. (see Fig. 2).
Fig. 2.
Nitrogen adsorption and desorption isotherms for thermally annealed np-Au samples. The sorption isotherms for the un-annealed np-Au (red), and thermally annealed np-Au at 100 °C (green), 200 °C (black), 300 °C (pink), and 400 °C (blue) are shown. The isotherm of each substrate shows a distinct sharp “knee” point near Ps/Po around 0.01–0.02. The np-Au isotherms present a steep adsorption/desorption at very high relative pressures characteristic of an upper mesopore to low macropore range solid. The individually recorded data points are represented as crosses and the interpolating lines are those generated by the BET apparatus software.
The hysteresis loops observed in the isotherms of Fig. 2 resemble those classified as being of the H1 type.44–46 Such hysteresis is assumed to arise due to the different radii of curvature that enter the Kelvin equation when comparing capillary condensation and evaporation in a cylindrical pore open at both ends.44 Such hysteresis behavior appears in the steep region of the isotherm, 0.94 < Ps/Po < 1, of the true equilibrium, where the ascending and descending branches resemble two parallel lines, separated from each other evenly, as seen in Fig. 2. Based on experimental observation, the time required for the measurement to equilibrate point by point and traverse the steep regions of the isotherms may last several hours while the time required to complete the measurements is within 1 h in the lower Ps/Po regime.
The analysis of the pore size distribution in mesoporous and especially monolithic samples such as those studied here is subject to both pore network and pore blockage effects. A consequence of these effects is that the calculated pore size distributions can differ drastically depending on whether they are obtained by analysis of the adsorption branch or desorption branch of the isotherm.33,42 The analysis of the adsorption branch is deemed as giving a more realistic assessment of the pore size distribution in this sort of sample. During the condensation occurring along the adsorption branch, an empty pore surrounded by filled pores will be able to fill since the vapor bubble can condense and the pore filled by liquid flow. In contrast, as evaporation occurs along the desorption branch a gas bubble cannot nucleate if the pore pathway to the exterior is blocked by other filled pores. The np-Au samples studied here were found to exhibit very different pore size distributions for the adsorption versus desorption branches, and those found from analysis of the adsorption branches were in better qualitative agreement with the SEM observations.
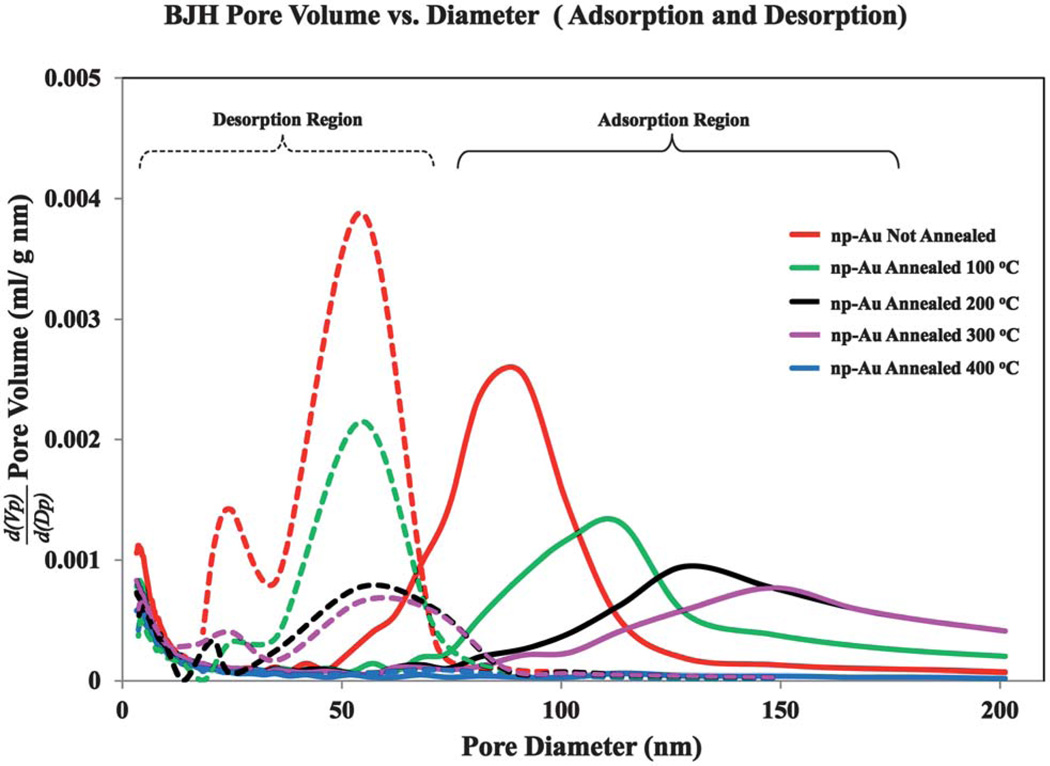
The Barrett-Joyner-Halenda (BJH) analysis results for np-Au that was not annealed and for np-Au annealed at 100 °C, 200 °C, 300 °C, and 400 °C are shown in Fig. 3. Although pores may not be truly cylindrical, we refer to their sizes as diameters consistent with this model. The pore size distributions obtained from the BJH analysis differ greatly depending on whether the distribution is taken from the adsorption branch of the isotherm or from the desorption branch. The most probable pore diameter is lower when obtained from the desorption branch, which is an outcome also reported for the case of silica gel GS80.33,42 The most probable pore diameter does not vary significantly as calculated from the desorption branch data, whereas for the adsorption branch data, the most probable pore diameter increases with the annealing temperature and gives a more realistic result. The area under the pore size distribution curves decreases with annealing temperature, consistent with the decrease in total surface area. The BET surface area, SBET, of un-annealed np-Au is 6.4 m2 g−1, and then decreases for np-Au annealed at progressively higher temperatures, as summarized in the data shown in Table 1. These results are consistent with the SEM images shown in Fig. 1I–L, which show a trend from little to no change in pore or ligament sizes for the samples treated at 100 °C, to extensive fusion for the sample annealed at 400 °C. The BET method was previously applied by Ji and Searson to study np-Au nanowires, and SBET of 3.7–6.9 m2 g−1 were reported.47 The BET and Langmuir surface areas, the pore volumes, and the most probable pore diameters (based on both the desorption and adsorption branches of the isotherms) of the np-Au annealed at various temperatures are summarized in Table 1. The values of the C parameter of the BET equation are also reported in Table 1. The C parameter is a measure of the strength of the interaction of the adsorbate (N2) with the surface and the higher the C parameter, the sharper the ‘knee point’ in the early part of the isotherm at low Ps/Po.33,42 The value of C for nitrogen adsorption at 77 K on porous inorganic materials such as alumina or silica is typically in the range of 80–150.18 The C values shown in Table 1 for the np-Au samples, both un-annealed and annealed at a series of temperatures, are similar and ~30.
Fig. 3.
Barrett-Joyner-Halenda (BJH) pore-size distribution and nitrogen adsorption-desorption isotherms for np-Au that was not annealed and for np-Au annealed at 100 °C, 200 °C, 300 °C, and 400 °C. The area under the pore size distribution curves decreases with annealing temperature, consistent with the decrease in total surface area.
Table 1.
Surface properties of nanoporous gold annealed at various temperatures
| Nanoporous Gold annealing T/ °C |
Pore Size (BJH), nm |
Surface Area (SBET), m2 g−1 |
Surface Area (Langmuir), m2 g−1 |
Pore Volume, cm3 g−1 |
C value of BET equation |
|---|---|---|---|---|---|
| Room temperature | 82 | 6.4 | 4.94 | 0.044 | 34.32 |
| 100 | 102 | 6.35 | 4.79 | 0.034 | 33.13 |
| 200 | 129 | 5.37 | 5.16 | 0.021 | 32.83 |
| 300 | 148 | 4.57 | 2.8 | 0.015 | 30.96 |
| 400 | 167 | 1.79 | 1.7 | 0.0076 | 26.52 |
The total BET surface areas steadily decrease as the annealing temperature of the np-Au samples increases. This phenomenon can be accounted for as a consequence of the surface self-diffusion of gold atoms, resulting in coarsened ligaments, increased pore diameters, and reduced surface area and surface energy. The total BET surface area can be seen to decrease from SBET ~ 6.4 m2 g−1 to SBET ~ 1.8 m2 g−1, together with steady contraction of the total pore volume from 0.04 cm3 g−1 to 0.008 cm3 g−1, as the annealing temperature increases. The BET obtained surface areas are always larger than those obtained from the simple Langmuir model. The SEM images shown in Fig. 1 indicate that at a low annealing temperature (<200 °C), minimal thermal coarsening was observed and the pore and ligament structures seen in the un-annealed sample were preserved to some extent. At elevated annealing temperatures (>200 °C), the pore structure of np-Au is altered more noticeably.
In comparing the surface area of np-Au with other materials, the high density of Au (19.8 g cm−3) versus that of other typical materials used to create porous monoliths should be kept in mind. For example, controlled pore glass, which bears some similarities in structure to np-Au, is made of silica with a density of 2.65 g cm−3. Typical surface areas for controlled pore glass are in the range 10–350 cm2 g−1, depending on its average pore size which can vary over a wide range and be as large as 400 nm.33,42 On a per gram basis, the surface area of np-Au is much lower; however, on a surface area per unit volume basis, the comparison of np-Au and controlled pore glass would be much more favorable, considering the density ratio of ~7.5.
Similar observations of the np-Au structural units (pore and ligament sizes) were reported by Hosson et al. in 2011.48,49 These authors employed a combination of analytical, computational, and experimental approaches to correlate the specific area of nanoporous materials to their solid bulk density and ligament size. Using dimensional analysis, the authors proved that the specific surface area S=(C/ρd), where d is the ligament diameter, ρ is the bulk density (19.8 g cm−3 for Au), and C is a parameter characteristic of the pore geometry. The relation for S is subject to the assumption that the pore and ligament dimensions are equivalent. For the case of a disordered (random) nanoporous material, the value C ~ 3.7 was determined by Hosson et al. from BET data and ligament diameters, and we use this value here also. To corroborate their findings, twenty ligaments were randomly selected from each SEM micrograph for np-Au samples that were annealed for 120 min at temperatures of 100 °C, 200 °C, and 300 °C(panel 3 in Fig. 1) and also for np-Au that was not annealed (image not show). The diameters of these ligaments were then determined from the SEM images. The ligament size based on these SEM micrographs, denoted as dSEM, was then compared with the ligament size estimated using the analytical expression of Hosson, denoted as dAE, with dAE = C/(ρSBET) using the BET surface areas determined herein for an annealing time of 120 min. The agreement of the measured values, dSEM, with the values of dAE obtained using the equation formulated by Hosson et al.49 is good (within 5%). For our np-Au sample without annealing we found dSEM = 28.8 × 2.6 nm, and dAE = 29.2 nm using the SBET value of 6.4 m2 g−1 for this sample. Similar calculations and measurements gave dSEM = 29.2 × 3.4 nm versus dAE = 28.9 nm for the sample annealed at 100 °C (SBET = 6.35 m2 g−1), dSEM = 35.1 × 3.4 nm versus dAE = 34.8 nm for the sample annealed at 200 °C (SBET = 5.37m2 g−1), and dSEM =41.9 × 5.2 nm versus dAE = 40.9 nm for the sample annealed at 300 °C (SBET = 4.57m2 g−1). Therefore, by utilizing Hosson’s equation, one can predict the specific surface area of arbitrary nanoporous materials given the average ligament diameter and the bulk density. However, we have to stress that, at longer annealing times or more elevated temperatures we observed deviation of the ligament sizes as predicted from the BET data. It is possible that the assumption that the pore and ligament dimensions were equivalent no longer held for these samples. It is also worth noting that the ligament sizes of np-Au annealed at shorter annealing times (e.g., 30 and 60 min) at the indicated temperatures are similar to those for an annealing time of 120 min.
The interesting coarsening phenomena of np-Au at elevated temperature led us to investigate the interior of the annealed np-Au. Examination of the interior is made possible by the fact that we have discovered that macroscopic np-Au samples can be cleaved.32 The top layer (at various depths, for repeated data acquisition on a same sample) of the sample is physically peeled off from the np-Au sample with the assistance of a piece of conventional double sided tape. The newly exposed inner surface of the np-Au was then characterized using SEM without any modification to the sample. From Fig. 4E, the image of the interior of a typical np-Au sample annealed at 100 °C resembles that of the exterior np-Au annealed at the same temperature, seen in Fig. 4A, with the exception of a general increase in pore size. However, the obvious fusion associated features were not observed until the annealing temperature reached 200 °C for the interior and 300 °C for the exterior of np-Au. Red arrows in Fig. 4 represent the fusion or nucleation features of the annealed np-Au. The dramatic differences in the morphology of interior versus the exterior of the np-Au lead us to believe that the diffusion routes of the gold atoms during thermal treatment differ at various depths of the np-Au monolith. An additional contribution to the differences between the exterior and interior may be due to differences in the original np-Au structure on the exterior versus in the interior due to variation of the rate of dealloying in nitric acid with depth into the sample. The loss of the typical appearance of the np-Au pores and ligaments begins at 400 °C, where the once observed pores and ligaments may be seen neither in the interior nor exterior of the gold substrates. It is also worth mentioning that during the thermal annealing process under various temperature or time points the visual appearance of the np-Au changed. The np-Au showed a bright shiny yellowish and lustrous appearance after heat treatment of 400 °C. In comparison, np-Au at room temperature has a dark brown color. This is consistent with the SEM images in Fig. 1, in which the drastic morphology change of np-Au structures around 400 °C shows a more continuous surface due to apparent ligament fusion, suggesting that it will be more reflective of light.
Fig. 4.
Exterior and interior SEM images of thermally annealed nanoporous gold after 120 min of annealing time. Nitric acid dealloyed np-Au samples were subjected to 2 h annealing at various temperatures. [A–D] typical morphology of np-Au subjected to the indicated annealing temperature, [C] exterior of np-Au, the fusion/nucleation of the np-Au ligaments was not obvious until the annealing temperature reached 300 °C, [E–H] at the same annealing time and temperature, the interior of the np-Au appeared to be more coarsened that the exterior, [F] interior of np-Au, fusion/nucleation of the np-Au was observed near 200 °C, gradual coarsening of the np-Au is apparent for the exterior and interior of np-Au, as the annealing temperature increases. Red arrows represent the typical fusion/nucleation features on the surface of the np-Au.
Morphological modification of nanoporous gold
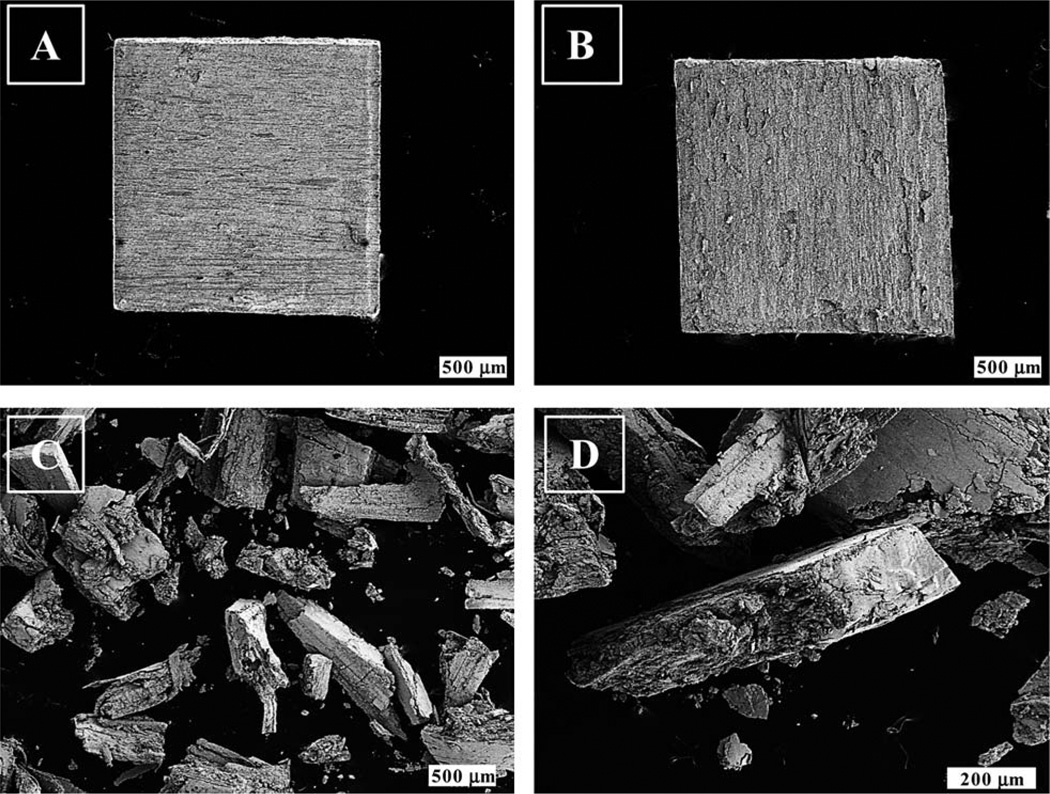
Many studies focus on using np-Au for catalytic, optical, and biological applications.31,40 Depending on specific goal, np-Au most likely will need to be physically or chemically modified to suit the research objective. In addition to modifying the np-Au via annealing, we have also carried out functionalization with an alkanethiol and also mashed the np-Au into fragments and then studied the effect of these changes on the BET surface area and pore size distribution results. A set of samples subjected to the above mentioned modification strategies were investigated by BET analysis of nitrogen adsorption isotherms and the BJH analysis to determine the pore size distributions. Fig. 5 shows the SEM images showing the exterior and interior of the untreated np-Au and mashed np-Au.
Fig. 5.
Scanning electron microscopy images of physical modified porous gold substrates. [A] and [B] are the exterior and interior of the np-Au dealloyed in trace metal grade nitric acid solution. [C] and [D] showing typical mashed fragments of the np-Au. These np-Au substrates were subjected to chemical (functionalization with an alkanethiol) and physical (thermal annealing or mashing) modification before BET and BJH analysis.
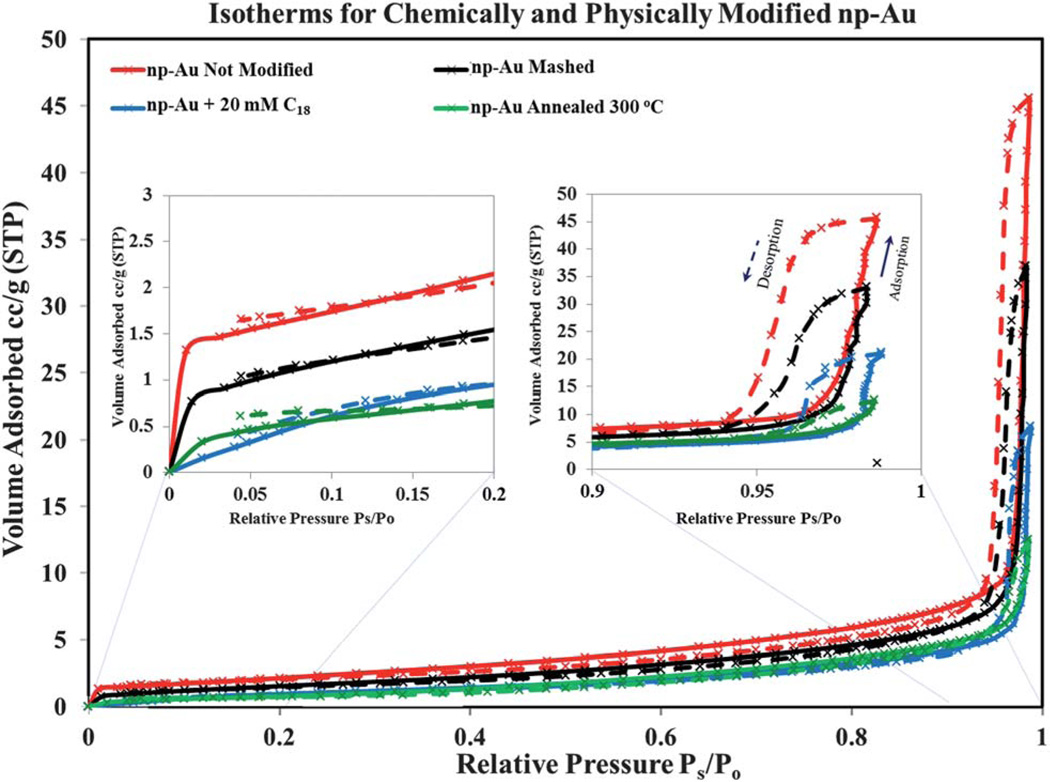
Fig. 6 shows the adsorption-desorption isotherms for these modified samples. The isotherms all display class IV behavior. The isotherms have a concave–convex shape with a sharp “knee” point near Ps/Po around 0.01 to 0.02 for all samples, except the C18-SH modified np-Au for which the “knee” point is not sharp and the isotherm may border class V behavior of a mesoporous material with weak adsorbent-adsorbate interaction. The strength of the interaction of the adsorbent with the adsorbate in these np-Au monoliths is thus reduced by surface modification with the long-chain alkanethiol such that the N2 molecules are interacting predominantly with hydrocarbon surface instead of with a gold surface for this sample. The total BET surface area decreases from unmodified np-Au to the mashed substrate followed next by the C18-SH functionalized np-Au and finally the annealed np-Au sample shows the smallest BET surface area.
Fig. 6.
Nitrogen adsorption and desorption isotherms for chemically and physically modified np-Au samples. The sorption isotherms for the un-annealed np-Au (red), mashed (black), octadecanethiol functionalized (blue) and thermally annealed np-Au at 300 °C (green) are shown. With the exception of alkanethiol modified np-Au, the isotherm of each substrate shows a distinct sharp “knee” point near Ps/Po around 0.01–0.02. The np-Au isotherms present a steep adsorption/desorption at very high relative pressures characteristic of an upper mesopore to low macropore range solid. However, the lack of a sharp “knee” point indicates that the attractive adsorbate-adsorbent interactions are much weaker for N2 molecules interacting with the hydrocarbon chains than with the Au metal surface. The individually recorded data points are represented as crosses and the interpolating lines are those generated by the BET apparatus software.
One interesting observation from this part of the study is that the “knee” point observed for all the other np-Au samples was not clearly observed for the C18-SH functionalized np-Au. Traditionally, the “knee” point of the isotherm is taken as indicating the relative pressure at which monolayer coverage is complete and multilayer adsorption begins to occur. The presence of a well-defined “knee” point is related to the C parameter of the BET equation that is a measure of the strength of the adsorbate-adsorbent surface interaction. As shown in Table 2, the value of the C parameter is similar for un-annealed and mashed np-Au, and somewhat lower for the np-Au annealed at 300 °C. The C value varies somewhat between different preparations of np-Au. Most strikingly, the C value is reduced to near 7 for the C18-SH modified np-Au sample, and this is visually evident in the isotherm, which lacks as distinct of a ‘knee’ as the other isotherms. The lack of a sharp “knee” point suggests that the attractive adsorbate-adsorbent interactions are weaker for N2 molecules interacting with the hydrocarbon chains than with the bare Au metal surface.33,42 For the other np-Au samples, the different knee point in the low relative pressure range (Ps/Po < 0.05) of the isotherm clearly arises from a different pore size distribution and hence different surface area in the samples. A quantitative analysis of the surface properties of the above discussed np-Au samples whose isotherms are shown in Fig. 7 is summarized in Table 2.
Table 2.
Surface properties of modified nanoporous gold
| Nanoporous Gold sample | Pore Size (BJH), nm | Surface Area (SBET), m2 g−1 |
Pore Volume, cm3 g−1 |
C value of BET equation |
|---|---|---|---|---|
| Not annealed | 91 | 7.83 | 0.052 | 42.38 |
| Mashed | 74, 102, 129 | 5.89 | 0.044 | 41.86 |
| C18-modified | 114 | 5.35 | 0.015 | 6.96 |
| Annealed 300 °C | 129 | 3.01 | 0.012 | 30.84 |
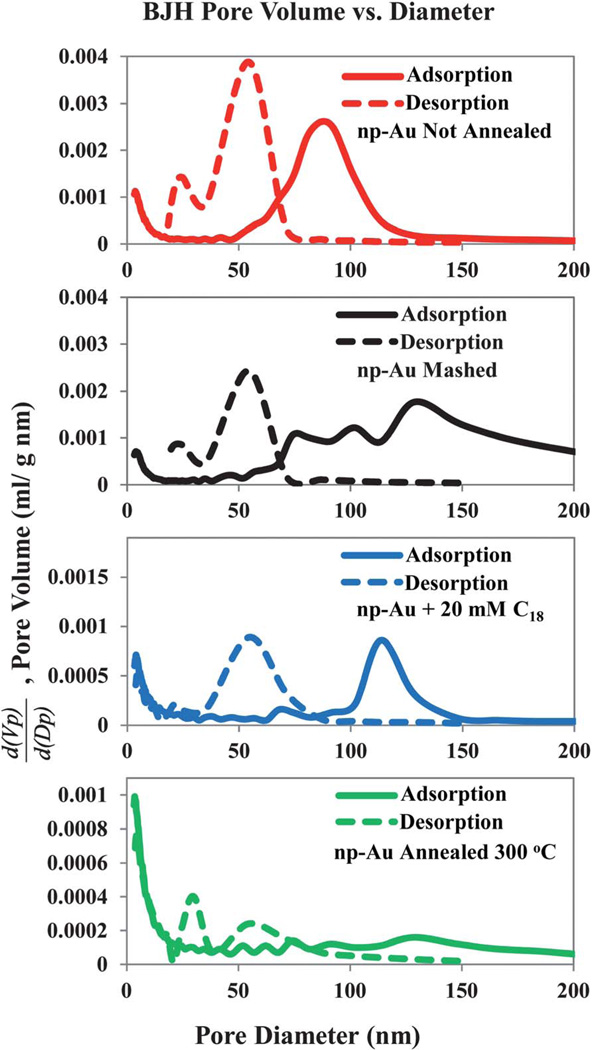
Fig. 7.
Barrett-Joyner-Halenda (BJH) pore-size distributions as determined from both the adsorption and desorption branches of the isotherms for N2 gas on np-Au that was subjected to the indicated chemical or physical modifications.
The surface area found by BET is less for the sample annealed at 300 °C than for the un-annealed np-Au, as expected. It is notable that the BET surface area is lower than that of the un-annealed sample for both the C18-SH functionalized np-Au and for the mashed np-Au samples. It is speculated that the mashing process may close off some of the pore entrances into the np-Au and thus make them possibly inaccessible to adsorption. It was not initially expected that mashing the np-Au sample would decrease the measured surface area. The case of the reduction in BET surface area upon C18-SH functionalization is also surprising, but could be attributed partly to the reduction in pore diameter by approximately 3 nm due to the space occupied by the alkanethiol. However, this geometric reduction is not sufficient to account for the observed reduction, and some other effect such as pore blockage from non-specifically bound C18-SH. The most probable pore sizes, as found by the BJH analysis of the adsorption branch are also found in Table 2; for the samples where there is more than one peak in the calculated distribution, more than one value is reported. As noted previously, the pore size distribution derived from the desorption branch is much different from that obtained from the adsorption branch. It is shown here only for the reason of documenting this interesting difference in outcomes of the BJH analysis.
Electrochemical estimation of surface area: Cyclic voltammetry analysis of nanoporous gold monoliths
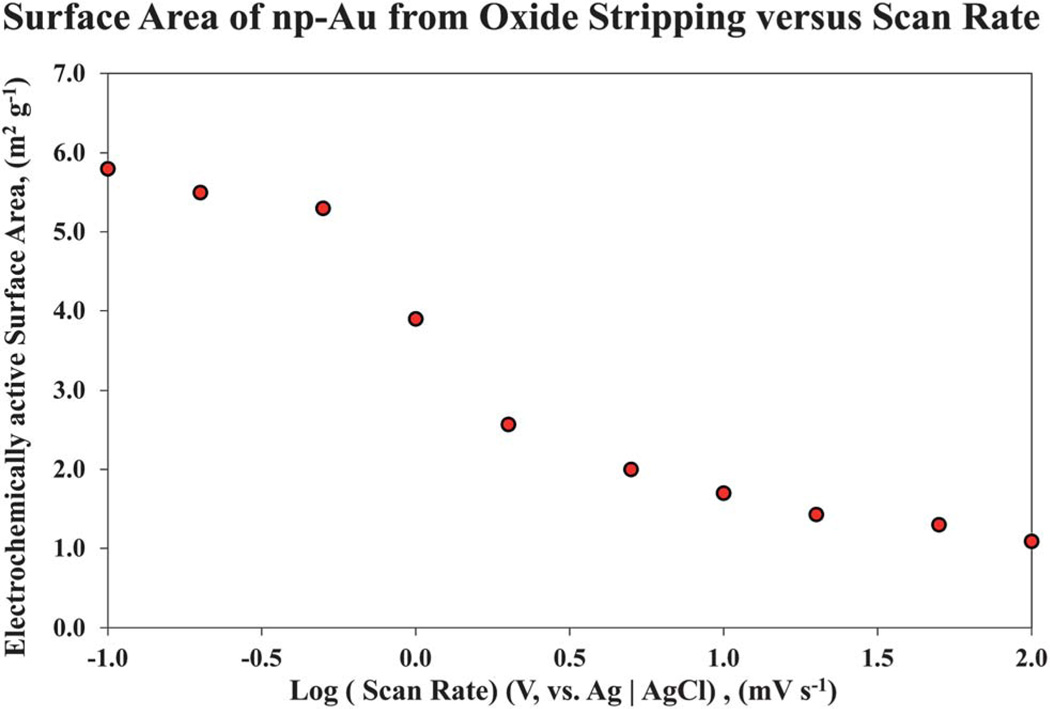
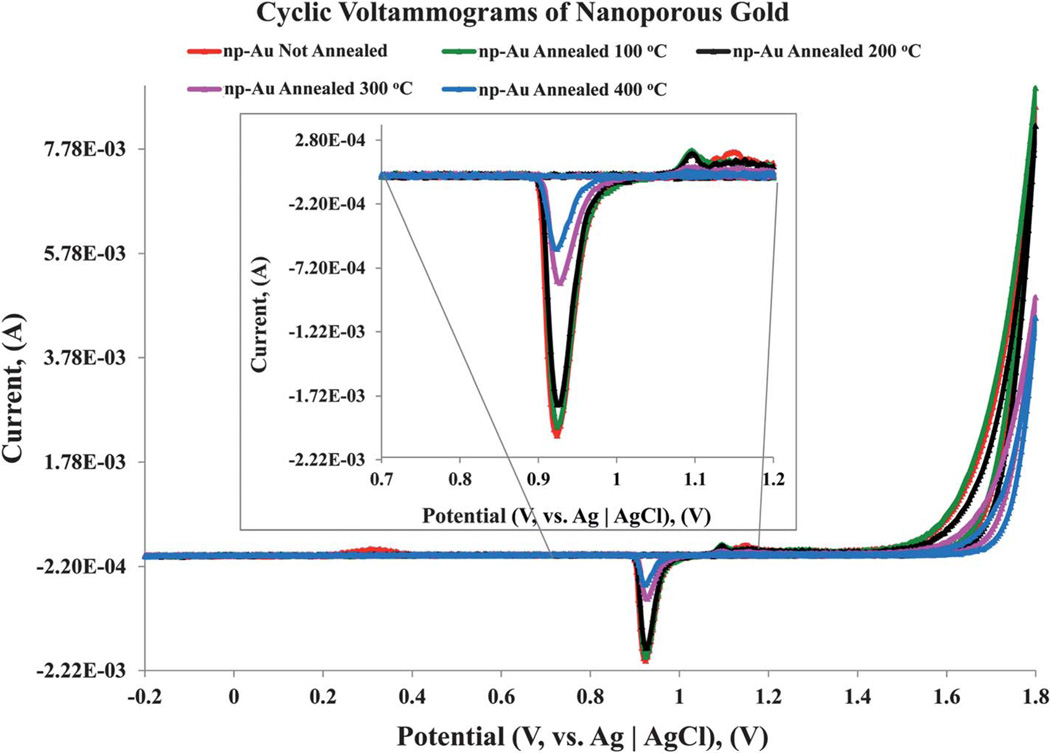
In parallel to the surface area analysis of np-Au using the BET method, determination of the surface area of the np-Au monoliths was performed using cyclic voltammetric analysis of gold oxide formation and stripping. The un-annealed np-Au and samples annealed at 100 °C, 200 °C, 300 °C, and 400 °C were prepared using similar conditions as those shown in Fig. 3. Cyclic voltammetry was employed to obtain the electrochemically active area of the different np-Au substrates. The electrochemically active surface areas of the np-Au were estimated by integrating the charge passed in the surface oxide stripping reaction recorded in 0.5 M sulfuric acid.34,50 A scan rate of 0.2 mV s−1 was used for the surface oxide stripping reaction. This value was determined after a wide range of scan rates from 100 mV s−1 to 0.1 mV s−1 was investigated, as shown in Fig. 8. Reliable electrochemically surface active areas were obtained using 0.2 mV s−1, with successfully reproducible results. A scan rate of 0.1 mV s−1 was attempted for the un-annealed np-Au, but requires −12 h to acquire the voltammogram. The surface area obtained from charge under the gold oxide reduction peak falls off at higher scan rates and becomes significantly less than the values from the BET data. As the scan rate is decreased to very slow rates, the charge passed under the reduction peak is observed to increase much more slowly and approaches but does not reach the value determined by BET. The decrease in surface charge passed as the scan rate increases suggests that there is a mass transfer related limitation on the reduction reaction inside the np-Au monolith, perhaps due to limitations on the diffusion of protons, and/or adsorbed sulfate anions involved in the surface reaction. The onset of significant anodic current due to surface oxide formation for these monolithic np-Au electrodes takes place at a higher potential than observed in the application of gold oxide formation and stripping on np-Au electrodes of much lower mass and dimension, for which a reversal potential of 1.6 V (versus Ag|AgCl) is typically used,51 and oxide formation related peaks are seen starting near 1.3–1.4 V. For these monolithic electrodes, we found it necessary to increase the reversal potential to 1.8 V. It has been reported that just above −1.77 V, an onset of hydrous oxide multilayer formation will occur.52 In the scans on these samples with reversal at 1.8 V, no visual changes were evident for the electrodes.
Fig. 8.
Surface area of np-Au monoliths estimated using cyclic voltammetry, shown as a function of the potential scan rate. Data are shown for the un-annealed np-Au. Scan rates ranging from 0.1 to 100 mV s−1 were used over the potential range from Ei (initial value) at −200 mV, to Es (switching potential) at 1800 mV, and back to Ef (final value) at −200 mV (vs. Ag | AgCl).
The charge passed during the gold oxide reduction for the above mentioned thermally annealed np-Au samples was compared with that obtained for un-annealed sample. The measured charge and therefore the active surface area decrease with the increase in annealing temperature, consistent with the BET and SEM analysis. Assuming a specific charge of 450 µC cm−2 for gold oxide reduction, the un-annealed sample gave a total active surface area of 5.34 m2 g−1.34,53,54 Similarly, total active surface areas of 4.85 m2 g−1, 4.34 m2 g−1, 3.92 m2 g−1, and 1.65 m2 g−1 were obtained for np-Au annealed at 100 °C, 200 °C, 300 °C, and 400 °C, respectively. The surfaces of np-Au have been reported from X-ray diffraction data to be primarily Au (111) in nature, but with significant contributions from other faces including Au (200), Au (220), Au (222), and Au (311).55–57
The surface areas obtained using cyclic voltammetry at very slow scan rates is comparable to those measured from nitrogen gas adsorption, although generally lower. In both cases, the un-annealed and np-Au annealed at 100 °C have relatively close surface areas. The surface areas of the np-Au annealed at elevated temperatures determined from cyclic voltammetry, Fig. 9, follow the same trend as those found in the BET surface area study; as the annealing temperature increases, the total surface area decreases. A summary of the surface areas acquired using both techniques is shows in Table 3.
Fig. 9.
Cyclic voltammograms of np-Au, un-annealed and annealed at a series of temperatures. A scan rate of 0.2 mV s−1 was used to measure the electroactive surface area of np-Au annealed at the indicated temperatures. The un-annealed and annealed np-Au substrates were scanned over the potential range of Ei (initial value) at −200 mV, to Es (switching potential) at 1800 mV, and Ef (final value) at −200 mV. For ease of rendering data/readability, the portion (0.7 V to 1.2 V) of the cyclic voltammogram trace emphasizing the reduction peak is magnified and shown in inset. The measurements of the electrochemically active surface areas of the np-Au samples were made based on the charge associated with stripping of the oxide film.
Table 3.
Electroactive surface area of nanoporous gold samples
| Nanoporous Gold annealing T/°C |
np-Au Substrate Mass,a (g) | Gold Oxide Stripping Peak, (µC) |
Electrochemically active Surface Area, (m2 g−1) |
Surface Area SBET, (m2 g−1) |
|---|---|---|---|---|
| room temperature | 0.256804 | 617.3 | 5.34 | 6.4 |
| 100 | 0.268556 | 585.9 | 4.85 | 6.35 |
| 200 | 0.230166 | 449.2 | 4.34 | 5.34 |
| 300 | 0.265356 | 467.6 | 3.92 | 4.57 |
| 400 | 0.249948 | 185.7 | 1.65 | 1.79 |
Mass of the np-Au sample was measured using Mettler Toledo XP26 microbalance with 1 µg readability.
Pyrolysis of octadecanethiol functionalized on nanoporous gold
In a close packed crystalline monolayer of alkanethiol on gold, each thiolate occupies 21.4 Å2 equivalent to ca. 4.67 × 1014 molecules cm−2.58 Since the surface area of a typical nanoporous gold sample was found to be 7.82 m2 g−1 (based on the BET surface area analysis of the same batch of np-Au used for the cyclic voltammetry measurements), and the amount of the thiolate functionalized onto the surface of np-Au can be measured based on the amount of thiolate decomposed using thermogravimetric analysis (TGA), the total number of C18-SH functionalized onto the surface of given np-Au sample can be estimated in terms of thiolate molecules cm−2.
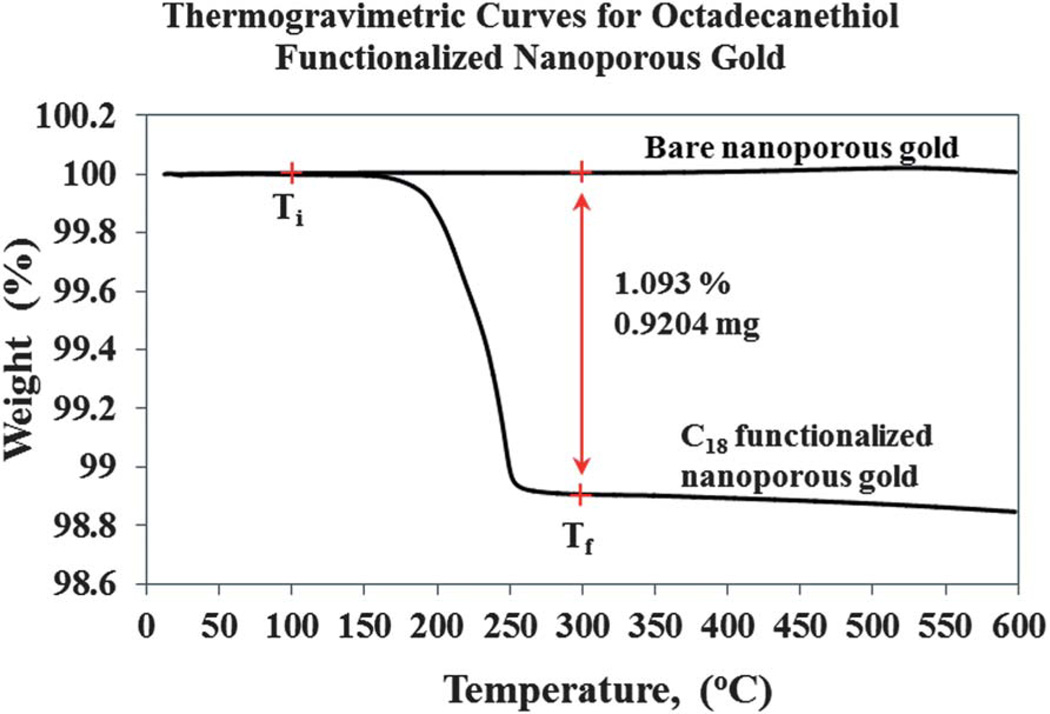
The thermogravimetric method is known as a destructive analytical method, which allows for quantitative measurement of the change or rate of change in the weight of a material as a function of temperature or time. This approach is applicable in our study, due to the high melting point of gold, 1064.18 °C, and the relatively low temperatures (in the range of 170–250 °C) at which the alkanethiol decomposes on the metal surface.59 In our experimental approach, a platinum weighing boat is used for pyrolysis carried out between 10 °C to 600 °C. To ensure that enough data points (or resolution) are obtained, dynamic thermogravimetry of the C18-SH functionalized np-Au, subjected to a constant temperature ramp of 5 °C min−1 over a period of time (or approximately 120 min) is used and any changes in mass are noted. The same batch of nanoporous gold plates dealloyed using nitric acid solution used in this study was not subject to any thermal annealing process. TGA curves for the bare nanoporous gold were recorded, as seen in Fig. 10, and used as control. Alternatively, the rest of the nanoporous gold was passivated with 20 mM C18-SH (in ethanol) using a static incubation approach, for 48 h at room temperature. These samples were then rinsed with hexane to remove the physisorbed alkanethiol and a final rinsing with ethanol was carried out before TGA analysis.
Fig. 10.
Thermogravimetry curve measured for octadecanethiol functionalized nanoporous gold monolith. A single decomposition step of the C18 between 100 °C to 300 °C, assigned as Ti and Tf regions, respectively, was observed. The decomposition of the C18 from the porous gold surface yields a mass change of 0.9204 mg, and there is no decomposition step observed for the bare nanoporous gold. The analysis was carried out at a ramping temperature of 5 °C min−1 under nitrogen carrier gas at a flow rate of 40 mL min−1.
In Fig. 10, the mass change of the decomposed thiolate molecules can be seen based on the single decomposition step. From this analysis, a total loss of 0.92 mg of C18-SH is obtained between the two plateaus at 100 °C and 300 °C (Ti and Tf region, respectively). The interval of Ti–Tf is arbitrarily set; Ti has no fundamental significance, but is a good indication that no water or any volatile solvent remains trapped in the porous environment that would contribute to the mass loss and give a false reading. However, the Tf region is set as final temperature, at which the decomposition of thiolate molecules appears to be complete. A mass change of 0.92 mg of C18-SH obtained using the thermogravimetric curve is equivalent to a monolayer surface coverage of 2.94 × 1014 molecules cm−2 (based on a single 8.0 mm × 8.0 mm × 0.25 mm C18-SH functionalized nanoporous gold with mass of 84.2 mg before TGA analysis) on the surface of the nanoporous gold. The amount of thiolate functionalized and decomposed on nanoporous gold is reproducible over numerous experimental trials, and robustness tests using different incubation times or different alkanethiol (data not shown, to be published). From our estimation, the loading of the C18-SH onto the nanoporous gold surface is approximately 63%. This indicates a relatively good penetration depth of the thiolate molecules into the porous environment during long periods of static incubation. Due to the complex porosity and ligament network of np-Au, vacant areas of the gold surface likely exist due to lack of accessibility of thiolate molecules to some regions within np-Au. It is possible that some parts of the interior gold surface may be blocked by trapped air or were not wet by the solvent. A resolution to this will be implementing a flow through method to force thiol solution into and through the nanoporous gold interior. This approach should, in theory, displace any trapped air inside the gold monoliths with the mobile phase containing the alkanethiol molecules. Such a flow system has been established and a study is currently being carried out in our laboratory and results will be reported in a future publication.
Conclusions
The present study demonstrates that np-Au monoliths of sufficient size can be investigated using gas adsorption-desorption isotherms, similar to the manner in which other materials, such as porous glasses and other monolithic porous materials, have been studied.33,42 The amount of sample required for evaluation of np-Au of a few hundred mg corresponds to a cost of about $12 US (assuming a gold price of $1700 US per ounce). Since BET is a non-destructive method, the np-Au sample can be characterized and then used in other experiments. The results for np-Au monoliths are of particular interest for applications using these structures, such as those involving flow of solutions through the monoliths, which we have recently demonstrated.32 Self-assembled monolayers should be able to be removed and flushed out of monoliths,60 leaving the bare Au surface available for reuse. The studies are also relevant to applications of these monoliths in supported synthesis, as pursued in our labs.6
The isotherms found are of type IV and show H1 type hysteresis, as expected for a mesoporous material. The BET surface areas and BJH pore size distribution of both chemically and physically modified np-Au samples were analyzed. The results of the BJH analysis indicate that the behavior of the adsorptive N2 within the pores is subject to pore network and pore blockage effects. Modification of np-Au by a self-assembled monolayer of C18-SH was found to have significant impact on the isotherm behavior, diminishing the sharpness of the ‘kneepoint’ at low relative pressure. The evolution of the np-Au under various thermal annealing treatments was examined using scanning electron microscopy. Voltammetry of the np-Au in acid at very slow scan rates was found to provide a method by which to estimate the electrochemically active surface area of the np-Au using the charge associated with stripping of the gold oxide film to estimate the electrode area. At sufficiently low scan rates, the electrochemically determined surface area compares well to that obtained by the BET surface area analysis although it is found to be somewhat smaller.
Based on TGA analysis, a mass change of 0.92 mg upon the decomposition of a SAM of C18-SH from the nanoporous gold surface was obtained and equated using the BET surface area to a monolayer surface coverage of 2.94 × 1014 molecules cm−2. TGA analysis appears to be an attractive analytical method for determining surface loading of SAMs inside np-Au monoliths of sufficient size, and will allow much better defined studies of such systems. The surface coverage of −63% for octadecanethiol in the np-Au monolith is encouraging, but points out that forming SAMs in these np-Au structures by static exposure to solution may not be optimal. The distribution of thiolate species within the monolith remains an open question, which could be probed given our demonstrated ability to cleave off layers of these np-Au structures to expose internal surfaces.
Investigation of np-Au monoliths by mercury porosimetry61 would also be of interest, as the pore sizes studied are nearing the upper limits of what is practically accessible to BET analysis of N2 adsorption. Mercury porosimetry is typically applied to porous inorganic or organic materials, and analysis of porosimetry data is based upon the Washburn equation and a contact angle of Hg of 140°. However, the contact angle of Hg on the gold surface should yield a low value of contact angle (θ). It is also possible that Hg forced through a gold material may be subject to amalgam formation, or that the pressures involved may damage the np-Au.
The findings are also of relevance for the development of potential biosensor substrate platforms/materials based on np-Au. Since the sensitivity of a sensor platform is dictated by the amount of receptive ligand molecules immobilized on the surface, our findings may provide insight into the SBET used to determine the capacity of an np-Au structure for receptor molecule immobilization. The impact of the SBET and pore sizes based on the modification approach may influence the substrate preparation method chosen for the potential application. Thus, the pore and ligament sizes can be tuned as required, depending on the dimensions of the reactive bio-macromolecules or proteins to be attached to the np-Au surfaces.
Further studies of np-Au monoliths could extend to investigate the effect of further variations in pore size, surface modification, and identity of the adsorptive. The use of krypton gas as the adsorptive would be of interest, as krypton is employed to determine lower specific surface areas,33,42 and may reduce the amount of np-Au sample required for analysis. The applications of np-Au in catalysis of gas phase reactions, for instance, could benefit from characterization of np-Au surface behavior.62 An understanding of the effect of chemical and physical modification of np-Au, in terms of further variations in pore size, surface modification, and identity of the adsorptive, will undoubtedly contribute to developing applications of these materials. Furthermore, such investigations can aid the design of applications of np-Au in biomaterials, biosensors, and bioanalytical systems.
Acknowledgements
The authors thank Dr. David Osborn for helpful discussions concerning the BET, TGA, and SEM instrumentation located in the UM-St. Louis Center for Nanoscience. This work was supported by UM-St. Louis and by the NIGMS award R01-GM090254.
References
- 1.Kafi AKM, Ahmadalinezhad A, Wang J, Thomas DF, Chen A. Biosens. Bioelectron. 2010;25:2458–2463. doi: 10.1016/j.bios.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Manso J, Mena ML, Yáñez-Sedeño P, Pingarrón JM. Electrochim. Acta. 2008;53:4007–4012. [Google Scholar]
- 3.Shulga OV, Jefferson K, Khan AR, D’Souza VT, Liu J, Demchenko AV, Stine KJ. Chem. Mater. 2007;19:3902–3911. doi: 10.1021/cm070238n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ertenberg RW, Andraka B, Takano Y. Phys. B. 2000;284–288:2022–2023. [Google Scholar]
- 5.Ding Y, Chen M, Erlebacher J. J. Am. Chem. Soc. 2004;126:6876–6877. doi: 10.1021/ja0320119. [DOI] [PubMed] [Google Scholar]
- 6.Pornsuriyasak P, Ranade SC, Li A, Parlato MC, Sims CR, Shulga OV, Stine KJ, Demchenko AV. Chem. Commun. 2009:1834–1836. doi: 10.1039/b817684a. [DOI] [PubMed] [Google Scholar]
- 7.Kramer D, Viswanath RN, Weissmüller J. Nano Lett. 2004;4:793–796. [Google Scholar]
- 8.Biener J, Wittstock A, Zepeda-Ruiz LA, Biener MM, Zielasek V, Kramer D, Viswanath RN, Weissmüller J, Bäumer M, Hamza AV. Nat. Mater. 2009;8:47–51. doi: 10.1038/nmat2335. [DOI] [PubMed] [Google Scholar]
- 9.Erlebacher J, Sieradzki K. Scr. Mater. 2003;49:991–996. [Google Scholar]
- 10.Qian LH, Chen MW. Appl. Phys. Lett. 2007;91 083105. [Google Scholar]
- 11.Ge X, Yan X, Wang R, Tian F, Ding Y. J. Phys. Chem. C. 2009;113:7379–7384. [Google Scholar]
- 12.Lang XY, Guan PF, Fujita T, Chen MW. Phys. Chem. Chem. Phys. 2011;13:3795–3799. doi: 10.1039/c0cp01571g. [DOI] [PubMed] [Google Scholar]
- 13.Chen LY, Lang XY, Fujita T, Chen MW. Scr. Mater. 2011;65:17–20. [Google Scholar]
- 14.Fujita T, Qian L-H, Inoke K, Erlebacher J, Chen M-W. Appl. Phys. Lett. 2008;92:251902–251903. [Google Scholar]
- 15.Ruffato G, Romanato F, Garoli D, Cattarin S. Opt. Express. 2011;19:13164–13170. doi: 10.1364/OE.19.013164. [DOI] [PubMed] [Google Scholar]
- 16.Florea I, Roiban L, Hirlimann C, Tihay F, Pham-Huu C, Werckmann J, Pham C, Nguyen P, Drillon M, Ersen O. Adv. Eng. Mater. 2011;13:122–127. [Google Scholar]
- 17.Rösner H, Parida S, Kramer D, Volkert CA, Weissmüller J. Adv. Eng. Mater. 2007;9:535–541. [Google Scholar]
- 18.Rouquerol F, Rouquerol J, Sing K. Adsorption by Powders and Porous Solids. London: Academic Press; 1999. pp. 51–92. [Google Scholar]
- 19.Brunauer S, Emmett PH, Teller E. J. Am. Chem. Soc. 1938;60:309–319. [Google Scholar]
- 20.Denoyel R, Barrande M, Beurroies I. In: Studies in Surface Science and Catalysis. Llewellyn PL, Rodriquez-Reinoso F, Rouqerol J, Seaton N, editors. Elsevier; 2007. pp. 33–40. [Google Scholar]
- 21.Erlebacher J, Aziz MJ, Karma A, Dimitrov N, Sieradzki K. Nature. 2001;410:450–453. doi: 10.1038/35068529. [DOI] [PubMed] [Google Scholar]
- 22.Biener J, Hodge AM, Hayes JR, Volkert CA, Zepeda-Ruiz LA, Hamza AV, Abraham FF. Nano Lett. 2006;6:2379–2382. doi: 10.1021/nl061978i. [DOI] [PubMed] [Google Scholar]
- 23.Volkert CA, Lilleodden ET, Kramer D, Weissmuller J. Appl. Phys. Lett. 2006;89:061920–061923. [Google Scholar]
- 24.Hakamada M, Mabuchi M. Scr. Mater. 2007;56:1003–1006. [Google Scholar]
- 25.Ding Y, Kim YJ, Erlebacher J. Adv. Mater. 2004;16:1897–1900. [Google Scholar]
- 26.Hodge AM, Biener J, Hayes JR, Bythrow PM, Volkert CA, Hamza AV. Acta Mater. 2007;55:1343–1349. [Google Scholar]
- 27.Hakamada M, Mabuchi M. Mater. Lett. 2008;62:483–486. [Google Scholar]
- 28.Li R, Sieradzki K. Phys. Rev. Lett. 1992;68:1168. doi: 10.1103/PhysRevLett.68.1168. [DOI] [PubMed] [Google Scholar]
- 29.Seker E, Gaskins JT, Bart-Smith H, Zhu J, Reed ML, Zangari G, Kelly R, Begley MR. Acta Mater. 2007;55:4593–4602. [Google Scholar]
- 30.Wang Y, Teitel S, Dellago C. Nano Lett. 2005;5:2174–2178. doi: 10.1021/nl051149h. [DOI] [PubMed] [Google Scholar]
- 31.Shulga OV, Zhou D, Demchenko AV, Stine KJ. Analyst. 2008;133:319–322. doi: 10.1039/b712760j. [DOI] [PubMed] [Google Scholar]
- 32.Tan YH, Schallom J, Ganesh NV, Fujikawa K, Demchenko AV, Stine KJ. Nanoscale. 2011;3:3395–3407. doi: 10.1039/c1nr10427f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregg SJ, Sing KSW, Salzberg HW. J. Electrochem. Soc. 1967;114:279C. [Google Scholar]
- 34.Guo Y-G, Zhang H-M, Hu J-S, Wan L-J, Bai C-L. Thin Solid Films. 2005;484:341–345. [Google Scholar]
- 35.Van Orden AC. In: ASTM International. Baboian R, editor. 2005. pp. 278–289. [Google Scholar]
- 36.Erlebacher J. J. Electrochem. Soc. 2004;151:C614–C626. [Google Scholar]
- 37.Ehrlich G. Surf. Sci. 1991;246:1–12. [Google Scholar]
- 38.Hagan HP, Campbell PA, Smith KW, Turner RJ, Walmsley DG. Ultramicroscopy. 1992;42–44:587–593. [Google Scholar]
- 39.Göbel H, von Blanckenhagen P. Surf. Sci. 1995;331–333:885–890. [Google Scholar]
- 40.Ding Y, Chen M. MRS Bull. 2009;34:569–576. [Google Scholar]
- 41.Qian LH, Yan XQ, Fujita T, Inoue A, Chen MW. Appl. Phys. Lett. 2007;90:153120–153120. [Google Scholar]
- 42.Gregg SJ, Sing KSW. Adsorption, surface area, and porosity. London; New York: Academic Press; 1982. [Google Scholar]
- 43.Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T. Pure Appl. Chem. 1985;57:603–619. [Google Scholar]
- 44.Cohan LH. J. Am. Chem. Soc. 1938;60:433–435. [Google Scholar]
- 45.Chang S-S, Clair B, Ruelle J, Beauchêne J, Di Renzo F, Quignard F, Zhao G-J, Yamamoto H, Gril J. J.f Experimental Botany. 2009 doi: 10.1093/jxb/erp133. [DOI] [PubMed] [Google Scholar]
- 46.Casanova F, et al. Europhys. Lett. 2008;81:26003. [Google Scholar]
- 47.Ji C, Searson PC. J. Phys. Chem. B. 2003;107:4494–4499. [Google Scholar]
- 48.Detsi E, van de Schootbrugge M, Punzhin S, Onck PR, De Hosson JTM. Scr. Mater. 2011;64:319–322. [Google Scholar]
- 49.Detsi E, De Jong E, Zinchenko A, Vuković Z, Vuković I, Punzhin S, Loos K, ten Brinke G, De Raedt HA, Onck PR, De Hosson JTM. Acta Mater. 2011;59:7488–7497. [Google Scholar]
- 50.Huang J-F. Talanta. 2009;77:1694–1700. doi: 10.1016/j.talanta.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Dong H, Cao X. J. Phys. Chem. C. 2008;113:603–609. [Google Scholar]
- 52.Burke L, Nugent P. Gold Bull. 1997;30:43–53. [Google Scholar]
- 53.Cao L, Yan P, Sun K, Kirk DW. Electroanalysis. 2009;21:1183–1188. [Google Scholar]
- 54.Finklea HO, Snider DA, Fedyk J. Langmuir. 1990;6:371–376. [Google Scholar]
- 55.Mohl M, Kumar A, Reddy ALM, Kukovecz A, Konya Z, Kiricsi I, Vajtai R, Ajayan PM. J. Phys. Chem. C. 2009;114:389–393. [Google Scholar]
- 56.Huang JF, Sun IW. Adv. Funct. Mater. 2005;15:989–994. [Google Scholar]
- 57.Kim M, Jeong GH, Lee KY, Kwon K, Han SW. J. Mater. Chem. 2008;18:2208–2212. [Google Scholar]
- 58.Ulman A. Chem. Rev. 1996;96:1533–1554. doi: 10.1021/cr9502357. [DOI] [PubMed] [Google Scholar]
- 59.Yang B, Tian S, Zhao S. Fuel Process. Technol. 2006;87:673–678. [Google Scholar]
- 60.Yuan M, Zhan S, Zhou X, Liu Y, Feng L, Lin Y, Zhang Z, Hu J. Langmuir. 2008;24:8707–8710. doi: 10.1021/la800287e. [DOI] [PubMed] [Google Scholar]
- 61.Kim H, Kim Y, Joo JB, Ko JW, Yi J. Microporous Mesoporous Mater. 2009;122:283–287. [Google Scholar]
- 62.Wittstock A, Zielasek V, Biener J, Friend CM, Bäumer M. Science. 2010;327:319–322. doi: 10.1126/science.1183591. [DOI] [PubMed] [Google Scholar]