Abstract
Rationale
Morphine relieves pain, in part, by acting on neurons within the periaqueductal gray (PAG). Given that the PAG contains a subpopulation of dopamine neurons, dopamine may contribute to the antinociceptive effects mediated by the PAG.
Methods
This hypothesis was tested by measuring the behavioral and electrophysiological effects of administering dopamine agonists and antagonists into the ventrolateral PAG (vPAG). An initial histological experiment verified the existence of dopamine neurons within the vPAG using dopamine transporter and tyrosine hydroxylase antibodies visualized with confocal microscopy.
Results
Microinjection of cumulative doses of morphine into the vPAG caused antinociception that was dose-dependently inhibited by the dopamine receptor antagonist α-flupenthixol. α-Flupenthixol had no effect on nociception when administered alone. Injection of the dopamine receptor agonist (-) apomorphine into the vPAG caused a robust antinociception that was inhibited by the D2 antagonist eticlopride but not the D1 antagonist SCH-23390. The effects of dopamine on GABAA-mediated evoked inhibitory post-synaptic potentials (eIPSCs) were measured in PAG slices. Administration of met-enkephalin inhibited peak evoked inhibitory post-synaptic potentials (eIPSCs) by 20-50%. Dopamine inhibited eIPSC by approximately 20-25%. Administration of α-flupenthixol (20 μM) attenuated eIPSC inhibition by dopamine, but had no effect on met-enkephalin-induced inhibition.
Conclusions
These data indicate that PAG dopamine has a direct antinociceptive effect in addition to modulating the antinociceptive effect of morphine. The lack of an effect of α-flupenthixol on opioid-inhibition of eIPSCs indicates that this modulation occurs in parallel or subsequent to inhibition of GABA release.
Introduction
The periaqueductal gray (PAG) plays an important role in the modulation of nociception. Several studies have localized the antinociceptive effects of morphine to the vPAG and other midbrain areas (Manning et al. 1994; Pert and Yaksh 1975; Yaksh et al. 1976), and interference with neural transmission within the PAG alters the antinociceptive effects of systemically administered morphine (Lane et al. 2005). Electrophysiological experiments demonstrate that opioids activate PAG output neurons that project to the rostroventral medulla and the spinal cord (Reichling and Basbaum 1990). This activation appears to occur via inhibition of GABAergic neurons in the PAG (Vaughan and Christie 1997).
Although the mechanisms underlying opioid antinociception in the PAG have been well studied, much less is known about the role of other neurotransmitters. For example, a subpopulation of neurons within the ventrolateral PAG (vPAG) is dopaminergic. These neurons project to the central nucleus of the amygdala, bed nucleus of the stria terminalis, sublenticular extended amygdala, substantia innominata, and locally within the PAG (Dong and Swanson 2006; Hasue and Shammah-Lagnado 2002). Relatively little is known about the contribution of these vPAG dopamine neurons to morphine-induced antinociception, although one study reported that removal of dopaminergic input into the vPAG using 6-hydroxydopamine (6-OHDA) or dopamine antagonists attenuated the antinociceptive effect of systemically administered morphine (Flores et al. 2004).
There is substantial overlap in the neural systems containing opioid and dopamine receptors (Nestler 1996; Wood 1983; Wood et al. 1980). On the cellular level, agonist binding to opioid or dopamine receptors activate GIRK channels (Davila et al. 2003; Pillai et al. 1998), alter cAMP formation (Self 2004), and modulate GABA transmission (Cameron and Williams 1995; Vaughan and Christie 1997). These findings indicate that dopamine receptor activation within the vPAG may have opioid-like effects on nociception.
The current studies used anatomical, behavioral, and electrophysiological techniques to examine the role of PAG dopamine in nociception and morphine-induced antinociception. Specifically, the presence of dopamine transporter (DAT) and tyrosine hydroxylase (TH) immunoreactive neurons in the PAG was confirmed using confocal microscopy, and the effect of microinjecting dopamine agonists and antagonists into the PAG on nociception was measured. Finally, cellular interactions between opioids and dopamine were analyzed in PAG slices. Given the similarities between dopamine and opioid mechanisms of action, it was hypothesized that dopamine agonists would produce antinociception and dopamine antagonists would attenuate morphine antinociception.
Methods
Subjects
Adult male Sprague-Dawley rats (230-375 g) were used in all behavioral experiments. Young male and female rats (50-200 g) were used in electrophysiological experiments because age-dependent increases in brain connective tissue makes slice recording difficult in older rats. No systematic differences between male and female rats were observed in the electrophysiological experiments. Our previous research shows that the behavioral and electrophysiological effects of opioids are similar between these ages of rats (Ingram et al. 2007). Experiments were conducted in accordance with the animal care and use guidelines outlined by the Committee for Research and Ethical Issues of the International Association for the Study of Pain, and were approved by the Animal Care and Use Committee at Washington State University.
Experiment 1: Localization of Dopamine neurons and fibers in the PAG
Previous experiments identified TH-positive dopamine neurons in the vPAG (Flores et al. 2004; Flores et al. 2005; Lu et al. 2006). We sought to verify this finding using anti-DAT antibodies in addition to anti-TH antibodies. Rats were deeply anesthetized using pentobarbital before they were perfused using a PB-heparin solution followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.3). The brain was removed and post-fixed overnight in ice-cold (4°C) 4% paraformaldehyde in PB. The brains were rinsed extensively in PB and then stored at 4°C in PB until they were sectioned. Brain sections (100 μM) were collected in PB using a vibratome.
For immunostaining, free-floating slices containing the PAG were rinsed three times for 5 minutes in phosphate buffer (PB) and then placed on a rotating shaker in blocking buffer (PB containing 1% Triton-X 100 and 5% normal goat serum) for 45-60 min at room temperature. The tissue was incubated overnight at 4°C in PB containing 0.1% Triton-X 100, 5% normal goat serum and either anti-mouse TH (final dilution 1:10,000; Sigma-Aldrich, St. Louis MO), anti-rat DAT (final dilution 1:400; Chemicon, a subdivision of Millipore, Billerica MA) or both. The tissue was rinsed 3 times (5 min) on a rotating shaker. Sections stained only for TH were incubated with anti-mouse Alexa Fluor 488 (Molecular Probes, a subdivision of Invitrogen, Carlsbad CA). Sections stained for DAT alone were incubated in anti-rat Alexa- Fluor 488. For both TH and DAT staining in the same slice, the sections were incubated in both anti-rat Alexa-Fluor 488 and anti-mouse Alexa-Fluor 568 for 30 min at room temperature. The sections were mounted onto slides, dried for 10 min and then coverslipped using Prolong (Molecular Probes).
Image capture was carried out using a TCS SP1 Laser Scanning Spectral confocal microscope (Leica Microsystems, Mannheim, Germany) equipped with four laser bands, which excite at wavelengths of 476, 488, 568 and 633 nm. Lasers were applied separately for emission at 488 (red, Alexa-Fluor 488) and 568 (green, Alexa-Fluor 568) nm. The images were digitally captured at 1024 × 1024 pixels in size. Sixteen serial sections were made through the sample down to a depth of approximately 20-40 μm. Serial reconstructions and imaging analysis was carried out using software by either Leica Microsystems (Leica Microsystems. Mannheim, Germany) or IPLabTM (Scanalytics Inc Fairfield, VA).
Experiment 2: The effect of α-flupenthixol on morphine antinociception
Each rat was anesthetized with pentobarbital (60 mg/kg). After placement in a stereotaxic frame, the skull was exposed by removal of a 15 mm diameter section of the scalp and sterilized with 70% ethanol in water using a cotton swab. Two anchor screws were then placed approximately 2.5 mm rostral and 2.0 mm to the right of lambda. A stainless steel guide cannula (21 gauge, 9 mm) was surgically placed through a hole drilled above the vPAG so that the tip was positioned above the vPAG, using the following coordinates (relative to lambda): AP: +1.7 mm, ML: +0.6 mm, DV: -4.6 mm. A stainless steel stylette was inserted into the cannula to prevent clogging. The cannula was secured with dental acrylic that encompassed the anchor screws, and the acrylic was allowed to dry before the rat was removed from the stereotaxic stage. Rats were given 4-10 days to recover before testing. Food and water were available ad libitum except during testing.
Rats were tested in a dimly illuminated room during the dark cycle of a 12h: 12h reverse light/dark schedule (lights off at 7:00 a.m.). Nociception was assessed with the hot-plate test by measuring the latency to lick the hind paw, or attempt to jump from the apparatus, when placed on a 52.5 °C plate. The rat was removed from the apparatus if it did not respond within 50 s. Drugs were injected into the vPAG through a 23-gauge microinjector that extended 2 mm beyond the tip of the cannula. A sham microinjector was inserted into the cannula on the day before testing to habituate the rat to the microinjection procedure and prevent possible behavioral effects produced by mechanical stimulation of neurons during initial tissue penetration. No drug was injected on this day.
Rats were injected with cumulative quarter log doses of morphine (1, 1.8, 3.2, 5.6, and 10 μg/0.4 μl) into the vPAG. Our previous research shows that cumulative dose microinjections reveal clear shifts in morphine dose-response curves (Morgan et al., 2006a). The competitive dopamine receptor antagonist α-flupenthixol (0, 10, 18, or 32 μg) or saline vehicle was injected in combination with the first morphine injection. Separate rats were used for each α-flupenthixol dose. Following baseline hot plate testing, rats were injected every 20 min and tested on the hot-plate 15 min after each injection. The doses and interdose intervals used in this cumulative dosing procedure were adapted from previous studies (Duttaroy et al. 1997; Kalant et al. 1971; Morgan et al. 2006a; Morgan et al. 2006b). An additional control group was injected with α-flupenthixol (32 μg) in the absence of morphine and tested on the hot plate following saline injections every 20 min for five trials.
Rats were killed with an overdose of halothane following testing. Brain slices (100 μm) were cut with a vibratome and viewed under a microscope to localize the microinjector placement. Only rats with microinjector placements within or immediately adjacent to the vPAG were included in data analysis.
Experiment 3: vPAG microinjection of (-) apomorphine
Rats were surgically prepared and tested as described in Experiment 2. As dopamine may be metabolized rapidly in the brain, we used the dopamine agonist (-) apomorphine, which has more robust effects on nociception than dopamine in other brain areas (Phillips et al. 1992). Rats were weighed and baseline hot-plate latency was assessed prior to microinjection of the dopamine D1 receptor antagonist SCH-23390 (4 μg), D2 receptor antagonist eticlopride (4 μg), or saline vehicle into the vPAG. Two minutes later, the first of four cumulative doses of (-) apomorphine (10 μg/0.4 μl each) were microinjected into the vPAG. Injections were administered every 7 min, and the hot-plate test occurred 5 min after each injection. A within-groups design was used in this study, in which each rat was tested with (-) apomorphine in combination with eticlopride, SCH-23390, or vehicle in a counterbalanced fashion on three separate test session. These doses were chosen from previous studies in which similar doses were microinjected into the vPAG and other brain areas (Bari and Pierce 2005; Flores et al. 2004; Sun and Rebec 2005). Each test session was separated by one week.
Experiment 4: In vitro analysis of α-flupenthixol/opioid interactions
Rats were deeply anesthetized with halothane (Sigma, St. Louis), and the brain rapidly dissected. Coronal slices (250 μm) were obtained using a vibratome, and stored in external buffer (126 mM NaCl, 2.5 mM KCl, 1.4 mM NaH2PO4, 1.2 mM MgCl2, 11 mM glucose, and 25 mM NaHCO3) equilibrated with 95% O2, 5% CO2 gas at 32°C. Slices containing the vPAG were placed in an electrophysiological recording chamber and superfused with the external buffer described above with the addition of 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo(F)quinoxaline (NBQX; 5 μM) and strychnine (1 μM) to block glutamatergic and glycinergic input, respectively. vPAG neurons were visualized using infra-red Nomarski optics. Evoked inhibitory postsynaptic currents were measured with whole-cell voltage clamp recordings using patch electrodes (2-5 MOhms) containing 140 mM CsCl, 10 mM EGTA, 5 mM HEPES, 2 mM CaCl2, 2 mM MgATP (pH 7.3, 270-290 mosmol/l). Series resistance was compensated by 80% and the access resistance was continuously monitored for changes. The membrane was clamped at -70 mV during all experiments. To elicit IPSCs, bipolar tungsten electrodes delivered 1-10 mV stimuli at 30 mHz to the slice. Dopamine receptor ligands and met-enkephalin were administered in the superfusate.
Met-enkephalin produces antinociception by inhibiting GABAergic neurons in the PAG. The objective of this experiment was to determine whether α-flupenthixol modulates met-enkephalin signaling. Met-enkephalin, which has mixed effects at the μ and δ-opioid receptors, was chosen instead of morphine, because its effects are reversible upon washout, and because there are no functional δ-opioid receptors in the PAG in the naïve rat (Ma et al. 2006). PAG slices were perfused with α-flupenthixol (20 μM) or external buffer vehicle for at least 10 min. Slices were stimulated every 30 s for 2 min prior to superfusion of met-enkephalin (5, 10, or 20 μM) for 2-5 min. At this point, all drugs were washed out for at least 10 min, and the experiment was repeated using the complementary α-flupenthixol/vehicle condition in a counterbalanced fashion. Only one cell was recorded per slice. For data analysis, peak eIPSCs were expressed as change from baseline, defined as the average of the four eIPSCs immediately before perfusion of met-enkephalin.
Experiment 5: Cellular effects of dopamine on GABAergic neurotransmission
PAG slices were prepared as described in Experiment 4. The direct effect of dopamine was assessed by measuring changes in eIPSCs following administration of dopamine. As in Experiment 4, slices were perfused with α-flupenthixol (20 μM) or external buffer vehicle for at least 10 min. Slices were stimulated every 30 s for 2 min, at which point dopamine (10, 30, or 60 μM) was perfused over the slices for an additional 2 min. Each cell received both α-flupenthixol/vehicle conditions in a counterbalanced fashion.
Statistics
Repeated-measures analysis of variance (ANOVA) was used to analyze changes in hot plate latency. D50 values, defined as the dose that results in half-maximal antinociception (Tallarida 2000), were calculated to compare shifts in morphine potency. For electrophysiological experiments, changes in peak eIPSC magnitudes were analyzed. Baseline eIPSC was derived as the mean of four stimulation trials. The last 4 eIPSCs obtained after drug application were averaged to obtain a “post-drug” value. Repeated measures ANOVA were used to measure the effect of drug with Time (baseline/post-drug) as the repeated measure and α-flupenthixol (0, 20 μM) as the between-groups measure. Specific differences were analyzed further using Tukey's post-hoc tests.
Results
Experiment 1: Localization of Dopamine neurons and fibers in the PAG
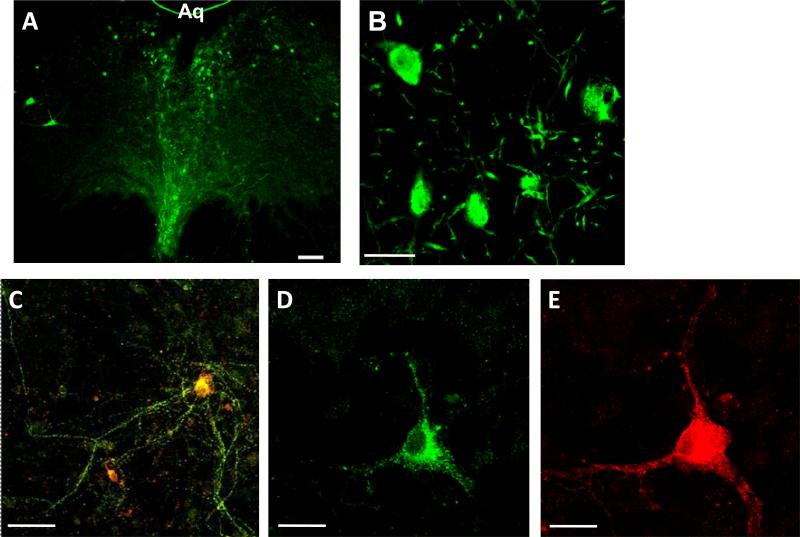
Previous studies have demonstrated that the PAG contains both small, rounded neurons and large, multipolar dopamine neurons within the PAG (Flores et al. 2004; Flores et al. 2005; Lu et al. 2006). TH and DAT immunohistochemical staining was identified within the ventral portions of the vPAG (Figure 1). TH labeled neurons were diffusely localized throughout the ventral PAG and were concentrated near the ventral region of the aqueduct (Figure 1A). Higher magnification of this staining identified the presence of cell bodies as well as neuronal processes (Figure 1B). These neurons also labeled for DAT (Figure 1C-E), demonstrating that they are dopaminergic.
Figure 1.
Dopaminergic neurons within the vPAG. A) Low magnification (5x) of a TH-stained brain slice of the vPAG. Arrows indicate examples of TH-stained cell bodies. Aq = Aqueduct. B) Confocal magnification (40x) showing TH-stained cell bodies and processes within the vPAG. C) Composite image of DAT (green, panel D) and TH (red; panel E) staining of a vPAG neuron. Scale bars are as follows: A) 100μm; B,C) 40 μm; D,E) 20μm.
Experiment 2: The effect of α-flupenthixol on morphine antinociception
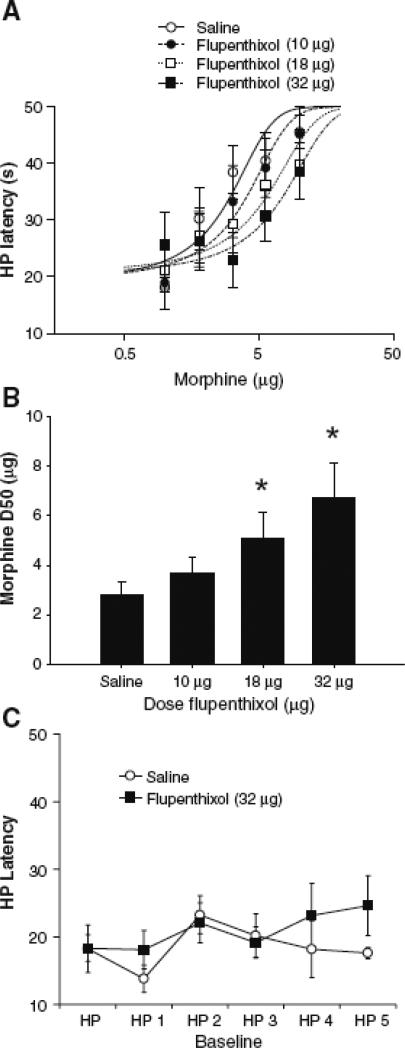
Microinjection of morphine into the vPAG produced a dose-dependent increase in hot-plate latency (Figure 2), as revealed by a main effect of morphine Dose [F (25, 125) = 6.2; p < 0.01]. Administration of α-flupenthixol caused a dose-dependent reduction in morphine antinociception [F (3, 166) = 3.4; p < 0.05]. Post-hoc comparisons indicate that the morphine D50 was significantly shifted from 2.8 μg (in saline-pretreated rats) to 5.1 and 6.8 μg in rats pretreatment with 18 and 32 μg α-flupenthixol, respectively (Figure 2B; ps < 0.05). Multiple injections of α-flupenthixol or saline into the vPAG had no effect on nociception (Figure 2C). Mean hot-plate latency varied between 18 and 23 s across trials despite repeated microinjection of α-flupenthixol into the vPAG.
Figure 2.
The antinociceptive effect of morphine is attenuated using the non-specific dopamine receptor antagonist α-flupenthixol. A) Morphine dose response curves were calculated using non-linear regression in the presence of 0, 10, 18, and 32 μg α-flupenthixol (n = 7-8 per dose). B) Summary of data presented in panel A, showing that α-flupenthixol caused a dose-dependent reduction in morphine potency. Data are expressed as mean D50s ± SEM for morphine antinociception. Asterisks indicate significant D50 shifts from the saline treatment condition. C) To determine whether flupenthixol altered nociception, the highest dose of flupenthixol (32 μg) was injected into the PAG and rats were tested for their hotplate latencies after saline microinjections at 20-min intervals (n = 7 per group). No consistent change in hot plate latency was evident despite repeated microinjections of saline.
Experiment 3: vPAG microinjection of (-) apomorphine
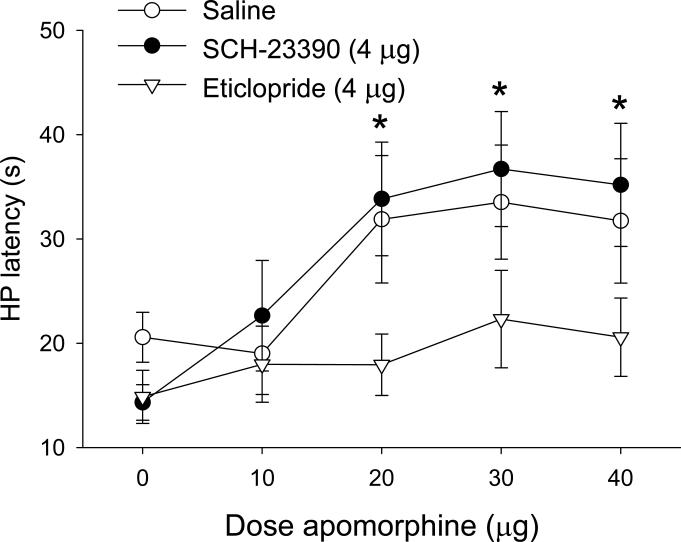
Microinjection of (-) apomorphine into the vPAG caused a dose-dependent increase in hot plate latency (Figure 3), as indicated by a main effect of Dose [F (4, 105) = 5.47; p < 0.01]. This effect was blocked by pretreatment with eticlopride, but not by SCH-23390, as indicated by a main effect of Antagonist [F (2, 105) = 6.83; p < 0.01]. Post-hoc comparisons indicated that latencies were increased at the 20, 30, and 40 μg doses of (-) apomorphine in the saline and SCH-23390 groups only. Infusions of apomorphine (n = 4) outside of the vPAG did not result in significant antinociception (data not shown). These results indicate that intra-vPAG (-) apomorphine induces antinociception through a dopamine D2 receptor mechanism.
Figure 3.
Microinjection of (-) apomorphine into the vPAG produced antinociception. (-) Apomorphine was administered cumulatively in combination with vehicle, the D1 receptor antagonist SCH-23390, or the D2 receptor antagonist eticlopride (n = 9 per treatment). Co-administration of eticlopride, but not SCH-23390 reversed the antinociceptive effect of apomorphine. Data are expressed as mean ± SEM. Asterisks indicate doses that caused significant increases in latencies relative to baseline.
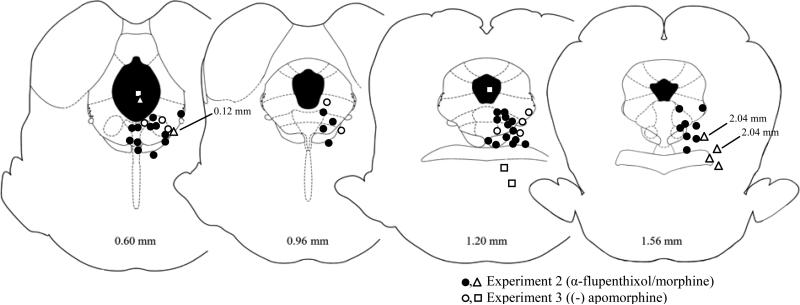
The placements of microinjectors used in the behavioral experiments are shown in Figure 4. Only rats with placements within or immediately adjacent to the vPAG were included in the analyses. Injections of morphine outside the PAG (n = 6) did not produce reliable antinociceptive effects.
Figure 4.
Location of microinjectors for behavioral experiments. Only rats with microinjector placements within or immediately adjacent to the vPAG were included in data analyses. Numbers refer to anterior/posterior coordinates relative to the interaural line (Paxinos and Watson 2005). Closed and open circles refer to Experiments 2 and 3, respectively. Triangles and squares refer to injections of (-) apormorphine and morphine that occurred outside of the vPAG, respectively. No difference in the location of the microinjector sites between groups was evident.
Experiment 4: In vitro analysis of α-flupenthixol/opioid interactions
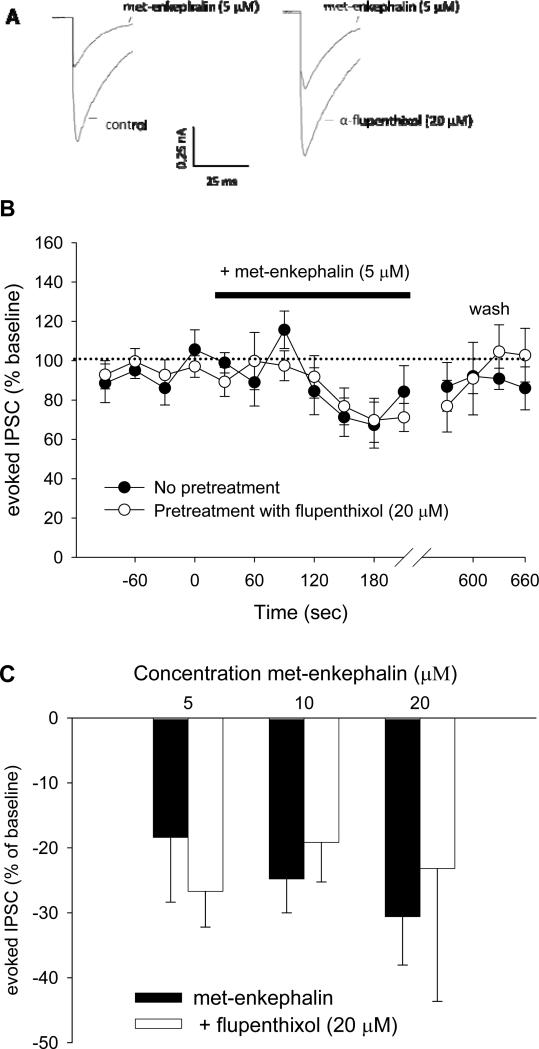
Met-enkephalin was applied to the PAG slice with or without α-flupenthixol in a counterbalanced fashion. Example traces from an experiment in which 5 μM met-enkephalin was administered are shown in Figure 5A. Met-enkephalin inhibited eIPSC amplitude by 20-50%; this inhibition peaked approximately 2 min following drug superfusion (Figure 5B). As shown in summary Figure 5C, the dose-dependent inhibition of eIPSCs by met-enkephalin [F (1, 45) = 41.7; p < 0.01 for the effect of Time] was unaffected by co-treatment with α-flupenthixol.
Figure 5.
α-Flupenthixol does not alter met-enkephalin induced inhibition of eIPSCs. A) Examples of GABAergic eIPSCs and their inhibition by met-enkephalin (5 μM) in the absence (left) and presence (right) of α-flupenthixol (20 μM). B) Summary of experiments (n = 8-9 cells per treatment group) in which slices were stimulated every 30 s, and met-enkephalin (5 μM) was superfused over the slices in the absence or presence of α-flupenthixol (20 μM). Data are expressed as change from baseline, defined as the average of eight eIPSCs before application of met-enkephalin. Only the last four basal timepoints are shown. C) Summary data showing the lack of effect of α-flupenthixol on increasing concentrations of met-enkephalin (5, 10, and 20 μM). Data are expressed as mean ± SEM.
Experiment 5: Cellular effects of dopamine on GABAergic neurotransmission
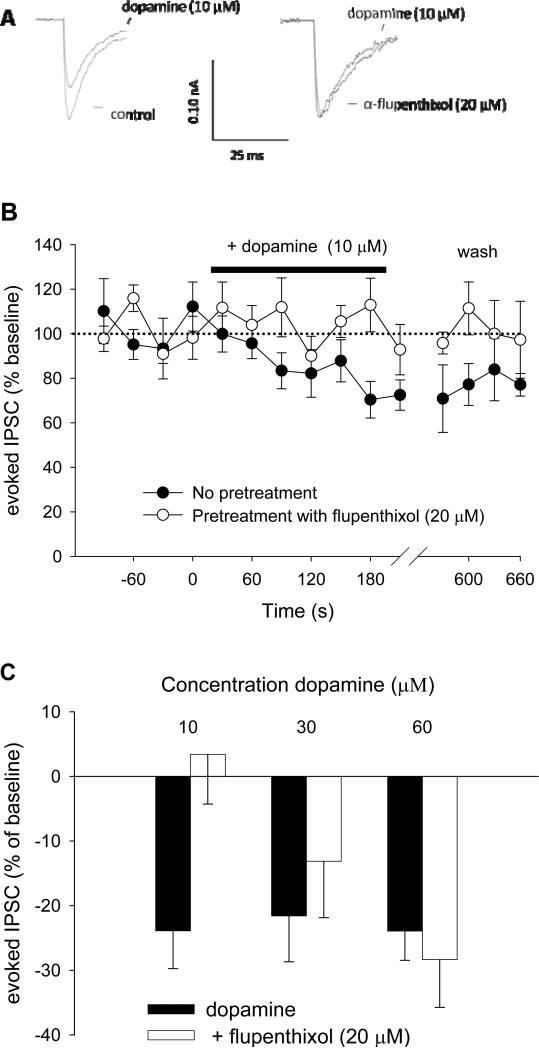
Example traces from an experiment in which 10 μM dopamine was administered are shown in Figure 6A. This dose of dopamine inhibited eIPSC amplitude by 20-25% (Figure 6B). As shown in Figure 6C, dopamine inhibited eIPSCs overall [F (1, 54) = 11.8; p < 0.01 for the effect of Time]. α-Flupenthixol blocked the effect of 10 μM dopamine [F (1, 27) = 6.1; p < 0.05 for the α-Flupenthixol × Time interaction]. However, there was no effect of α-flupenthixol on higher doses of dopamine. This is likely due to the displacement of the competitive antagonist α-flupenthixol by the high dopamine doses.
Figure 6.
Dopamine inhibits eIPSCs in the vPAG. A) Examples of GABAergic eIPSCs and their inhibition by dopamine (10 μM) in the absence (left) and presence (right) of α-flupenthixol (20 μM). B) Summary of experiments (n = 14-15 cells per treatment group) in which slices were stimulated every 30 s, and dopamine (10 μM) was superfused over the slices in the absence or presence of α-flupenthixol (20 μM). Data are expressed as change from baseline, defined as the average of eight eIPSCs before application of met-enkephalin. Only the last four basal timepoints are shown. C) Summary data showing the effect of α-flupenthixol on increasing concentrations of dopamine (10, 30, and 60 μM). Note that the effect of the competitive antagonist α-flupenthixol was reversed by increasing concentrations of dopamine. Data are expressed as mean ± SEM.
Discussion
These data provide anatomical, behavioral, and electrophysiological evidence for dopaminergic modulation of nociception in the PAG. We verified the presence of dopamine neurons within the vPAG using immunohistochemical stains for TH and DAT, finding an abundance of dopamine cell bodies and fibers within the vPAG. Administration of morphine directly into the vPAG caused marked antinociception, which is consistent with previously published research (Jensen and Yaksh 1986; Morgan et al. 1998). Recent studies (Flores et al. 2004) have found that dopamine antagonists injected directly into the vPAG attenuate the antinociceptive effects of systemically injected morphine and heroin. The present data extend these findings by demonstrating that morphine and dopamine interact within the vPAG.
Previous studies have reported antinociceptive effects of dopamine ligands when injected systemically or into specific brain areas. For example, (-) apomorphine injected into the nucleus raphe magnus (NRM) or intrathecally produced antinociception (Jensen and Smith 1982; Phillips et al. 1992). Microinjection of dopamine into the NRM had a similar although smaller and more transient effect (Phillips et al. 1992). This difference is likely caused by the rapid clearance of dopamine following microinjection. For this reason, we injected (-) apomorphine into the PAG and found substantial antinociception. Activation of dopamine receptors within the vPAG had opioid-like effects in producing antinociception and inhibiting GABA-mediated IPSCs, but there was no effect of the dopamine antagonist α-flupenthixol on opioid-induced inhibition of GABA mediated IPSCs in vPAG slices. Together, these experiments indicate that dopamine modulation of morphine antinociception does not occur by altering opioid inhibition of GABAergic transmission within the vPAG.
Morphine has been shown to increase dopamine release in several brain areas, including the nucleus accumbens and prefrontal cortex (Di Chiara and Imperato 1988). While it is unknown whether morphine causes an increase in dopamine release within the vPAG, our behavioral data suggest that a portion of morphine's antinociceptive effects are mediated by increases in vPAG dopamine. Studies that have manipulated dopamine in other brain areas support a role for dopamine in morphine-induced antinociception. For example, microinjection of morphine into the ventral tegmental area (VTA), which provides substantial dopaminergic inputs to many brain areas, results in antinociception (Manning et al. 1994). Depletion of dopamine and other biogenic amines with reserpine or 6-OHDA attenuates morphine analgesia (Kaneto and Kihara 1986; Nakamura et al. 1973). In addition, removal of dopaminergic input into the vPAG using 6-hydroxydopamine (6-OHDA) or dopamine antagonists attenuates the antinociceptive effect of systemically administered morphine (Flores et al. 2004). On the cellular level, however, we found that α-flupenthixol did not alter the effect of morphine on GABA eIPSCs.
Alternatively, the finding that intra-vPAG (-) apomorphine and morphine both have antinociceptive effects raises the possibility that dopamine and opioid receptors activate PAG output neurons through a similar mechanism. There is substantial evidence that both dopamine D2 and mu opioid receptors inhibit adenylyl cyclase (for review see Missale et al. 1998; Williams et al. 2001) and activate potassium channels (Davila et al. 2003; Ingram et al. 1998), which would result in the inhibition of neuronal activity by dopamine and morphine within the vPAG. Further, it is well established that morphine and other opioids activate PAG output neurons through inhibition of GABA release within the vPAG (Ingram et al. 1998; Vaughan et al. 1997). (-) Apomorphine may activate a similar mechanism, as dopamine-mediated inhibition of GABA release has been reported in other brain areas (Nicola and Malenka 1997). In this manner, stimulation of presynaptic dopamine and opioid receptors would result in parallel disinhibition of PAG output neurons. Our current studies support this model by demonstrating that dopamine reduces GABA-mediated IPSCs, an effect blocked by α-flupenthixol. This effect was long lasting; IPSCs were reduced for at least 10 min following dopamine application (Figure 6B). A similar long-term inhibitory effect of dopamine on GABA IPSCs has been reported in the prefrontal cortex (Seamans et al. 2001). This timecourse also matches the behavioral data: antinociception caused by intra-vPAG (-) apomorphine peaked within 5 min and lasted up to 15 min post-injection (Figure 3).
This model, however, does not explain the finding that α-flupenthixol inhibited morphine-induced antinociception. It is possible that, in an intact animal, tonic dopamine input into the vPAG modulates opioid effects, and that removal of this input reduces the antinociceptive effect of morphine and other opioids. This would suggest that morphine and dopamine interact in a more complex way than through the activation of parallel pathways as described above. However, our electrophysiological data show no evidence for an interaction. Even though the presence of intact dopamine terminals in the PAG can not be ruled out, it is possible that tonic dopaminergic input from other brain areas is removed in our slice preparation and, therefore, the interaction between dopamine and opioid systems could not be observed. Dopaminergic connections with limbic structures (Beckstead et al. 1979; Simon et al. 1979) would explain why nociception is altered in the absence of an interaction in vPAG slices. Likewise, it is possible that feedback systems necessary for dopamine modulation of opioid antinociception are disrupted by removing the PAG for in vitro recordings.
It also is possible that dopamine modulates opioid effects within the vPAG via a mechanism other than modulation of GABA-mediated eIPSCs. For example, the rightward shift of morphine's effects could be caused by morphine releasing the inhibition of dopamine terminals. This would result in increases in dopamine within the vPAG, resulting in dopamine-mediated antinociception. We chose to study the inhibition of GABA-mediated IPSCs because other studies have suggested that antinociception induced by opioids within the vPAG is due to their effects on GABA neurotransmission (Osborne et al. 1996; Vaughan et al. 1997). However, opioids have other cellular effects within the vPAG, including the activation of voltage-gated potassium channels, calcium channels, and other currents. It is therefore possible that dopamine may modulate the effects of opioids on these channels. In addition, dopamine inhibits the release of glutamate within many brain regions (Manzoni and Williams 1999; Margolis et al. 2005). Future studies in our laboratory will investigate whether opioid antagonists alter the antinociceptive effects of dopamine agonists within the vPAG.
α-Flupenthixol is a non-specific antagonist of the D1 and D2 subtypes of dopamine receptors, therefore it is unknown which subtype mediates the effects on morphine observed in this study. Studies using systemic injections of specific dopamine receptor ligands reported antinociception produced by D2 agonists, but not D1 agonists (Rooney and Sewell 1989). We have shown that the antinociceptive effects of (-) apomorphine were blocked by the D2 receptor antagonist eticlopride, and not by the D1 antagonist SCH-23390. However, previous studies have suggested that the D1 receptor is critical for the attenuation seen after systemic opioid injection (Flores et al. 2004). This difference suggests that dopamine receptors modulate morphine antinociception in unique ways in different parts of the nervous system. It may be that dopamine induced antinociception via the direct activation of D2 receptors, while opioids can indirectly modulate D1 receptors that participate in opioid-induced antinociception. Future studies are needed to fully understand the role of dopamine receptor subtypes in opioid-induced antinociception.
In conclusion, these experiments demonstrate a role for dopamine in nociception and in the modulation of opioid-mediated antinociception. Our data suggest that dopamine inhibits the GABAergic system in the vPAG similar to opioids. The dopaminergic modulation of nociception within the PAG is likely involved in more complex behavioral phenomena, including the development of opioid tolerance and drug seeking. The vPAG is known to play an important role in tolerance to opioids (Lane et al., 2005; Meyer et al., 2007; Morgan et al., 2006; Tortorici et al., 1999). In addition, the development of opioid-mediated reinforcement and sensitization has been shown to be dependent on PAG dopamine (Flores et al. 2005). Our data suggest that dopamine/opioid interactions within the vPAG have implications for the treatment of pain as well as drug addiction.
Acknowledgements
The authors declare that, except for income received from our primary employers, no financial support or compensation has been received from any individual or corporate entity over the for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. This investigation was supported in part by funds provided for medical and biological research by the State of Washington Initiative Measure No. 171, and by NIH grant DA015498.
References
- Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–68. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Opposing roles for dopamine and serotonin at presynaptic receptors in the ventral tegmental area. Clin Exp Pharmacol Physiol. 1995;22:841–5. doi: 10.1111/j.1440-1681.1995.tb01947.x. [DOI] [PubMed] [Google Scholar]
- Davila V, Yan Z, Craciun LC, Logothetis D, Sulzer D. D3 dopamine autoreceptors do not activate G-protein-gated inwardly rectifying potassium channel currents in substantia nigra dopamine neurons. J Neurosci. 2003;23:5693–7. doi: 10.1523/JNEUROSCI.23-13-05693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006;494:142–78. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttaroy A, Kirtman R, Farrell F, Phillips M, Philippe J, Monderson T, Yoburn BC. The effect of cumulative dosing on the analgesic potency of morphine in mice. Pharmacol Biochem Behav. 1997;58:67–71. doi: 10.1016/s0091-3057(96)00463-7. [DOI] [PubMed] [Google Scholar]
- Flores JA, El Banoua F, Galan-Rodriguez B, Fernandez-Espejo E. Opiate anti-nociception is attenuated following lesion of large dopamine neurons of the periaqueductal grey: critical role for D1 (not D2) dopamine receptors. Pain. 2004;110:205–14. doi: 10.1016/j.pain.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Flores JA, Galan-Rodriguez B, Ramiro-Fuentes S, Fernandez-Espejo E. Role for Dopamine Neurons of the Rostral Linear Nucleus and Periaqueductal Gray in the Rewarding and Sensitizing Properties of Heroin. Neuropsychopharmacology. 2005;31:1475–88. doi: 10.1038/sj.npp.1300946. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Fossum EN, Morgan MM. Behavioral and Electrophysiological Evidence for Opioid Tolerance in Adolescent Rats. Neuropsychopharmacology. 2007;32:600–6. doi: 10.1038/sj.npp.1301139. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci. 1998;18:10269–76. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Smith DF. Role of 5-HT and NA in spinal dopaminergic analgesia. Eur J Pharmacol. 1982;86:65–70. doi: 10.1016/0014-2999(82)90397-1. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of antinociceptive action of morphine in the periaqueductal gray, medial and paramedial medulla in rat. Brain Res. 1986;363:99–113. doi: 10.1016/0006-8993(86)90662-1. [DOI] [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE, Gibbins RJ. Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacol Rev. 1971;23:135–91. [PubMed] [Google Scholar]
- Kaneto H, Kihara T. Morphine analgesia without development of tolerance in reserpinized mice. Jpn J Pharmacol. 1986;42:169–73. doi: 10.1254/jjp.42.169. [DOI] [PubMed] [Google Scholar]
- Lane DA, Patel PA, Morgan MM. Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience. 2005;135:227–234. doi: 10.1016/j.neuroscience.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26:193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol Pharmacol. 2006;69:1137–45. doi: 10.1124/mol.105.019109. [DOI] [PubMed] [Google Scholar]
- Manning BH, Morgan MJ, Franklin KB. Morphine analgesia in the formalin test: evidence for forebrain and midbrain sites of action. Neuroscience. 1994;63:289–94. doi: 10.1016/0306-4522(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999;19:6629–36. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons. J Neurophysiol. 2005;93:3086–93. doi: 10.1152/jn.00855.2004. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Fossum EN, Ingram SL, Morgan MM. Analgesic tolerance to microinjection of the μ-opioid agonist DAMGO into the ventrolateral periaqueductal gray. Neuropharmacology. 2007;52:1580–1585. doi: 10.1016/j.neuropharm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Levine CS, Ingram SL. Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav. 2006a;85:214–9. doi: 10.1016/j.pbb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Stalding BM, King MM. Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain. 2006b;7:358–66. doi: 10.1016/j.jpain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whitney PK, Gold MS. Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray. Brain research. 1998;804:159–166. doi: 10.1016/s0006-8993(98)00669-6. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kuntzman R, Maggio A, Conney AH. Restoration of morphine analgesia in morphine-tolerant rats after the intraventricular administration of 6-hydroxydopamine. J Pharm Pharmacol. 1973;25:584–7. doi: 10.1111/j.2042-7158.1973.tb09166.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Under siege: The brain on opiates. Neuron. 1996;16:897–900. doi: 10.1016/s0896-6273(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J Neurosci. 1997;17:5697–710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne PB, Vaughan CW, Wilson HI, Christie MJ. Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. J Physiol. 1996;490(Pt 2):383–9. doi: 10.1113/jphysiol.1996.sp021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; 2005. Elsevier Academic Press. [Google Scholar]
- Pert A, Yaksh T. Localization of the antinociceptive action of morphine in primate brain. Pharmacol Biochem Behav. 1975;3:133–8. doi: 10.1016/0091-3057(75)90092-1. [DOI] [PubMed] [Google Scholar]
- Phillips S, Gelgor L, Mitchell D. Antinociceptive action of dopamine agonists in the nucleus raphe magnus of rats is mediated by D2 receptors. Arch Int Pharmacodyn Ther. 1992;319:66–75. [PubMed] [Google Scholar]
- Pillai G, Brown NA, McAllister G, Milligan G, Seabrook GR. Human D2 and D4 dopamine receptors couple through betagamma G-protein subunits to inwardly rectifying K+ channels (GIRK1) in a Xenopus oocyte expression system: selective antagonism by L-741,626 and L-745,870 respectively. Neuropharmacology. 1998;37:983–7. doi: 10.1016/s0028-3908(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Basbaum AI. Contribution of brainstem GABAergic circuitry to descending antinociceptive controls: II. Electron microscopic immunocytochemical evidence of GABAergic control over the projection from the periaqueductal gray to the nucleus raphe magnus in the rat. J Comp Neurol. 1990;302:378–93. doi: 10.1002/cne.903020214. [DOI] [PubMed] [Google Scholar]
- Rooney KF, Sewell RD. Evaluation of selective actions of dopamine D-1 and D-2 receptor agonists and antagonists on opioid antinociception. Eur J Pharmacol. 1989;168:329–36. doi: 10.1016/0014-2999(89)90794-2. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–38. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(Suppl 1):242–55. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Simon H, Le Moal M, Calas A. Efferents and afferents of the ventral tegmental-A10 region studied after local injection of [3H]leucine and horseradish peroxidase. Brain Res. 1979;178:17–40. doi: 10.1016/0006-8993(79)90085-4. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;177:315–23. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose Effect Data Analysis. Chapman & Hall/CRC; 2000. Chapman & Hall/CRC. [Google Scholar]
- Tortorici V, Robbins CS, Morgan MM. Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not leteral-dorsal periaqueductal gray of the rat. Behav Neurosci. 1999;113:833–839. doi: 10.1037//0735-7044.113.4.833. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol. 1997;498(Pt 2):463–72. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–4. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Wood PL. Opioid regulation of CNS dopaminergic pathways: a review of methodology, receptor types, regional variations and species differences. Peptides. 1983;4:595–601. doi: 10.1016/0196-9781(83)90003-7. [DOI] [PubMed] [Google Scholar]
- Wood PL, Stotland M, Richard JW, Rackham A. Actions of mu, kappa, sigma, delta and agonist/antagonist opiates on striatal dopaminergic function. J Pharmacol Exp Ther. 1980;215:697–703. [PubMed] [Google Scholar]
- Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]