Abstract
Background
Oculodentodigital syndrome (ODD; OMIM #164200) is a rare autosomal dominant disorder with pleiotropic effects. It is caused by mutation in gap junction protein α 1 (GJA1) gene which encodes connexion 43. ODD is characterised by symptoms i.e. craniofacial, neurologic, limb, ocular abnormalities, syndactyly type III of the hands, phalangeal abnormalities, diffuse skeletal dysplasia, enamel dysplasia, and hypotrichosis.
Objectives
To study the Molecular Genetics of Oculodentodigital syndrome.
Patients/materials and methods
Our current study includes a Pakistani family affected with ODD. Clinical evaluation revealed that this family shows typical form of ODD with Syndactyly type III. Mutations in GJA1 have been reported in ODD and also in syndactyly type III. In this study we sequenced the coding exons of GJA1 gene in affected and normal individuals of the family for mutation detection.
Results
Direct sequencing of the affected individuals showed a mutation at the nucleotide position 389 T>C. This mutation changed the codon 130 from Isoleucine to Threonine. Normal family members did not show this mutation.
Conclusion
Our study showed no gross neurological upset with I130T mutation in GJA1 gene. This may present novel phenotypic outcome with the I130T. The study will help in better understanding of pathophysiology of oculodentodigital syndrome and type III syndactyly.
Keywords: diffuse skeletal dysplasia, GJA1 gene, Oculodentodigital Syndrome, Syndactyly Type III
Introduction
Oculodentodigital Syndrome (ODD)
Oculodentodigital syndrome (ODD) is a rare autosomaldominant congenital abnormality. It was first recognised in 1920 by Lohmann[1] and is characterized by developmental abnormalities of the craniofacial, ocular, neurological and limbs.
Characteristic facial featuresinclude a narrow, pinched nose with hypoplastic alae nasi, prominent columella and thin anteverted nares together with a narrow nasal bridge, and prominent epicanthic folds giving the impression of hypertelorism.[2] The hair may be thin and sparse, the teeth small and carious, and the head circumference somewhat reduced. Eye defects consist of microcornea, iris anomalies, and in some cases secondary glaucoma, small palpebral fissures, persistent pupillary membrane, and microphthalmos. The pupil may be eccentric. Bilateral complete syndactyly of the fourth and fifth fingers (type III syndactyly) is the characteristic digital malformation. The third finger may also occasionally be involved and associated comptodactyly is a common finding.[3,4]
Genetics of ODD
Mostly ODD shows autosomal dominant mode of inheritance but recessive form of ODD has also been reported.[5,6]
A number of studies have confirmed that ODD caused by mutations in the gap junction protein alpha-1 (GJA1), mapped to chromosomal region 6q22-q23, which codes for the gap junction protein connexin 43 (Cx43).[7,9] These findings have been further evaluated extensively which detail that pleiotropic feature in ODD results from GJA1 mutations.[10–14]
The GJA1 is composed of two exons with an intervening intron 11 kb in size. The mRNA sequence contains 191 bp of the first exon, which is untranslated. The second exon consists of 16 bp of additional 5'untranslated sequence, 1,149 bp of coding sequence, and ˜1,732 bp of 3'untranslated sequence.
Syndactyly
Syndactyly can be simple or complex: In simple syndactyly, adjacent fingers or toes are joined by soft tissue where in complex syndactyly; the bones of adjacent digits are fused. Furthermore, syndactyly can be complete or incomplete: In complete syndactyly, the skin is joined all the way to the tip of the finger, where as in incomplete syndactyly; the skin is only joined part of the distance to the fingertip.
Genetics of Syndactyly
Five types of syndactyly have been identified in humans.[15] The corresponding loci associated with these types and their common phenotype expression is as follows:
Type I: 2q34-q36;16 webbing occurs between middle and ring fingers and/or second and third toes.
Type II: 2q31; 17 also involves long and ring fingers, but has a sixth finger merged in between.
Type III: 6q21-q23; small finger is merged into the ring finger.
Type IV: 7q36; 18 involves all fingers and/or toes.
Type V: 2q31-q32; similar to type I, but the metacarpals and metatarsals may also be fused.
In syndactyly type III, which is often clusters with ODD, there is usually complete and bilateral syndactyly between the fourth and fifth fingers. Usually it is soft tissue syndactyly but occasionally the distal phalanges are fused. The fifth finger is short with absent or rudimentary middle phalanx. The feet are not affected.
Syndactyly type III is caused by mutation in the GJA1 gene. Syndactyly type III is the characteristic digital anomaly in oculodentodigital dysplasia which is also caused by mutation in the GJA1 gene.[19]
Our current study comprises of a Pakistani family affected with autosomal dominant ODD. Clinical evaluation revealed that this family shows typical form of ODD with syndactyly type 3; the skin and distal phalanges of fourth and fifth or third, fourth and fifth fingers are fused, thin nose, prominent nose bridge, diastema and voice stuttering. GJA1 gene was sequenced for its coding exon 2 to identify the mutation.
Materials and methods
Subjects and Methods
Subjects
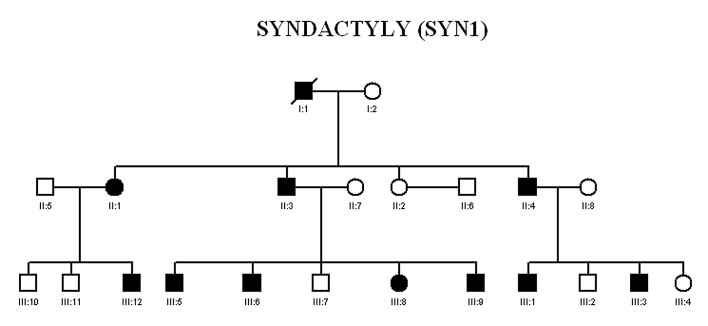
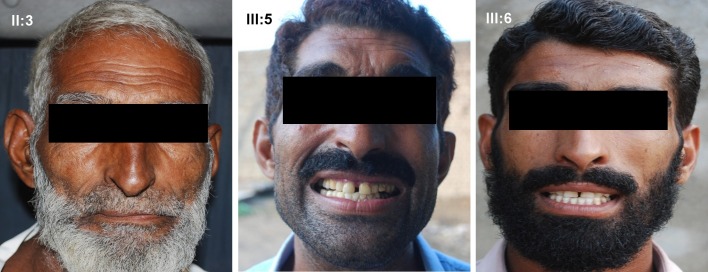
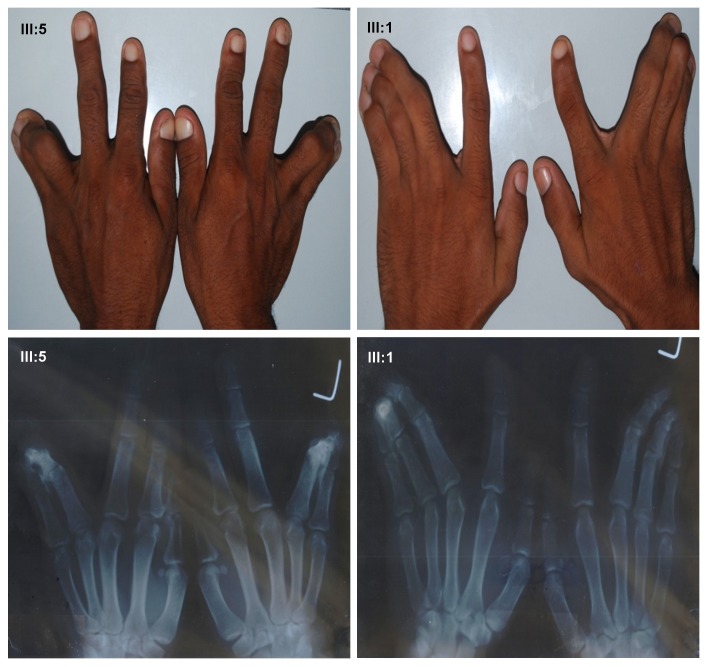
A consanguineous family with autosomal dominant ODD with syndactyly type III was collected after taking consent of the family, from remote area of Punjab, Pakistan. Out of 21 family members, 12 members; six affected (II-3, III-12, III- 5, III-8, III-9, and III-1) and six normal subjects (II-7, II-2, II-8, III-7, III-2, and III-4) were available for clinical and genetic evaluations [Fig. 1]. All affected subjects have thin nose, prominent nose bridge, diastema [Fig. 2] and mild stuttering in speech. There was cutaneous fusion either between the fourth and fifth finger and webbing of third, fourth and fifth finger along with the fusion of distal phalanges as shown in X-ray finding [Fig. 3].
Figure 1.
Pedigree of family with Occulodentodigital syndrome (ODD) with Syndactyly type III; Circles represent females and squares represent males. Filled circles and squares represent affected individuals. Double lines indicate consanguineous marriages. Cross lines on the symbol represent deceased Individuals.
Figure 2.
Facial features of ODD with syndactyly type III Pakistani family. Pedigree number II:3, III:5 and III:6.
Figure 3.
Hands features and its X-ray views of ODD with syndactyly type III Pakistani family. Pedigree number III:5 and III:1.
Methods
This study was approved by Ethical review board and Institutional Board of Study of Institute of Biomedical and Genetic Engineering and International Islamic University, Islamabad respectively.
DNA from peripheral blood lymphocytes of 12 members of the family was extracted using Phenol-Choloroform-Isoamylalcohal method.[20] Exon 1 and 2 of GJA1 were amplified for the pedigree members in 10 uL final volume using following PCR conditions: 1X PCR buffer (Fermentas), 0.2mM dNTPs (Fermentas), 0.2 U Taq (Fermentas), 1 nM forward and reverse primers, 6ng DNA as template. PCR was performed at initial melting at 94°C for 4 min, 35 cycles each consisting of (i) melting at 94°C for 45 sec, (ii) annealing at 55°C for 45 sec, (iii) extension at 72°C for 45 sec and final extension for 10 min at 72°C.
PCR products were run on 2% w/v agarose gel at constant power supply of 200 volts for 35 minutes. The gel was analyzed in gel documentation system (syngene, Cambridge, UK).
Direct sequencing of the PCR product for GJA1 exons amplified in patient and normal members of the pedigree was done in 3130 Genetic Analyzer. (Applied Biosystems).
Results and Discussion
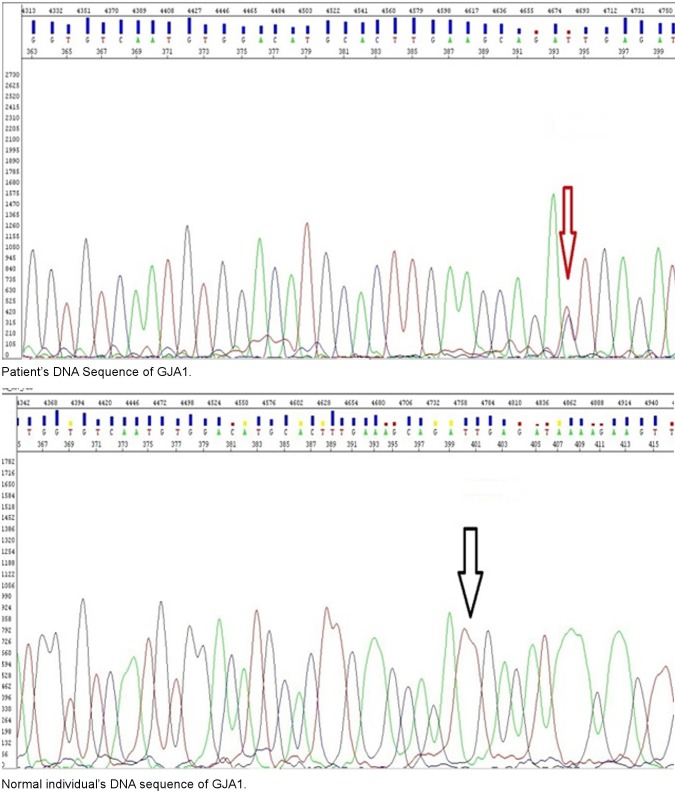
We found autosomal dominant mutation in GJA1 exon 2 at nucleotide position 389 T>C [Fig. 4]. This mutation resulted in change of I130T (ATT → ACT). This mutation did not appear in unaffected members of the family. Isoleucine at codon 130 position is present in the cytoplasmic domain of the GJA1 protein. This change has changed the isoleucine, a hydrophobic amino acid, to the threonine which is polar. This mutation in GJA1 gene resulted in the formation of an aberrant gap junction protein which was responsible for syndactyly and ODD in the family under study. Other mutations have been reported in GJA1 gene for ODD and Syndactyly in white families. This shows that this disorder is mostly present in families of white origin. Although mutation reported in this study has been reported by Paznekas et al. in 2003 in a white family.[8] As neurological symptoms often cluster with the other salient phenotypic features of the ODD; this white family had neurologic symptoms with white matter degeneration. But we are reporting the first ODD family with syndactyly type III from Pakistan. This family did not show any neurological disorder in any individual of age from 18 to 56 years in any generation. This might present a novel phenotype including no gross neurological ailment with the I130T mutation in GJA 1 gene. Previous families with the similar mutation; reported by Paznekas et al. and Amador et al. showed a wide range of neurological dysfunction.[8,20] ODD observed in the family shows typical feature i.e. partial webbing of third and fourth or webbing of third, fourth and fifth fingers, thin nose, prominent nose bridge, diastema and minor stuttering. Moreover it should be noted that the typical features of ODD segregate with the mutation in the entire affected individuals of the family [Fig. 2 and 3].
Figure 4.
Sequence of patient and normal individual.
Previously Richardson et al. in 2004 reported occulodentodigital syndrome (ODD) due to mutation R33X in the transmembrane domain of GJA 1 in a family of Pakistani origin.[6] This finding showed the autosomal recessive inheritance of ODD. In another finding by Malik et al. showed no linkage of the anomaly to chromosomal regions 2q34-q36, 2q31, and 6q22-q23 encompassing loci for syndactyly types I, II, and III.[21] No case of ODD has been reported from Pakistan before our current report.
The symptoms of the family reported in this study are quite variable than the symptoms already reported for ODD due to I130T mutation. Since neurological abnormalities do not always accompany ODD as we reported in this study. But in cases where it happens along with mutation at the similar location in GJA1 gene, prospective study needs to find the exact molecular mechanism to isolate or combine neuropatholgical manifestations in ODD.
References
- Lohmann W. Beitrag zur Kenntnis des reinen Mikrophthalmus. Arch Augenheilkd. 1920;86:136–141. [Google Scholar]
- Fará M, Gorlin RJ. The question of hypertelorism in oculodentoosseous dysplasia. Am J Med Genet. 1981;10:101–102. doi: 10.1002/ajmg.1320100112. [DOI] [PubMed] [Google Scholar]
- Brueton LA, Huson SM, Farren B, Winter RM. Oculodentodigital dysplasia and type III syndactyly: separate genetic entities or disease spectrum? J Med Genet. 1990;27:169–175. doi: 10.1136/jmg.27.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner SH, Kott E, Bornstein B, Salinger H, Kaplan I, Gorlin RJ. Oculodentodigital dysplasia. Am J Dis Child. 1969;118:600–607. doi: 10.1001/archpedi.1969.02100040602013. [DOI] [PubMed] [Google Scholar]
- Judisch GF, Martin-Casals A, Hanson JW, Olin WH. Oculodentodigital dysplasia: four new reports and a literature review. Arch Ophthalmol. 1979;97:878–884. doi: 10.1001/archopht.1979.01020010436007. [DOI] [PubMed] [Google Scholar]
- Richardson RJ, Joss S, Tomkin S, Ahmed M, Sheridan E, Dixon MJ. A nonsense mutation in the first transmembrane domain of connexin 43 underlies autosomal recessive oculodentodigital syndrome. J Med Genet. 2006;43:e37. doi: 10.1136/jmg.2005.037655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski R, Sommershof A, Willecke K. Some oculodentodigital dysplasia-associated Cx43 mutations cause increased hemichannel activity in addition to deficient gap junction channels. J Membr Biol. 2007;219:9–17. doi: 10.1007/s00232-007-9055-7. [DOI] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of Oculodentodigital Dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick A, Richardson RJ, Butterworth J, Barron MJ, Dixon MJ. Novel mutations in GJA1 cause oculodentodigital syndrome. J Dent Res. 2008;87:1021–1026. doi: 10.1177/154405910808701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Donnai D, Meire F, Dixon MJ. Expression of Gja1 correlates with the phenotype observed in oculodentodigital syndrome/type III syndactyly. J Med Genet. 2004;41:60–67. doi: 10.1136/jmg.2003.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer KW, Hansen L, Eiberg H, Leicht P, Opitz JM, Tommerup N. Novel Connexin 43 (GJA1) mutation causes oculo‐dento‐digital dysplasia with curly hair. Am J Med Genet A. 2004;127A:152–157. doi: 10.1002/ajmg.a.20614. [DOI] [PubMed] [Google Scholar]
- Pizzuti A, Flex E, Mingarelli R, Salpietro C, Zelante L, Dallapiccola B. A homozygous GJA1 gene mutation causes a Hallermann‐Streiff/ODDD spectrum phenotype. Hum Mutat. 2004;23:286. doi: 10.1002/humu.9220. [DOI] [PubMed] [Google Scholar]
- van Steensel MA, Spruijt L, van der Burgt I, Bladergroen RS, Vermeer M, Steijlen PM, van Geel M. A 2‐bp deletion in the GJA1 gene is associated with oculo‐dento‐digital dysplasia with palmoplantar keratoderma. Am J Med Genet A. 2005;132A:171–174. doi: 10.1002/ajmg.a.30412. [DOI] [PubMed] [Google Scholar]
- Vitiello C, D'Adamo P, Gentile F, Vingolo EM, Gasparini P, Banfi S. A novel GJA1 mutation causes oculodentodigital dysplasia without syndactyly. Am J Med Genet A. 2005;133A:58–60. doi: 10.1002/ajmg.a.30554. [DOI] [PubMed] [Google Scholar]
- Flatt AE. Webbed fingers. Proc (Bayl Univ Med Cent) 2005;18:26–37. doi: 10.1080/08998280.2005.11928029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse K, Betz RC, Lee YA, Wienker TF, Reis A, Kleen H, Propping P, Cichon S, Nöthen MM. Localization of a gene for syndactyly type 1 to chromosome 2q34-q36. Am J Hum Genet. 2000;67:492–497. doi: 10.1086/303028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfarazi M, Akarsu AN, Sayli BS. Localization of the syndactyly type II (synpolydactyly) locus to 2q31 region and identification of tight linkage to HOXD8 intragenic marker. Hum Mol Genet. 1995;4:1453–1458. doi: 10.1093/hmg/4.8.1453. [DOI] [PubMed] [Google Scholar]
- Sato D, Liang D, Wu L, Pan Q, Xia K, Dai H, Wang H, Nishimura G, Yoshiura K, Xia J, Niikawa N. A syndactyly type IV locus maps to 7q36. J Hum Genet. 2007;52:561–564. doi: 10.1007/s10038-007-0150-5. [DOI] [PubMed] [Google Scholar]
- Gladwin A, Donnai D, Metcalfe K, Schrander-Stumpel C, Brueton L, Verloes A, Aylsworth A, Toriello H, Winter R, Dixon M. Localization of a gene for oculodentodigital syndrome to human chromosome 6q22-q24. Hum Mol Genet. 1997;6:123–127. doi: 10.1093/hmg/6.1.123. [DOI] [PubMed] [Google Scholar]
- Amador C, Mathews AM, Del Carmen Montoya M, Laughridge ME, Everman DB, Holden KR. Expanding the neurologic phenotype of oculodentodigital dysplasia in a 4-generation Hispanic family. J Child Neurol. 2008;23:901–905. doi: 10.1177/0883073808317730. [DOI] [PubMed] [Google Scholar]
- Malik S, Arshad M, Amin-Ud-Din M, Oeffner F, Dempfle A, Haque S, Koch MC, Ahmad W, Grzeschik KH. A novel type of autosomal recessive syndactyly: Clinical and molecular studies in a family of Pakistani origin. Am J Med Genet A. 2004;126A:61–67. doi: 10.1002/ajmg.a.20555. [DOI] [PubMed] [Google Scholar]