Summary
In human gene therapy applications, lentiviral vectors may have advantages over gamma-retroviral vectors because of their ability to transduce non-dividing cells, their resistance to gene silencing, and a lack of integration site preference. In this study, we utilized VSV-G pseudotype third generation lentiviral vectors harboring specific anti-tumor T-cell receptor (TCR) to establish clinical-scale lentiviral transduction of PBL. Spinoculation (1000 × g, 32°C for 2 h) in the presence of protamine sulfate represents the most efficient and economical approach to transduce a large number of PBLs compared to RetroNectin-based methods. Up to 20 million cells per well of a 6-well plate were efficiently transduced and underwent an average 50-fold expansion in two weeks. TCR transduced PBL mediated specific anti-tumor activities including IFN-γ release and cell lysis. Compared to gamma-retroviral vectors, the TCR transgene could be preferentially expressed on a less-differentiated cell population.
Keywords: Lentivirus, T-cell receptor, Adoptive immunotherapy, Gene therapy
Introduction
We recently showed that administration of peripheral blood lymphocytes transduced with a MART-1 reactive TCR gene using a gamma-retroviral could lead to tumor regression in patients with metastatic melanoma1. However, there are several limitations to current gamma-retroviral vector-based gene transfer systems. Unlike gamma-retroviral vectors, lentiviral vectors have been showed to be less prone to gene silencing in certain cell types2,3, including mammalian embryonic stem cells and preimplantation embryos, and they lack a preference for integration near transcription start sites4, which may reduce the potential for insertional mutagenesis. Furthermore, current gamma-retroviral vector based protocols require T cells to be fully activated for efficient transduction and this may be deleterious to their function5,6. Full activation of T cells may impair their half-life, repertoire, and the immune competence of the transduced cells5. It has been reported that lentiviral vectors can efficiently transduce cytokine stimulated PBLs in the absence of TCR activation and maintain intact immune competence 7.
Lentiviral vectors with their larger packaging capacity and ability to express complex expression cassettes may have a significant advantage compared to gamma-retroviral vectors8,9. An example of a clinically important multi-gene construct is a tumor-associated antigen T-cell receptor, which is a heterodimer of TCR alpha and beta chains10. The TCRs for a variety of tumor-associated antigens (TAA) have been identified including the MART-1 and gp100 melanoma differentiation antigens, the NY-ESO-1 cancer-testis antigen and the p53 tumor suppressor11–16. An important aspect of TCR expression vector design is that the T-cell receptor alpha and beta chains must be coordinately expressed for proper biologically activity10. In this study, we utilized lentiviral vectors encoding TCRs against the gp100:154–162 and MART-1:27–35 tumor antigen peptides in which the alpha and beta chains were linked by a modified 2A peptide17.
A clinical trial using a lentiviral vector reported by Levine et al18 demonstrated efficient and safe gene delivery to PBL with cell persistence in vivo. These studies stimulated interest in the clinical application of TCR expressing lentiviral vectors19. We established a clinical-scale lentiviral vector transduction protocol using anti-CD3/CD28 bead activation, and demonstrated that transduced cells expressed a high percentage of biological active TCRs. Using this approach we could express these TCRs in relatively less-differentiated populations of PBL.
Materials and Methods
Cell culture
PBL used in this study were obtained from metastatic melanoma patients seeking treatments at the Surgery Branch, National Cancer Institute. Briefly, PBL were collected by leukapheresis, and lymphocytes were separated by Ficoll/Hypaque cushion centrifugation, washed in HBSS and resuspended at a concentration of 1 × 106/ml in AIM-V medium (Invitrogen, Carlsbad, CA) supplemented with 300 IU/ml IL-2 and 5% heat-inactivated human AB serum (Valley Biomedical, Winchester, VA). Melanoma cell lines included MART-1 positive HLA-A2+ 624, 526 and two MART-1 positive HLA-A2− cells 888 and 938. 293T and 293 FT (Invitrogen, Carlsbad, CA) were cultured in DMEM supplemented with 10% FCS, 100 U/ml penicillin/streptomycin, 2 mM L-glutamine, 20 μM 2-mercaptoenthanol and 25 mM HEPES buffer solution. All cell lines were cultured at 37 °C in a 5% CO2 humidified incubator.
Vector construction
The lentiviral constructs utilized were derived from pRRLSIN.cPPT.MSCV/GFP.WPRE harboring a green fluorescent protein (GFP) gene driven by the murine stem cell virus (MSCV) U3 promoter (S. Jones, unpublished data). Mouse CTL clone sp0.01 A (alpha chain DQ452619 and beta chain DQ452620) targeting gp100:154–162 melanoma differentiation antigen was screened and established in Surgery Branch (L. Cassard, unpublished data). A lentiviral vector expressing the gp100 TCR alpha and beta chains was previously described17. In brief, PCR was used to amplify the individual alpha and beta chains that were linked by (in order); a furin cleavage site, V5 peptide, SGSG amino acid linker, and F2A ribosomal skip peptide. The promoter used to drive expression of the TCR was from the MSCV virus20.
The TCR alpha and beta chains from the human T cell clone DMF5 targeting melanoma antigen MART-1 21 were subcloned into lentiviral vector with the following primers; 5′ DMF5 TCR alpha chain (acccctcactcggcgcgccgatggatctgtacgacgatgacgataagatgttgaaatccttgagagt) and 3′ DMF5 beta chain (ctctcaaggatttcaacatcttatcgtcatcgtcgtacagatccatcggcgcgccgagtgaggggttg), F5P3alphaR (ctttacaggggctccgcttccggacgtagaatcgagaccgaggagagggttagggataggcttacctcgcttggcacggctggaccacagccgcagc) and F5P4betaF (agcggagcccctgtaaagcagactttgaattttgaccttctcaagttggcgggagacgtcgagtccaaccctgggcccatgaga atcaggctcctgtgc). The resulting lentiviral vector was designated, fuV5F2AF5. MSGV1-154-AIB (retroviral construct with gp100 (154) TCR gene linked with IRES) and MSGV1-F5AfT2aB (retroviral construct with MART-1 TCR gene linked with furin plus T2A peptide) were used as controls to transduce PBL. All the constructs are confirmed by restrictive enzyme digestion and sequencing.
Lentivirus preparation and transduction
293FT cells (Invitrogen, Carlsbad, CA) were cultured in complete culture medium (DMEM containing 10% FBS) supplemented with Geneticin (500 μg/ml). The day before transfection, 20 × 106 293 FT cells were plated onto 150 mm2 poly-D-Lysine coated plates (BD Biosciences, San Jose, CA) using 15 ml of culture medium. On the day of transfection, the culture medium was replaced with 15 ml of normal growth medium containing serum without antibiotics. Pseudotyped lentiviral vector was produced by transfection using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) of 150 mm2 using a four plasmid system of pMDLg/pRRE plus pRSV-Rev, the gp100 (154) TCR, MART-1 TCR or GFP vectors and the plasmid pMD2-G encoding the VSV-G envelope protein. Each plate received plasmid DNA 60 μg (transfer vector 22.5 μg, VSV-G 7.5 μg, pMDLg/pRRE 15 μg and pRSV-Rev 15 μg) and 180 μl lipofectamine 2000. Six hours after transfection, the plates were washed 3 times with PBS and 20 ml fresh medium was added to each plate. The supernatant was collected 30–48 hr post-transfection and cell debris was removed by centrifugation at 6000g for 10 min, then passed through a 0.45 μm PVDF filter. Supernatant containing viruses was tittered using a p24 kit (ZeptoMetrix, Buffalo, NY) and was either used directly or stored at −80°C.
At day 0 (counting when experiment starts), PBLs were transduced with the vector by spinoculation using 6-well or 24-well tissue culture plates. Briefly, PBLs were washed twice with PBS and pre-incubated with Dynal anti-CD3/CD28 beads (Invitrogen, Carlsbad, CA) (cells to beads ratio 1:3) for 10 min at room temperature, 5 ml vector plus 1 ml AIM-V (with 10% FBS, IL-2 300 IU/ml) were applied in the presence of 10 μg/ml protamine sulfate, and the plates were centrifuged at 1000 g, 32 °C for 2 h, then incubated overnight. The next day (day 1) a 2nd transduction was performed by replacing 5 ml of culture media with vector supernatant, (the concentration of IL-2 was maintained at 300 IU/ml) and spinoculation performed as above. At day 2, the medium was replaced with AIM-V containing 5% human serum, 300 IU/ml IL-2. At day 3, cells were transferred to 75 cm2 culture flask. For transduction using PBLs activated by anti-CD3 antibody OKT3, transduction was performed either immediately upon addition of OKT3 (50 ng/ml) or following stimulation for 1 day. The lentiviral titer was determined by p24 ELISA assay as described22.
FACS analysis
Cell surface expression of gp100 (154) TCR, MART-1 TCR, CD3, CD4, CD8, CD27, CD25, CD28, CD62L, CD45RO and murine Vβ was measured using fluorescein isothiocyanate (FITC), APC, or phycoerythrin (PE)-conjugated antibody or tetramers. Custom designed anti-gp100:154–162 and MART-1:27–35 tetramers were used (iTAg MHC Tetramer, Beckman Coulter, Fullerton, CA), all other reagents were commercially available. Immunofluorescence staining was analyzed as the relative log fluorescence of live cells, determined using a FACscan flow cytometer (BD Biosciences, San Jose, CA). A combination of forward angle light scatter and propidium iodide staining was used to gate out the dead cells, and 1 × 105 cells were analyzed.
Analysis of the DNA and total cellular protein content was carried out as described23. Briefly, PBLs were pelleted by centrifugation 500 g for 5 min and thoroughly resuspended in 1 ml cold PBS containing 0.5 mM EDTA, then 3 ml cold ethanol was added and cells incubated overnight. After fixation, cells were washed once with PBS containing 5 mM EDTA and stained using 1 ml PBS containing 30 μg/ml propidium iodide, 0.005 ug/ml fluorescein isothiocyanate (Invitrogen, Carlsbad, CA) and 0.3 mg/ml RNase A (Sigma, St. Louis, MO). After 1 h incubation at room temperature, cells were filtered through a 30 μm nylon mesh (Spectrum, Los Angeles, CA) and analyzed. All FACS data was analyzed using FlowJo 8.1.1 software (FlowJo, Ashland, OR).
Measurement of lymphocyte reactivity to antigen
Transduced PBL effector cells (1 × 105) were cocultured with melanoma lines (1 × 105) in a final volume of 0.2 ml in each well of a round-bottom 96-well plate. Cell culture supernatants were harvested and assayed 16 hr later for IFNγ by ELISA (Pierce Endogen, Rockford, IL). The culture supernatants were diluted to be in the linear range of the assay. Results representing mean (±SD) cpm of duplicate cultures.
51Cr release assay
The ability of transduced PBL to lyse HLA-A2+/gp100 (154) melanoma cells was evaluated using a 51Cr assay as described24. Briefly, 106 tumor cells were labeled for 1 h at 37 °C with 100 μCi of 51Cr (Amersham Biosciences, Pittsburgh, PA). Labeled target cells (2 × 103) were co-cultured with effector cells at the ratios indicated in the figures for 4 h at 37 °C in 0.15 ml of complete medium. Harvested supernatants were counted using a MicorBeta TriLux instrument (Perkin Elmer, Waltham, MA). Total and spontaneous 51Cr release was determined by incubating 2 × 103 labeled target cells in either 2% SDS or medium alone for the above conditions respectively. Each data point was determined as a mean of quadruplicate wells. The percentage of specific lysis was calculated as indicated in figure legend.
Results
Efficient transduction of minimally stimulated PBL by lentiviral vector
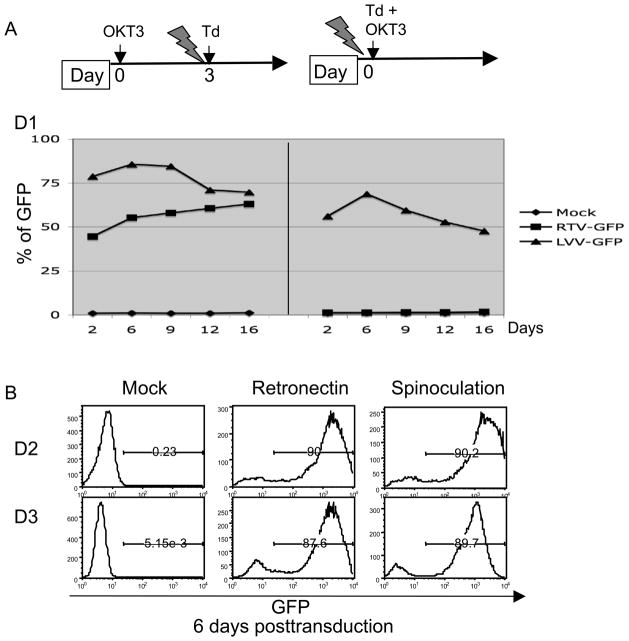
Unlike gamma-retroviruses, which require cell division for productive infections, lentiviruses can transduce non-dividing cells, though not cells in the G0 phase of the cell cycle. Because more than 90% of T cells from PBMC are in G025, T cells need to be stimulated with agents such as cytokines, anti-CD3 antibody, or mitogen to drive the T cells into the G1 phase. To compare the ability of lentiviral and gamma-retroviral vectors to transduce PBL for adoptive immune therapy, we transduced fully activated PBL (PBL were stimulated with anti-CD3 antibody, OKT3 for 3 days). As shown in Figure 1A (left panel), both vectors could efficiently transduce PBL measured by the expression of GFP. When vectors were added at the same time as stimulation with OKT3, the lentiviral vector effectively transduced PBL while the gamma-retroviral vector was inactive. RetroNectin is a truncated form of fibronectin that has been shown to significantly enhance retroviral vector-mediated gene transfer in certain type of cells26 including PBL27 without the use of polycations. In figure 1B, we compared the transduction efficiency from RetroNectin based transduction and spinoculation in PBLs from 2 donors, we found both methods were equally efficient at transduction of activated human PBL.
Figure 1.
Lentiviral vector mediated transduction of PBL. A, left panel: PBL were activated using soluble OKT3 for 3 days before starting transduction by gamma-retroviral or lentiviral vector harboring GFP; right panel: PBL were transduced with gamma-retroviral or lentiviral vector harboring GFP with simultaneous OKT3 activation. GFP was measured by FACS at 2 days post-transduction for 16 days. B, PBL were transduced with lentiviral vector harboring GFP upon bead (anti-CD3/CD28) activation using RetroNectin or spinoculation in the presence of 10 μg/ml protamine sulfate. For RetroNectin, the transduction was conducted using vector coated plates while for spinoculation, PBL were mixed with vector and centrifuged at 1000 g, 32 °C for 2 h. Six days post-transduction, the expression of GFP was measured by FACS.
Anti-CD3/CD28 bead activation facilitates lentiviral transduction of PBL
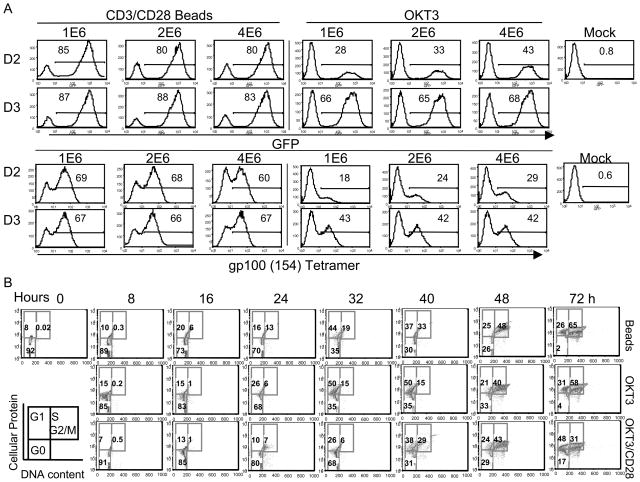
We next investigated the influence of cell stimulation methods on PBL transduction, and to test maximal density of cells for transduction. Using 24-well plates, we compared activation by anti-CD3/CD28 beads versus OKT3 using GFP and gp100 (154) TCR lentiviral vectors. Compared to OKT3 activation, bead activation mediated higher levels of transduction of PBL as shown in Figure 2A. Although there is a trend for transgene expression to decrease with increasing number of cells per well from 1 million to 4 million per well, this decrease was modest. To investigate the mechanism for the observed differences in transduction efficiency between OKT3 and CD3/CD28 beads, we compared the kinetics of cell cycle progression with beads, OKT3, and OKT3 supplemented with anti-CD28. In Figure 2B, we observed a similar kinetics of cell cycle progression, suggesting that other mechanisms may account for the differences in transduction.
Figure 2.
Bead activation facilitates lentiviral transduction of PBL. A, PBLs from two donors were transduced with lentiviral vectors harboring GFP or gp100 (154) TCR using anti-CD3/CD28 bead or OKT3 activation. 1 × 106, 2 × 106 and 4 × 106 cells were added per well of a 24-well plate. Transduction was performed by spinoculation as described. Six days post transduction, the expression of GFP or gp100 (154) TCR was measured by FACS. B, The kinetics of PBL cell cycle progression was measured by cellular protein and DNA content. PBL from leukapheresis were activated using anti-CD3/CD28 beads, OKT3, or OKT3 plus anti-CD28 antibody as indicated. Cells in each group were collected every 8 h up to 72 h post stimulation. The cells in G1 phase were distinguished from G0 by staining the cellular protein content (FITC), and G1 from S + G2/M by DNA content staining (PI). The percentage of cells at G0, G1, S and G2/M phases were as shown.
Clinical-scale lentiviral transduction of a gp100 (154) TCR
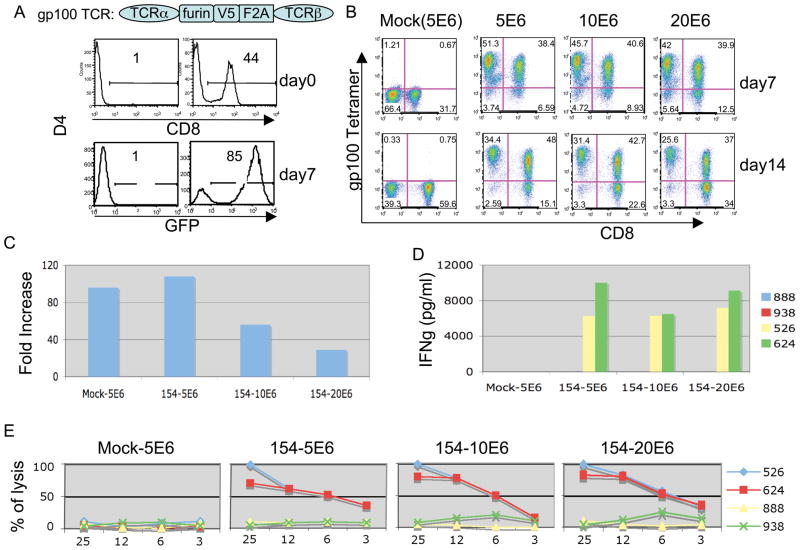
To determine the maximum the number of PBL that could be transduce, 5, 10, and 20 million PBL were added per well of a 6-well plate, activated using anti-CD3/CD28 beads, and subject to spinoculation using the anti-gp100(154) TCR lentiviral vector. Results from one of three independent experiments were shown in Figure 3. In Figure 3A, the percentage of CD8 was measured prior to activation, and the expression of control vector GFP was measured at day 7 post-transduction to compare to the TCR vector. Figure 3B demonstrated effective transduction of 20 million cells per well of 6-well plate using a vector expressing an anti-gp100 TCR (82% tetramer positive cells at day 7 post-transduction). We observed a modest decrease in tetramer staining at 14 days post-transduction that coincided with an increase in the percentage of CD8 cells in culture. There was about 50 fold expansion over 2 weeks culture (ranging from 100 to 30 fold) as shown in Figure 3C. In all three conditions, we found similar biological activities when transduced cells were co-cultured with melanoma lines (Figure 3D and 3E).
Figure 3.
Clinical-scale lentiviral transduction and analysis of a gp100 (154) TCR vector. A, Diagram of gp100 (154) TCR showing linkage of alpha and beta chains using furin, V5 peptide, and the F2A ribosomal skip peptide. Percent of CD8 was measured by FACS prior to activation. GFP vector transduced PBL were measured at day 7 to monitor transduction efficiency. B, PBLs were stimulated with anti-CD3/CD28 beads in 6-well plates (5 × 106 to 20 × 106 cells/well) and transduced using a constant amount of lentiviral vector expressing a gp100 (154) TCR. Six hours post-transduction, the supernatant was replaced with fresh medium, and a second transduction was performed. The expression of TCR was measured by gp100 (154) tetramer at day 7 and day 14 post- transduction. C, At day 14 the total number of cells was and plotted as fold expansion. D, Transduced PBL were co-cultured with tumor cells for 16 h, and the concentration of IFNγ determined by ELISA, data plotted as the mean of duplicate cultures. E, The specific lytic activities of transduced PBL were determined by co-cultured with 51Cr- labeled tumor cells at the indicated ratio and the release of 51Cr was measured. The percent cell lysis was calculated using the formula ((specific release-spontaneous release)/(total release-spontaneous release)) × 100. Results representing mean of triplicate cultures were plotted.
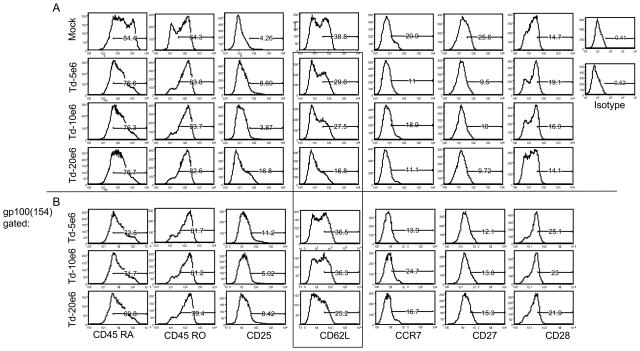
The phenotype of the cells at 2-weeks post-stimulation was investigated using T cell differentiation markers (CD45RA, CD45RO, CD25, CD27, CD28, CD62L and CCR7). Compared to untransduced cells all transduced cell cultures demonstrated similar phenotypes, figure 4. Overall, the PBL had a phenotype consistent with that of a differentiated effector T cells.
Figure 4.
T cell phenotype. The transduced cells described in Figure 3 were analyzed using a panel of differentiated markers, gp100 (154) tetramer positive cells (below line) were gated out of total population (above line), and the differentiated markers were plotted as above. The CD62L+ cells from the total population versus gp100 (154) tetramer positive population was highlighted by the box.
Establishing clinical-scale lentiviral transduction of a MART-1 TCR
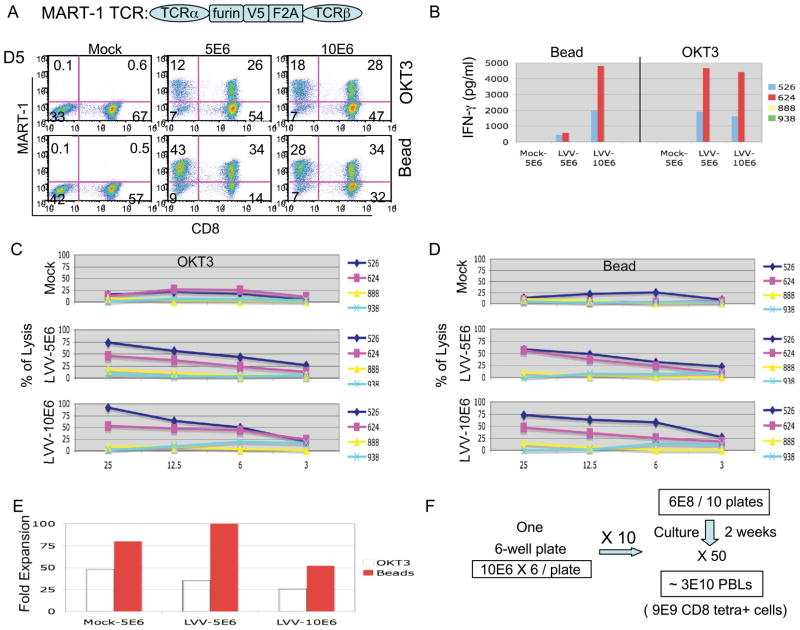
As a final example of a of clinical-scale lentiviral transduction we utilized a second anti-melanoma TCR vector targeting MART-1. In figure 5A, we compared transduction following 24 hr pre-stimulation with OKT3 versus simultaneous transduction and activation using anti-CD3/CD28 bead. After 2 weeks culture the expression of TCR was measured by tetramer. There was a lower transduction efficiency, 46% (18% in CD4 cells and 28% in CD8 cells) using soluble OKT3 activation compared to bead activation 62% (28% in CD4 and 34% in CD8), using 10 million cells per well (similar results were obtained using 5 million cells pre well, Figure 5B). Both transduced cell populations (OKT3 or anti-CD3/CD28 bead activated) exhibited similar biological activities when co-cultured with melanoma lines, except for cells transduced by bead activation using 5 million cells per well (likely due to a lower proportion of CD8 cells). In Figure 5C and 5D, both groups of transduced cells demonstrated specific anti-tumor cell lysis as evaluated by 51Cr release assay. The fold cell expansion was as shown in figure 5E. A proposed scale-up of a lentiviral vector-mediated TCR gene transfer protocol is shown in Figure 5F. Based on our data, we propose to transduce PBL using anti-CD3/CD28 bead activation with 10 million PBMC per well in each well of a 6-well plate using a total of 10 plates. After 2 weeks culture, an average of 50 fold expansion (Figure 5E) would yield an estimated 3 × 1010 PBLs with 9 × 109 CD8 positive tetramer positive cells (assuming 30% gene transfer into CD8 T cells, Figure 5A).
Figure 5.
Clinical-scale lentiviral transduction of a MART-1TCR vector.
A, Diagram of MART-1 TCR showing linkage of alpha and beta chains using furin, V5 peptide, and the F2A ribosomal skip peptide. PBLs were activated by OKT3 or CD3/CD28 beads. The expression of TCR was measured by MART-1 tetramer at day 14. B, Transduced PBL were co-cultured with melanoma tumor cells for 16 h, and the concentration of IFNγ determined by ELISA, data plotted as the mean of duplicate cultures. C and D, The specific lytic activities of transduced PBLs. The cells were co- cultured with 51Cr-labeled tumor cells at the indicated ratio and the release of 51Cr was measured. The percent of cell lysis was calculated using the formula ((specific release-spontaneous release)/(total release-spontaneous release)) × 100. Results representing mean of triplicate cultures were plotted. E, At day 14 the total number of cells were counted and plotted as fold expansion. F, The proposed scale-up for therapeutic preparation was illustrated that starting from ten 6-well plates containing 10 million cells per well cultured for 2 weeks could yield 3 × 1010 cells for treatment.
Lentiviral vector mediated transgene expression in a less-differentiated T cell population
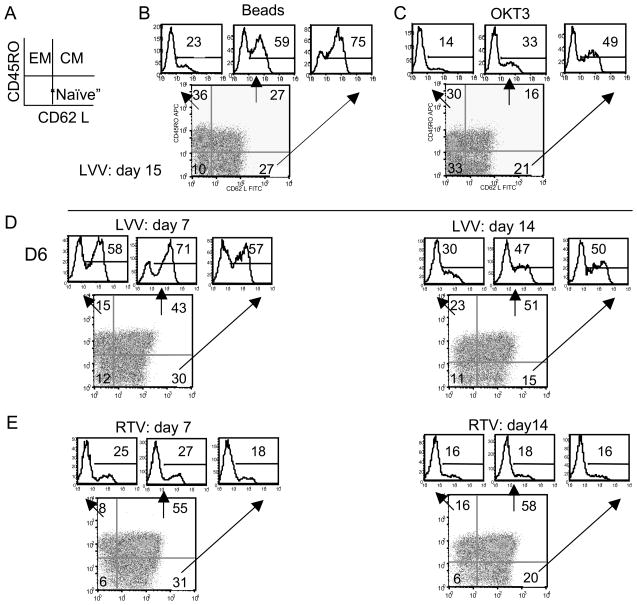
In humans, central-memory T cells (TCM) and effector-memory T cells (TEM) can be defined by the level of CCR7 and CD62L28 expression. At two weeks post-transduction, we evaluated the differentiation status of PBLs transduced by lentiviral vector harboring the MART-1 TCR using markers CD45RO and CD62 L (Figure 6A). Each quadrant of the FACS histogram was then analyzed for MART-1 TCR positive cells as shown (images above each histogram, Figure 6B, 6C). We observed a higher percentage of MART-1 TCR positive cells in the less-differentiated cells population (CD45RO-/CD62L+). To confirm this observation, we set up a second experiment and as shown in Figure 6D, we again observed disparity of transgene expression appearing as early as at day 7 (on left) that was more apparent on day 14 (on right). At the same time, we performed a parallel experiment using a gamma-retroviral vector and observed that the expression of the MART-1 TCR transgene was evenly distributed in TEM, TCM and “naive” populations at both day 7 (Figure 6E, left) and day 14 (Figure 6E, right).
Figure 6.
Lentiviral vector mediated transgene expression in less-differentiated cells. A, Diagram of hypothetical FACS of PBL using anti-CD62L and CD45RO. EM, effector memory; CM, central memory; naïve T cells. B, C, Transduced PBLs activated via anti-CD3/CD28 bead or OKT3 was analyzed for CD62L and CD45RO at day 15. The number in each plot represents the percentage of cells in each quadrant. Three histograms indicated by arrows display the percentage of MART-1TCR positive cells in each quadrant. D, E, PBLs were transduced with lentiviral vector in D or gamma-retroviral vector in E. PBLs were activated using OKT3, with transduction at day 1. FACS was performed at day 7 and day 14 using CD62L and CD45RO markers and MART-1 TCR positive cells were plotted in histograms as described above.
Discussion
Gattinoni et al reported that fully differentiated effector T cells in a murine model of adoptive cell transfer, although capable of in vitro tumor killing and high-level IFN-γ release, exhibited inferior activity for in vivo tumor treatment compared to naïve and less differentiated effectors29. Lentiviral vectors can efficiently transduce non-dividing cells, making them more effective than gamma-retroviral vectors for gene transfer to postmitotic or slowly dividing cells, which may include hematopoietic stem cells and less-differentiated T cells 19. The lentiviral vectors used in this study were 3rd generation vectors, which contain several elements previously shown to enhance vector function3,30–35. In addition, current lentiviral vectors contain a woodchuck hepatitis virus post-transcriptional responsive element (wPRE) for improved transcriptional termination36, but because of the theoretical concern that carcinogenesis maybe associated with the wPRE X protein, we are now testing an alternative truncated form of wPRE in our vectors37.
The ability of lentiviral vector to transduce minimally stimulated T cells7 may have significant advantages, as transgenic mouse models indicated that less-differentiated T cells, where superior for in vivo tumor treatment compared to differentiated T cells29,38. To transduce the least differentiated T cells, requires minimal ex vivo culture that must take into account the block to productive lentiviral vector transduction at the G0 phase of the cell cycle. While 90% of freshly isolated human PBMC are at G0, anti-CD3 stimulation rapidly induces G0 cells to enter into G125. Our data demonstrate that simultaneous transduction and stimulation with anti-CD3/CD28 beads mediates efficient gene transfer. Furthermore, the low level of metabolic activity in unstimulated lymphocytes makes transduction at high cell density possible. The establishment of this clinical-scale lentiviral transduction protocol in 6-well plates permits a simple scale-up procedure. Based on our data, we propose using 10 million cells per well in 6-well plates (10 plates total), which, with an average 50-fold expansion in 2 weeks of in vitro culture, would yield 3 × 1010 cells for patient treatment.
During the analysis of the phenotype of the TCR transduced cells, we noted a higher percentage of TCR expressing cells in the less-differentiated T cell population (defined by analysis of CD45RO and CD62L). Conversely, the expression of TCR transduced by gamma-retroviral vector harboring the same TCR did not show this preference (figure 6). The possible mechanism(s) for this observation is unknown, but could be mediated by differences in vector biology (e.g., integration dynamics and site preference, of different envelope pseudotype, VSV-G versus GaLV env). The potential for expression of lentiviral vector transgenes in less differentiated cells may be important, as Berger et al recently showed in macaques that antigen-specific CD8 T cell clones derived from TCM, but not TEM, persisted long-term in vivo28. Thus, the property of lentiviral vectors to transduce TCM and “naive” population may impart a potent in vivo anti-tumor advantage versus gamma-retroviral vectors.
Recently, Levine et al reported on a phase 1 clinical trial using lentiviral vectors that demonstrated efficient and safe gene delivery to patients’ T cells with good persistence in vivo18. The lentiviral vectors harboring specific anti-tumor TCRs described herein, in conjunction with establishment of clinical-scale lentiviral transduction protocol, and the observed transgene expression within less-differentiated T-cell populations, provide the opportunity not only for sustained transgene expression but also new methodologies to modify T lymphocytes at a less differentiated stage, and may have immediate applications in cancer immunotherapy.
Acknowledgments
We thank FACS lab and TIL lab in Surgery Branch for providing technical support and maintenance of tumor cells from patients. This work is supported by the Intramural Research Program of the National Institute of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Financial Disclosure: The authors have declared there are no financial conflicts of interest in regards to this work.
Competing interests statements:
The authors declare that they have no competing financial interests.
References
- 1.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikawa M, Tanaka N, Kao WW, et al. Generation of transgenic mice using lentiviral vectors: a novel preclinical assessment of lentiviral vectors for gene therapy. Mol Ther. 2003;8:666–673. doi: 10.1016/s1525-0016(03)00240-5. [DOI] [PubMed] [Google Scholar]
- 3.Pfeifer A, Ikawa M, Dayn Y, et al. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci U S A. 2002;99:2140–2145. doi: 10.1073/pnas.251682798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montini E, Cesana D, Schmidt M, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 5.Sauce D, Bodinier M, Garin M, et al. Retrovirus-mediated gene transfer in primary T lymphocytes impairs their anti-Epstein-Barr virus potential through both culture-dependent and selection process-dependent mechanisms. Blood. 2002;99:1165–1173. doi: 10.1182/blood.v99.4.1165. [DOI] [PubMed] [Google Scholar]
- 6.Sauce D, Tonnelier N, Duperrier A, et al. Influence of ex vivo expansion and retrovirus-mediated gene transfer on primary T lymphocyte phenotype and functions. J Hematother Stem Cell Res. 2002;11:929–940. doi: 10.1089/152581602321080592. [DOI] [PubMed] [Google Scholar]
- 7.Cavalieri S, Cazzaniga S, Geuna M, et al. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- 8.Chang AH, Sadelain M. The Genetic Engineering of Hematopoietic Stem Cells: the Rise of Lentiviral Vectors, the Conundrum of the LTR, and the Promise of Lineage-restricted Vectors. Mol Ther. 2007;15:445–456. doi: 10.1038/sj.mt.6300060. [DOI] [PubMed] [Google Scholar]
- 9.Wiznerowicz M, Trono D. Harnessing HIV for therapy, basic research and biotechnology. Trends Biotechnol. 2005;23:42–47. doi: 10.1016/j.tibtech.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Krogsgaard M, Davis MM. How T cells ‘see’ antigen. Nat Immunol. 2005;6:239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher TN. T-cell-receptor gene therapy. Nat Rev Immunol. 2002;2:512–519. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 12.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Zheng Z, Robbins PF, et al. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan RA, Dudley ME, Yu YY, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes MS, Yu YY, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen CJ, Zheng Z, Bray R, et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S, Cohen CJ, Peng PD, et al. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene therapy. 2008 doi: 10.1038/gt.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine BL, Humeau LM, Boyer J, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci U S A. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohn DB. Lentiviral vectors ready for prime-time. Nat Biotechnol. 2007;25:65–66. doi: 10.1038/nbt0107-65. [DOI] [PubMed] [Google Scholar]
- 20.Hawley RG, Lieu FH, Fong AZ, et al. Versatile retroviral vectors for potential use in gene therapy. Gene therapy. 1994;1:136–138. [PubMed] [Google Scholar]
- 21.Johnson LA, Heemskerk B, Powell DJ, Jr, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geraerts M, Willems S, Baekelandt V, et al. Comparison of lentiviral vector titration methods. BMC Biotechnol. 2006;6:34. doi: 10.1186/1472-6750-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boquest AC, Day BN, Prather RS. Flow cytometric cell cycle analysis of cultured porcine fetal fibroblast cells. Biol Reprod. 1999;60:1013–1019. doi: 10.1095/biolreprod60.4.1013. [DOI] [PubMed] [Google Scholar]
- 24.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
- 25.Lea NC, Orr SJ, Stoeber K, et al. Commitment point during G0-->G1 that controls entry into the cell cycle. Mol Cell Biol. 2003;23:2351–2361. doi: 10.1128/MCB.23.7.2351-2361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimizuka F, Taguchi Y, Ohdate Y, et al. Production and characterization of functional domains of human fibronectin expressed in Escherichia coli. J Biochem. 1991;110:284–291. doi: 10.1093/oxfordjournals.jbchem.a123572. [DOI] [PubMed] [Google Scholar]
- 27.Pollok KE, Hanenberg H, Noblitt TW, et al. High-efficiency gene transfer into normal and adenosine deaminase-deficient T lymphocytes is mediated by transduction on recombinant fibronectin fragments. Journal of virology. 1998;72:4882–4892. doi: 10.1128/jvi.72.6.4882-4892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouneaud C, Garcia Z, Kourilsky P, et al. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dardalhon V, Herpers B, Noraz N, et al. Lentivirus-mediated gene transfer in primary T cells is enhanced by a central DNA flap. Gene Ther. 2001;8:190–198. doi: 10.1038/sj.gt.3301378. [DOI] [PubMed] [Google Scholar]
- 31.Barklis E, Mulligan RC, Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986;47:391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- 32.Colicelli J, Goff SP. Isolation of a recombinant murine leukemia virus utilizing a new primer tRNA. J Virol. 1986;57:37–45. doi: 10.1128/jvi.57.1.37-45.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther. 2005;16:1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- 34.Lois C, Hong EJ, Pease S, et al. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 35.Ma Y, Ramezani A, Lewis R, et al. High-level sustained transgene expression in human embryonic stem cells using lentiviral vectors. Stem Cells. 2003;21:111–117. doi: 10.1634/stemcells.21-1-111. [DOI] [PubMed] [Google Scholar]
- 36.Schambach A, Galla M, Maetzig T, et al. Improving Transcriptional Termination of Self-inactivating Gamma-retroviral and Lentiviral Vectors. Mol Ther. 2007;15:1167–1173. doi: 10.1038/sj.mt.6300152. [DOI] [PubMed] [Google Scholar]
- 37.Schambach A, Bohne J, Baum C, et al. Woodchuck hepatitis virus post-transcriptional regulatory element deleted from X protein and promoter sequences enhances retroviral vector titer and expression. Gene Ther. 2006;13:641–645. doi: 10.1038/sj.gt.3302698. [DOI] [PubMed] [Google Scholar]
- 38.Abad JD, Wrzensinski C, Overwijk W, et al. T-Cell Receptor Gene Therapy of Established Tumors in a Murine Melanoma Model. J Immunother. 2008;31:1–6. doi: 10.1097/CJI.0b013e31815c193f. [DOI] [PMC free article] [PubMed] [Google Scholar]