Abstract
Patients with irritable bowel syndrome (IBS) show decreased discomfort and pain thresholds to visceral stimuli, as well hypervigilance to gastrointestinal sensations, symptoms, and the context in which these visceral sensations and symptoms occur. Previous research demonstrated normalization of visceral hypersensitivity following repeated exposure to experimental rectal stimuli over a 12 month period that was associated with reduction in cortical regions functionally associated with attention and arousal. Building upon these functional analyses, multivariate functional and effective connectivity analyses were applied to [15O] water positron emission tomography (PET) data from 12 IBS patients (male=4) participating in a PET study before and after 4 visceral sensory testing sessions involving rectal balloon distensions over a 1 year period. First, behavioral partial least squares was applied to test for networks related to reduced subjective ratings observed following repeated application of an aversive rectal stimulus. Next, path analysis within a structural equation modeling framework tested the hypothesis that perceptual habituation to the repeated visceral stimuli resulted in part from the reduced connectivity within a selective attention to threat network over time. Two independent, perception-related networks comprised of interoceptive, attentional and arousal regions were engaged differentially during expectation and distension. In addition, changes in the effective connectivity of an attentional network as well as modulatory amygdala influence suggested that perceptual habituation associated with repeated stimulus delivery results both in an increase in top down modulation of attentional circuits, as well as in a reduction of amygdala-related interference with attentional mechanisms.
Introduction
Multiple peripheral and central mechanisms (Mayer and Gebhart, 1994; Munakata et al., 1997; Verne et al., 2001) including central pain amplification have been implicated in the enhanced perceptual responsiveness of patients with irritable bowel syndrome (IBS) to experimental visceral stimuli [“visceral hypersensitivity”] (Kellow et al., 1991; Naliboff et al., 1997a; Whitehead and Palsson, 1998). Hypervigilance and selective attention to experimental visceral and somatic stimuli is a key feature of IBS (Naliboff et al., 2000), related functional pain disorders (Crombez et al., 2004; Eccleston et al., 1997; Roelofs et al., 2003) and anxiety disorders (Bishop, 2007; Paulus and Stein, 2006) and these factors may play an important role in this central pain amplification.
Studying IBS patients with 15O PET, we have previously reported that following repeated exposure to experimental rectal stimuli over a 12 month period, visceral hypersensitivity as indexed by patient ratings of stimulus intensity normalized and activity in cortical regions functionally associated with attention (parietal cortex (PC), Mid-cingulate cortex (MCC)) and arousal [dorsal brainstem including locus coeruleus complex (LCC) and amygdala (AMYG)] decreased (Naliboff et al., 2006a). Although the involvement of the parietal cortex was a post hoc finding in these analyses, our findings were consistent with the well established concept that repeated exposure to anxiety-provoking stimuli in the absence of aversive consequences leads to reduced vigilance and associated arousal (decreased salience of threat) (Lorenz and Tracey, 2008). Consistent with this interpretation, other reports have demonstrated that in healthy controls, reducing attention to an aversive visceral stimulus via distraction reduces the perceptual ratings of the stimulus (Coen et al., 2008).
Several distinct networks of attention have been extensively characterized in healthy control populations. The alerting network of attention (Posner, 2008; Posner and Dehaene, 1994) supports achieving and maintaining a high state of sensitivity to all incoming stimuli and includes prefrontal and parietal cortex regions (PFC, PC). The engagement of this alerting network is in part related to emotional arousal and the ascending noradrenergic influences from the LCC (Posner, 2008; Posner and Rothbart, 1980; Posner et al., 2007; Posner et al., 2006). Enhanced activity of the LCC and the closely connected AMYG has been well characterized in animal models of IBS (Valentino et al., 1999; Valentino and Van Bockstaele, 2008; Van Bockstaele et al., 1998). Supporting evidence has been reported in human patient populations (Berman et al., 2008a; Naliboff et al., 2006a) and suggests that the reported reduction in amygdala and dorsal pontine activity following repeated rectal stimulation may in part reflect reduced activation of ascending noradrenergic arousal mechanisms (Naliboff et al., 2006b).
An alternate mechanism of this reduction in arousal can be explained via the interaction of the alerting and executive control network of attention. The executive control network comprises top-down control mechanisms involved in allocation of attentional resources as well as resolving conflict among thoughts, feelings, and behavioral responses. It is supported by the lateral (l) PFC and rostral ACC/medial PFC (rACC/mPFC) (Botvinick, 2007; Bunge et al., 2001; Fan et al., 2003; Fan et al., 2005; Hopfinger et al., 2000; Posner et al., 2007). More specifically, research suggests that the rACC/mPFC, which has close connections to the anterior insula (aINS), detects conflicts in information processing (e.g., ‘something doesn't feel right’) and triggers reactive adjustments of cognitive control. The lPFC is believed to govern allocation of attention resources by governing selection of stimuli to optimize further processing in the posterior attention system (PC) (Botvinick, 2007; Sarter et al., 2003). This view is consistent with Corbetta et al's (2008) model of attention where afferents from a ventral network, including the aINS to the dorsal network (PC, PFC) can switch the focus of attention (Corbetta et al., 2008).
Extending Posner's work on attention networks and ‘biased competition models of attention’(Matthews and Mackintosh, 1998) to address selective attention to threat, Bishop (Bishop, 2008; Bishop et al., 2004; Bishop et al., 2007; Matthews and Mackintosh, 1998) has provided evidence to support a model in which selective attention to threat circuitry comprises the relative signal strength from a pre-attentive threat evaluation mechanism (AMYG) versus that from a top-down control mechanisms of information flow (lPFC, rACC/mPFC). In this model, inputs from pre-attentive threat detection/evaluation mechanisms and top-down information control mechanisms influence the outcome of this competition for attention resources. This model of selective attention to threat can easily be applied to IBS patients, a patient population with increased general (Mayer et al., 2001) as well as disease-related anxiety (Labus et al., 2007). Experimentally-induced visceral hypersensitivity in these patients is greatest when they are asked to focus their attention exclusively on the rating of an experimental visceral stimulus (as in all visceral sensitivity testing paradigms), and during ascending method of limit testing (Naliboff et al., 1997b), but is greatly reduced or normalized when patients’ attentional resources are engaged in another experimental task (Accarino et al., 1997) or by a specific context demanding attention , e.g. scanner environment.
Given the role of attention in the modulation of experimental pain (Bantick et al., 2002; Coen et al., 2008), and recent ERP findings indicating that attentional mechanisms as measured by the P300 component differ between IBS patients and healthy controls (Vianna et al., 2009),there is a need to evaluate the involvement of attentional networks in the modulation of the perception of experimentally induced pain. Although previous statistical parametric mapping of this data set via the general linear model provided evidence supporting the involvement of general attention mechanisms (Naliboff et al., 2006a), some of the results were based on posthoc analyses (parietal cortex), and multivariate techniques that apply system-level algorithms are better-suited to test hypotheses regarding networks of brain regions and the effective connectivity of underlying brain circuits. In the current paper, a behavioral partial least squares analysis (bPLS) was applied to determine the brain regions associated with reduced perceptual ratings of experimental induced pain after repeated exposure to the visceral distension paradigm over a 12 months period. Next, focusing on attentional mechanisms, path analysis within a structural equation modeling framework was applied to assess changes in the effective connectivity of attentional circuitry over time. This network approach complements previous work by evaluating possible changes in the engagement of a selective attention network (Naliboff et al., 2006a). Specifically, we test the hypothesis that perceptual habituation to the repeated visceral stimuli resulted in part from the reduced connectivity within a network involved in selective attention to threat over time.
Methods
Experimental design
Data from a previously published longitudinal [15O] water positron emission tomography (PET) neuroimaging study (Naliboff et al., 2006a) were analyzed. Specifically, 12 patients (male=4) with a diagnosis of IBS [Rome I criteria] (Thompson et al., 1994) participated in a PET study before and after 4 visceral sensory testing sessions involving rectal balloon distensions over a 1 year period. With the exception of 1 patient, all patients were free from centrally acting drugs from initial screening to final PET scan. One patient was started on a low-dose tri-cyclic antidepressant (imipramine) and an anxiolytic (buspirone) during the course of the study, which did not have a significant effect on IBS symptoms, and the medication was temporarily withdrawn 48 hours prior to the last PET scan. Patients had no history of substance abuse or psychiatric illness. On average, patients were 39.4 years old and reported usual symptoms in the past 6 months as 12.92 (.80) [Mean (SE)] and current symptoms as 6.91(1.30) on a 20-cm Verbal Descriptor Visual Analogue Scale (VDVAS). Similar levels of symptom severity were reported one year later.
The experimental protocol implemented during Day 1 and Day 2 (one year later) has been described in detail previously (Naliboff et al., 2006a). Briefly, PET counts (Siemens/CTI 953 tomograph, Siemens-Computer Technology, Knoxville TN) from 31 contiguous axial planes corresponding to an axial depth of 3.375 mm each in a 128x128 image matrix were summed over 90 seconds after intravenous administrations of 25 mCi [15O] water to construct volume images reflecting regional cerebral blood flow (rCBF) during 1) a resting baseline (BL) [5 mmHg], 2) expected moderate (45 and 60 mmHg) rectal balloon distensions (INF), and finally 3) an expected but undelivered distension (EXP). During the two INF conditions, the rectal balloon was inflated to 45 mmHg or 60 mmHg pressure. This pressure has been shown to be associated with a subjective rating of discomfort rather than pain (Posserud et al., 2007).
Scan order comprised a resting baseline (BL) scan with balloon inserted but not inflated (5mmhg), first inflation (INF) 45 mm Hg , BL, second INF 60 mmHg and an expectation (EXP) condition where subjects were informed that they may receive an even higher intensity visceral stimulus than they had already experienced but no balloon inflation occurred (5 mmHg). This experiment paradigm was repeated twice. After each 8 minute scan, patients rated their visceral sensation on a 20-cm verbal descriptor visual analogue scale (VD-VAS) of stimulus intensity for each condition (BL, INF, EXP). Patients were instructed to focus their attention exclusively on the experimental visceral stimulus as they would be asked to rate it.
All scans were preprocessed (SPM99, Wellcome Trust Centre for the Study of Cognitive Neurology, London, UK) by realignment to the initial scan for each subject, registration into the standardized space of the average MRI brain image provided by the Montreal Neurological Institute (MNI space), spatial smoothing with an isotropic 3 mm FWHM Gaussian filter, and reslicing to 4 mm isotropic voxels.
Statistical Analysis
Stimulus intensity ratings were analyzed using dependent t-tests in SPSS 16.0 (SPSS 16.0 for Windows, Rel. 16.0.1, 2007). A behavioral partial least squares analysis (bPLS) was applied to identify significant distributed patterns of activity that were functionally connected (correlated) with stimulus intensity ratings and compare these patterns between days and conditions. bPLS was applied to functionally ground the networks identified by PLS and to test the hypothesized involvement of regions functionally related to selective attention to threat in the intensity rating networks revealed by the bPLS. Given that perceived intensity ratings of the same visceral stimuli are decreased significantly with increased distraction (Coen et al., 2008) stimulus ratings were considered an indirect measure of attention, and the functional networks were expected to contain regions involved in attention.
bPLS (McIntosh et al., 1996; McIntosh et al., 2004; McIntosh and Lobaugh, 2004) is a multivariate covariance based technique that identifies voxels contributing to systematic brain-behavior correlations. The data matrix (matrix containing the normalized signal intensity measure at each voxel [i.e., rCBF adjusted for global CBF by dividing each voxel by the mean voxel activity of the whole-brain image]) is correlated with a behavioral measure to produce a “cross-block correlation” matrix or correlation map. Singular value decomposition is then performed on this correlation map to extract mutually orthogonal latent variables (LVs), distributed patterns of brain-behavior correlations corresponding to experimental patterns (e.g., days, condition) and accounting for the maximum amount of independent variance in the data. Regional CBF data and VD-VAS ratings were averaged across repetitions to produce one mean averaged image per day for the BL, INF and EXP conditions for entry into the partial least squares analysis. Thus, the data matrix comprised 72 (12 subjects × 3 conditions × 2 days) rows and one column for each voxel.
For the behavioral PLS, the significance of each LV was assessed via nonparametric permutation testing using 500 permutations. The exact number of times the permuted singular values exceeded the observed singular value was computed and p <.05 was considered significant. The experimental effects are depicted graphically by plotting the correlation of the stimulus intensity ratings with the latent variable score within group and condition. The numerical weights of the voxels comprising the brain LV are called “saliences” and can be positive or negative, indicating the magnitude and direction in which each voxel correlates with the ratings. Voxel saliences index reliability of the voxels comprising a LV and were computed via bootstrapping. The standard error for each voxel salience was calculated from a distribution of saliences derived from resampling subjects 100 times with replacement and recalculating the bPLS on each sample. The ratio of the observed salience to the bootstrapped standard error, which is approximately equivalent to a z score, was then calculated. Results from this reliability testing are displayed by projecting the bootstrap ratios onto a brain map and summarized in a cluster report where regions comprising a LV are reported in terms of clusters of voxels represented by a peak voxel, defined as the voxel in the cluster with the highest BSR. The cluster report was generated by thresholding voxels at a bootstrap ratio (BSR) of ±3.00 (p <.003) and defining a cluster as at least 10 reliable contiguous voxels. Where a cluster comprised several brain regions, the local maximum within each brain region was identified and reported. Regions were characterized in terms of anatomical region and Brodmann Area (BA). PLS analyses were implemented using freely available code (http://www.rotman-baycrest.on.ca:8080) in Matlab7.01 (Mathworks, Natick, MA).
Effective Connectivity
Path analysis via a structural equation modeling (SEM) framework and implemented in AMOS 17.0 (Arbuckle, 2005) was employed to characterize the selective attention to threat network and test for differences in this circuitry before and after repeated exposure to experimental rectal stimuli. SEM permits explicit testing of directional interactions of brain regions given the pairwise covariance between measures of rCBF activity in brain regions. Using a system of linear equations, ‘optimal’ path coefficients for each anatomical connection in the proposed network was determined using full information likelihood.
Temporal- or day-specific differences in the effective connectivity of the network were tested using multi-group tests for invariance (Joreskog, 1971). Specifically, day-specific differences in the circuitry of the network were localized using pair-wise comparisons between a completely unconstrained model and a partially constrained model using a chi-square difference test with 1 degree of freedom. Sequentially, each path of interest was restricted to be equal across sex and tested against a completely unconstrained model (e.g., all parameters estimated freely). Significance indicates that a pathway should be freely estimated in a model rather than constrained to be equal and denotes significant day-specific differences in the effective connectivity of the brain regions. These differences can involve both the sign and magnitude of the coefficient. Differences in sign reflect a reversal or qualitative change in regional interactions. Changes in magnitude reflect increase or decrease in the strength of the coupling between regions. Chi-square statistics for group differences and critical ratios for path coefficients were interpreted as significant at p <.05.
Specification of the selective attention to threat network
Effective connectivity analyses using SEM requires a priori specification of a structural (anatomical) model representing the hypotheses about the causal relations between brain regions. The regions and causal structure of this network were specified to test the hypothesized involvement of the ‘selective attention to threat’ network (Bishop, 2007) (see Figure 1). Regions comprising this circuitry included dlPFC, rACC/mPFC, AMYG, MCC, posterior cingulate cortex (PCC), and PC. Literature searches from Pubmed (http://www.ncbi.nlm.nih.gov/entrez) and the Collations of Connectivity on the Macaque Brain (CoCoMac) database (www.cocomac.org) (Kötter, 2004) were used to verify the anatomical projections among the nodes of the specified network. Representative nodes (voxels) for each region were selected based upon the most reliable loading on the relevant ratings network revealed by the bPLS.
Figure 1. Hypothesized selective attention to threat circuitry.
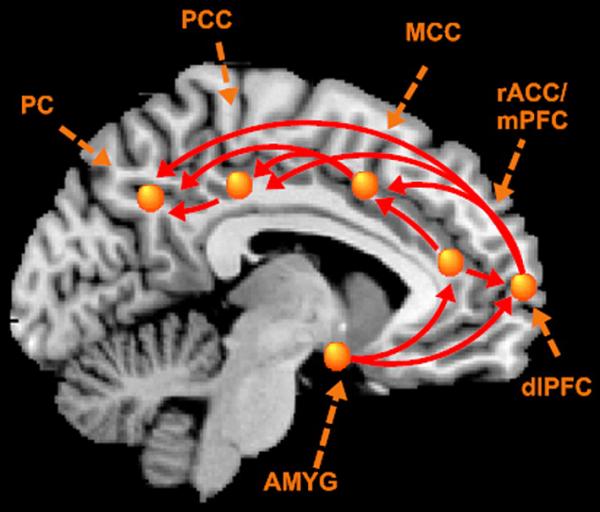
Abbrev: AMYG=amygdala, dlPFC=dorsolateral prefrontal cortex, MCC= mid-cingulate cortex, mPFC=medial prefrontal cortex, PCC=posterior cingulate cortex, PC= parietal cortex, rACC=rostral anterior cingulate cortex
The MCC and PCC were included in this circuitry due to their role in attentional processes. Specifically it has been proposed that the PCC works closely with the PC to integrate motivational information (motivational state and motivational value of the target) with spatial attention which is critical for the selective allocation of attention resources (Mohanty et al., 2008). The MCC has been attributed both attentional and affective function (Fan et al., 2008; Peyron et al., 2000; Posner and Petersen, 1990; Vogt, 2005) and has been most recently functionally associated automatically orienting attention toward pain (Brown and Jones, 2008).
RESULTS
Stimulus ratings
VD-VAS stimulus intensity ratings decreased Day 1 to Day 2 across all conditions (see Table 1). Statistically significant decreases were observed during the INF (mean difference=1.8, t(11)=3.2, p<.01, 2.70 VAS points), while the small decrease during BL and EXP did not reach significance.
Table 1.
Average Stimulus Intensity Ratings
| Condition | Stimulus intensity ratings | |
|---|---|---|
| Day 1 | Day 2 | |
| BL | 1.44 (0.64) | 0.90 (0.38) |
| INF | 14.24 (0.64) | 12.41 (1.01) |
| EXP | 1.58 (0.52) | 1.04 (0.26) |
*Stimulus intensity ratings are listed as Mean (Standard Error)
BL=resting baseline, EXP=expectation, INF=inflation
Behavioral PLS
When bPLS was applied to determine whether there was a network of regions functionally related to the stimulus intensity ratings, two significant “stimulus intensity rating” networks were observed.
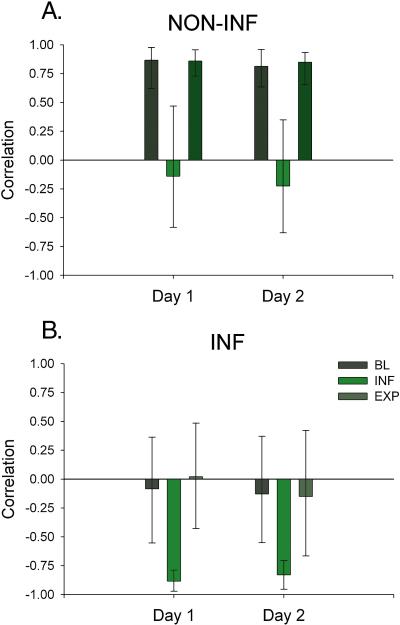
Non-INF conditions
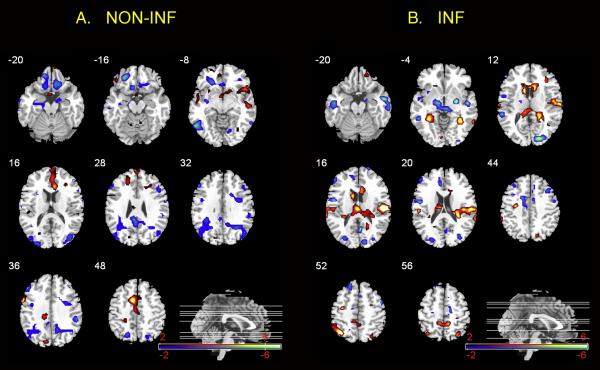
The first network was comprised of brain regions associated with the stimulus ratings only during BL and EXP but not INF and explained 34.43% of variance in the correlation matrix, p=.008 (See design plot, Figure 2a). A complete list of positively and negatively correlated regions is shown in supplemental Table 1 and these regions are displayed on the projection plot in Figure 3a. The non-INF network was comprised of brain regions correlated positively with the stimulus intensity rating during BL and EXP (e.g. showed lower activity on Day 2) including homeostatic afferent regions (anterior insula (aINS), MCC, rACC), prefrontal regions (mPFC (Brodmann Area (BA) 9), frontal pole (BA10), supplementary motor area (SMA) (BA 9/6)), and the hypothalamus. Regions in this network that were negatively correlated with the stimulus ratings (e.g. showed higher ratings on Day 2) included cingulate subregions (subgenual ACC, PCC), prefrontal regions [ventral medial (vm) PFC (BA 11), dlPFC (BA 46, BA 9)], PC (BA 40), precuneus, limbic regions (L AMYG, L hippocampus), midbrain, and periaqueductal gray (PAG).
Figure 2. NON-INF and INF design plots.
A. The first network of brain regions, the NON-INF network, was associated with the stimulus ratings only during BL and EXP but not INF. The second network of brain regions, the INF network, was associated with the stimulus ratings only during INF. Abbrev INF=inflation NON-INF=non-inflation
Figure 3. NON-INF and INF projection plots.
Average correlations from reliable network regions across conditions and days A. Regions project in red are positively correlated with stimulus ratings where as regions in blue are negatively. B. Regions project in blue are positively correlated where as regions in red are negatively correlated with the stimulus ratings. INF=inflation NONINF=non-inflation
INF conditions
The second network was comprised of brain regions correlated with the stimulus ratings only during INF and explained 29.52% of the variance in the correlation matrix, p=.03 (See design and projection plots, Figures 2b and 3b). A complete list of positively and negatively correlated regions is provided in supplemental Table 2. The INF network comprised brain regions positively correlated with the stimulus ratings (e.g. showed lower activity on Day 2) and included homeostatic afferent regions (middle INS, MCC/rACC), prefrontal regions (mPFC (BA 9), vlPFC (BA 47), dlPFC (BA 46)], the PCC, AMYG and PAG). Regions in this network that were negatively correlated with the stimulus intensity ratings (e.g. showing higher activity on DAY 2) included homeostatic afferent regions (R pINS, B thalamus), prefrontal regions [(vmPFC), parietal cortical regions (PC [BA 7/40], precuneus (BA 7)], and hippocampus.
Effective Connectivity Analysis
Selective attention to threat circuitry (Figure 1)
Nodes for the selective attention to threat circuitry were selected from the bPLS results as outlined in the methods section. Tables 2 and 3 contain the coordinate of the representative node from the bPLS results for each region. Note that since the MCC and the PCC actually comprised one large cluster in the INF stimulus rating network, only one node was selected to represent both regions. Tables 4-6 present the effective connectivity parameter estimates, (e.g., betas, standard errors) indexing functioning of the selective attention to threat circuitry on Day 1 and Day 2 during each condition as well as the chi-square difference statistics assessing day differences in the effective connectivity each circuit. The direction and significance of the connectivity between nodes of the attentional network during BL, EXP and INF on Day 1 and Day 2 are shown in Figure 4, and significant differences in the circuitry of the network between Day 1 and 2 are shown in Figure 5.
Table 2.
Representative nodes of the selective attention to threat circuit for BL and EXP
| MNI Coordinates (radiological convention) | |||
|---|---|---|---|
| Network Node | |||
| dlPFC | -48 | 32 | 32 |
| rACC | 8 | 32 | 16 |
| AMYG | 20 | -4 | -20 |
| MCC | -8 | 12 | 44 |
| PCC | -4 | -40 | 28 |
| PC | 56 | -36 | 36 |
AMYG=amygdala, dlPFC=dorsolateral prefrontal cortex, MCC= mid-cingulate cortex, PCC=posterior cingulate cortex, PC=parietal cortex, rACC=rostral anterior cingulate cortex
Table 3.
Representative nodes of the selective attention to threat circuit for INF
| MNI Coordinates (radiological convention) | |||
|---|---|---|---|
| Network Node | |||
| dlPFC | -48 | 44 | 12 |
| rACC | -4 | 32 | 20 |
| AMYG | 20 | -4 | -20 |
| MCC/PCC | -8 | -12 | 48 |
| PC | -36 | -60 | 52 |
AMYG=amygdala, dlPFC=dorsolateral prefrontal cortex, MCC= mid-cingulate cortex, PCC=posterior cingulate cortex, PC= parietal cortex, rACC=rostral anterior cingulate cortex
Table 4.
Selective attention to threat circuit Day 1 to Day 2 during resting baseline.
| DAY 1 | DAY 2 | |||||
|---|---|---|---|---|---|---|
| B | SE | B | SE | χ2Δ | ||
| AMYG → | rACC | 0.18 | 0.11 | -0.90 | 0.21 | 20.7 |
| rACC → | dlPFC | -0.34 | 0.24 | -0.88 | 0.30 | 2.0 |
| AMYG → | dlPFC | 0.01 | 0.16 | -0.66 | 0.50 | 1.6 |
| dlPFC → | MCC | -0.39 | 0.18 | 0.30 | 0.21 | 6.2 |
| rACC → | MCC | 0.27 | 0.29 | 0.48 | 0.23 | 0.3 |
| MCC → | PCC | -0.04 | 0.19 | -0.30 | 0.24 | 0.7 |
| dlPFC → | PCC | 0.02 | 0.17 | 0.33 | 0.17 | 1.6 |
| MCC → | PC | -0.60 | 0.13 | 0.25 | 0.37 | 4.8 |
| PCC → | PC | 0.36 | 0.12 | 0.61 | 0.43 | 0.3 |
| dlPFC → | PC | 0.18 | 0.12 | 0.15 | 0.27 | 0 |
*Bolded values are significant at p<.05
AMYG=amygdala, B =beta estimate, dlPFC=dorsolateral prefrontal cortex, MCC= mid-cingulate cortex, PCC=posterior cingulate cortex, PC=parietal cortex, rACC=rostral anterior cingulate cortex, SE=standard error, χ2Δ=chi square difference
Table 6.
Selective attention to threat circuit Day 1 to Day 2 during inflation.
| DAY 1 | DAY 2 | |||||
|---|---|---|---|---|---|---|
| EST | SE | EST | SE | χ2Δ | ||
| AMYG → | rACC | 0.79 | 0.33 | -0.01 | 0.22 | 4.1 |
| rACC → | dlPFC | 0.20 | 0.39 | -0.01 | 0.19 | 0.3 |
| AMYG → | dlPFC | 1.34 | 0.56 | 0.48 | 0.22 | 2.1 |
| dlPFC → | MCC /PCC | 0.59 | 0.27 | 0.75 | 0.16 | 0.3 |
| rACC → | MCC /PCC | 0.61 | 0.38 | 0.30 | 0.19 | 0.6 |
| MCC/PCC → | PC | -0.14 | 0.22 | -0.24 | 0.26 | 0.1 |
| dlPFC → | PC | -0.96 | 0.30 | -0.61 | 0.34 | 0.6 |
*Bolded values are significant at p<.05
AMYG=amygdala, B =beta estimate, dlPFC=dorsolateral prefrontal cortex, MCC= mid-cingulate cortex, PCC=posterior cingulate cortex, PC=parietal cortex, rACC=rostral anterior cingulate cortex, SE=standard error, χ2Δ=chi square difference
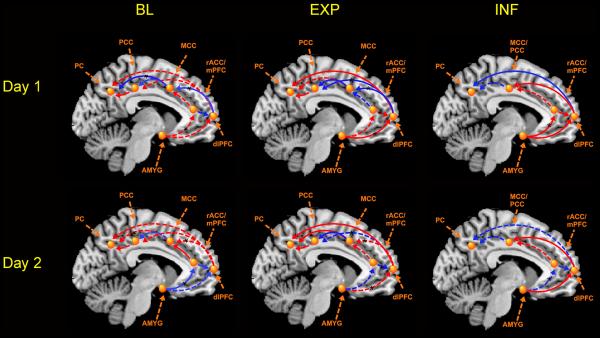
Figure 4. Effective connectivity of the selective attention to threat network.
Solid and dashed lines denote significant (p<.05) and nonsignificant connectivity as indexed by critical ratios (Beta/SE). Red and blue indicate =positive and negative betas, respectively. Black asterisks denote circuits which showed significant day specific changes. Abbrev: AMYG=amygdala, BL=baseline, dlPFC=dorsolateral prefrontal cortex, EXP=expectation, INF=inflation, MCC= mid-cingulate cortex, mPFC=medial prefrontal cortex, PCC=posterior cingulate cortex, PC= parietal cortex, rACC=rostral anterior cingulate cortex
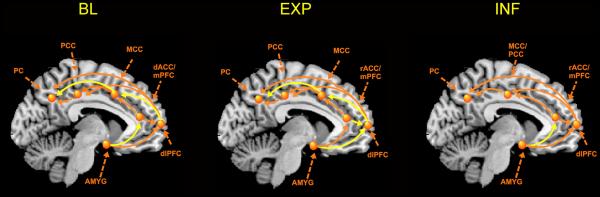
Figure 5. Day specific changes in effective connectivity Day 1 to Day 2.
Yellow lines denote circuitry was significantly altered day 1 to day2, chi square difference test significant at p<.05.
Abbrev: AMYG=amygdala, dlPFC=dorsolateral prefrontal cortex, MCC= mid-cingulate cortex, mPFC=medial prefrontal cortex, PCC=posterior cingulate cortex, PC= parietal cortex, rACC=rostral anterior cingulate cortex
Baseline
As can be seen in Table 4, on Day 1, significant connectivity was observed between several nodes of the attention to threat circuitry: Between dlPFC and MCC (β=-.39), between MCC and PC (β=-.60), and between PCC and PC (β=.36). On Day 2, significant connectivity was observed between the AMYG and rACC (β=-.90), and between rACC and dlPFC (β=-.88) and MCC (β=.48). Chi square difference testing indicated significant changes from Day 1 to Day 2 in the effective connectivity between AMYG and rACC (χ2=20.7), between dlPFC and MCC (χ2= 6.2), and between MCC and PC (χ2= 4.8.)
Expectation
As can be seen in Table 5, on Day 1, significant connectivity was observed between the dlPFC and several other nodes of the network: MCC (β=-.70), PCC (β=-.51), and PC (β=.50), and between AMYG and dlPFC(β=.54). In addition, significant coupling was observed between MCC and PC (β=-.50), and between PCC and PC (β=.48). On Day 2, significant connectivity was observed between rACC and dlPFC (β=-.83), between DLPFC and PC (β=.90), and between MCC and PCC (β=.68) and PC (β=1.41). Chi square differences indicated significant changes Day 1 to Day 2 in the coupling between dlPFC and rACC (χ2= 3.09), AMYG (χ2= 5.5) and MCC (χ2=10.9), and between MCC and PC (χ2=6.9).
Table 5.
Selective attention to threat circuit Day 1 to Day 2 during expectation.
| DAY 1 | DAY 2 | |||||
|---|---|---|---|---|---|---|
| EST | SE | EST | SE | χ2Δ | ||
| AMYG → | rACC | 0.12 | 0.14 | -0.15 | 0.10 | 2.6 |
| rACC → | dlPFC | 0.00 | 0.31 | -0.83 | 0.28 | 3.9 |
| AMYG → | dlPFC | 0.54 | 0.20 | -0.13 | 0.21 | 5.5 |
| dlPFC → | MCC | -0.70 | 0.23 | 0.23 | 0.17 | 10.9 |
| rACC → | MCC | -0.14 | 0.38 | 0.41 | 0.24 | 1.5 |
| MCC → | PCC | -0.50 | 0.20 | -0.68 | 0.19 | 0.4 |
| dlPFC → | PCC | -0.51 | 0.23 | -0.20 | 0.14 | 1.3 |
| MCC → | PC | 0.07 | 0.16 | 1.37 | 0.47 | 6.9 |
| PCC → | PC | 0.48 | 0.16 | 1.41 | 0.56 | 2.5 |
| dlPFC → | PC | 0.50 | 0.18 | 0.90 | 0.23 | 1.8 |
*Bolded values are significant at p<.05
AMYG=amygdala, B =beta estimate, dlPFC=dorsolateral prefrontal cortex, MCC= mid-cingulate cortex, PCC=posterior cingulate cortex, PC= parietal cortex, rACC=rostral anterior cingulate cortex, SE=standard error, χ2Δ=chi square difference
Inflation
On Day 1, significant connectivity was observed between the AMYG and rACC (β=.79), and dlPFC (β=1.34), and between the dlPFC and MCC/PCC (β=.59), and PC (β=-.96) [see Table 6]. On Day 2, significant connectivity was observed between the AMYG and dlPFC (β=.48), and between dlPFC and MCC/PCC (β=.75).Chi square tests examining day 1 to day 2 differences only reached significance for the connectivity between AMYG and rACC (χ2=4.2).
Discussion
Distinct, yet overlapping networks functionally correlated with perceptual habituation of the intensity ratings of visceral stimuli day 1 to day 2 were engaged during non-INF and during INF conditions. In addition to previously identified areas from a region of interest analysis (Naliboff et al., 2006a), these perception-related networks included many additional regions functionally related to cognitive and affective modulation of the afferent signal. Furthermore, network analysis indicated alterations in the coupling within an attention to threat network over time, and these changes differed between the non-INF and INF conditions. When viewed together with the previously reported decrease in activity in nodes of a general attention-related network (Naliboff et al., 2006a) these findings suggest that the observed habituation to the repeated experience of visceral aversive sensations is related to a decrease in the activity and functional connectivity within threat related attentional networks.
Functional Connectivity
Consistent with the generally accepted framework for the emotional modulation of attention (selective attention to threat) (Bishop, 2007), activity in several cortical regions (including dorsal ACC subregions [rACC, MCC], PCC, and dmPFC) was positively correlated with the stimulus ratings during expectation and distension, while other cortical regions (including vmPFC, dlPFC, PC [BA 40]) precuneus (BA 7), and hippocampus were negatively correlated with the stimulus ratings.
Although both the INF and the non-INF networks appear to be comprised of similar regions, the location and spatial extent of the overlap is small. Careful inspection of results indicates the regions comprising these networks, although similarly named, are generally juxtaposed with very little spatial overlap and are engaged differently depending on the task. For example, both networks contain the right MCC region but the cluster of voxels representing this area is represented by different maxima for each network and overlap of these clusters is minimal. The area of the MCC functionally related to the stimulus ratings during EXP and BL is more anterior and extends into the rACC. On the other hand, the MCC region functionally relevant for stimulus ratings during inflation is more posterior. Similarly, the anterior but not posterior region of the INS was functionally correlated with NON-INF stimulus ratings, whereas the pINS was functionally correlated with INF ratings. This shift from MCC to the rACC and from pINS to aINS is consistent with previous reports during anticipated (Ploghaus et al., 1999), empathic (Singer et al., 2004), or imagined pain when compared with actual sensory pain (Lorenz and Tracey, 2008).
Other similarly juxtaposed regions demonstrating qualitative differences in functional connectivity dependent on the rating task included the dlPFC, AMYG, and the PAG. As discussed above for INS and MCC subregions, the observed difference may be related to the functional specialization of subregions within the larger structures. For example, activation of different amygdala subregions has been demonstrated in connection with both nociceptive and antinociceptive responses (Neugebauer et al., 2004) and our own results have shown heterogeneity of activation within MCC subregions regions(Berman et al., 2006). Elaborate functional specialization of subregions within the dlPFC (Fuster, 2001) and the PAG (Keay and Bandler, 2001) have been reported.
When viewed together, the findings from the functional connectivity analysis suggest that in addition to the expected positive correlation of conscious perception of an interoceptive stimulus with the INS and the rACC/MCC (Craig, 2009), stimulus ratings of aversive rectal distension are positively correlated with activity in attentional and arousal circuits. We have previously reported a reduction in the activity in several nodes of these attentional/arousal networks (MCC, PCC, PC and AMYG) and an increase in dlPFC over time (Naliboff et al., 2006a). Based on these earlier findings, we had postulated that the associated reduction in perceptual ratings was related to habituation and changes in the activity within an attentional network over time. The current results on the same brain imaging data set confirms this hypothesis, and revealed additional changes in cognitive modulatory, pain modulatory, homeostatic afferent activity and limbic activity.
Effective connectivity
The results from the effective connectivity analysis, which focused on the circuitry of a selective attention to threat network, demonstrate that in addition to regional brain activity reliably correlating with the perceptual habituation of visceral stimuli ratings over the course of the year, there are also significant changes in the coupling between regions of the attention network and the AMYG.
During both non-INF and INF conditions, clear differences in the coupling between the amygdala and anterior nodes of the attentional network from day 1 to day 2 were observed (Table 4-6 and Figure 5). Specifically, the positive influence of the AMYG on the rACC or the dlPFC observed on day 1 was reduced on day 2. When viewed together with our previous demonstration of a reduction of both amygdala and MCC activity, and an increase in dlPFC activity over time (Naliboff et al. 2000), these findings are consistent with a reduced influence of emotional arousal circuits (including the amygdala) on attentional processes, and an improved top down inhibition by lateral PFC of AMYG activity. For both non-INF conditions, but not the INF condition, significant changes were observed in the interaction between the dlPFC and MCC and the posterior regions of the attentional network, in addition to the altered connectivity between AMYG and rostral regions of the attentional network (Table 4-6 and Figure 5). The fact that the nodes comprising the selective attention to threat circuit were associated with reduced perceptual ratings during all three conditions along with the observed significant changes in the effective connectivity between AMYG, dlPFC and the anterior nodes of the attentional network suggest that the observed perceptual habituation is at least in part related to a reduction in arousal and its influence on attentional processes.
Even though the current study was not specifically designed to test the competing influences of a threat distractor and another task for attentional resources (Bishop, 2008), this model which has been developed for anxiety disorders might be reasonably applied to explain our findings and put them into the context of IBS pathophysiology. IBS patients (who typically show evidence for both increased trait and state anxiety) may allocate excessive amounts of attention to gut related symptoms and circumstances in the absence of any noxious visceral stimuli. This increased attention to potential threats may in part be related to increased responsiveness of emotional arousal circuits (amygdala, LCC, corticotropin-releasing factor system) and its influence of the anterior nodes of the attention network (as shown in a recent study (Berman et al., 2008b) , and in part to alterations in top down control mechanisms in the lateral PFC allocating attentional resources to potentially relevant stimulus (reflected in alterations in the observed interactions between anterior and posterior nodes of the attentional network). It has been suggested that the former is related to increased state anxiety (Berman et al., 2008b)), while the latter may be related to trait anxiety (Bishop, 2008). According to Bishop's model, the greater selective attention to threat related distractors in individuals with higher trait anxiety is seen only under conditions of low perceptual load. Applied to the current study, increased attention to the possible threat of abdominal discomfort and pain is greatest during the expectation of the stimulus (both BL and EXP condition), while attentional resources will be fully absorbed during perceptual processing of the intense visceral stimulus. Even though state anxiety was not assessed in this study, the consistent reduction in coupling between the amygdala and attentional regions during all 3 conditions suggest that the observed habituation to the experimental stimulus was associated with a reduction in state anxiety, and that may have been the result of greater dlPFC inhibition of amygdala activity.
Study limitations and future directions
Although the current approach enabled testing of important hypotheses regarding percept-related networks and reductions in effective connectivity of an attention threat network over time, connectivity analyses with PET data have several limitations, including limited temporal and spatial resolution. For example, brain responses acquired with [15O] water PET are usually averaged over a 60–120 second period to achieve an acceptable signal to noise ratio (SNR). Such averaging results in the convolution of multiple neural processes (alerting, orienting, and executive attention), presumably occurring at different time scales in the overall picture of neural activation, and ignores the temporal engagement of this circuitry.
The finding that the functional networks revealed with bPLS comprised attentional regions was quite promising given that the perceived intensity ratings of the visceral stimuli were considered a proxy measure of attention and PET data is not well suited to study early attentional and arousal processes which occur in the millisecond domain. Ongoing fMRI studies have been designed to test specific attentional circuits using more powerful event-related designs and greater sample sizes including healthy controls. Although small, the current sample size of 12 is comparable to the sample size of other effective connectivity studies (de Marco et al., 2006; Palmer et al., 2009; Rosenbaum et al., 2008; Rowe et al., 2005). Given the small sample size and the limited number of available data points, a hypothesis driven SEM was applied to test for changes in a statistically economical selective attention to threat network. Many of the regions comprising the network have reciprocal connections with each other but could not be modeled due to mathematical constraints (Berry, 1984). In addition, while we fully appreciate the importance of the insula in interoceptive processes and the role of the aINS in interoceptive awareness, we decided to focus the effective connectivity analysis on attentional brain circuits. Sample size limited the incorporation of other brain regions involved in interoception, arousal and emotional modulation.
The current results build upon and extend the findings from the previous reported statistical parametric mapping analysis of this data set (Naliboff et al., 2006a). Major differences exist between the two analyses:
Unlike previous work (which focused on pre-hypothesized ROIs), in the current study, bPLS was applied to explicitly test for a network of regions associated with the changing perceptual ratings. This analysis approach identified regions belonging to interoceptive, attentional and emotional arousal circuits.
In the initial report, no behavioral measures were used to functionally ground the brain regions that showed differences over time.
The effective connectivity analysis focused on networks of attentional brain regions based on an a priori hypothesis about the selective attention to threat circuit. In the previous analysis, the involvement of the posterior attention system was based on a post hoc analysis of the parietal cortex. The results of this analysis are novel and demonstrate a relationship between regions comprising known attentional circuits (arousal, executive attention, attention to threat) and modulation of experimental pain perception. This examination of the involvement of selective attention to threat circuits and their behavioral correlates breaks new ground.
The functional connectivity of the LCC region with the rest of the brain in the original manuscript was examined via ANCOVA. This functional connectivity analysis indicated a bivariate relationship between the LCC and known arousal regions but did not provide satisfactory support for the interaction of these regions as a network. No work was done to look at the involvement of selective attention to threat circuitry in the initial report.
Only effective connectivity analysis can support inference regarding the interaction of brain regions as a network or circuit. Here we find alteration in a selective attention to threat network over time. The nodes of this network were associated with the changing perceptual rating in the bPLS analysis, implicating providing support for the involvement of this circuit in modulation experimental pain perception.
Summary and conclusions
In summary, we identified extensive percept-related networks which are engaged differentially during expectation and distension. These networks include interoceptive, attentional and arousal systems. In addition, we identified connectivity changes over time in both cortical attentional networks, as well as in the coupling of these cortical networks with the amygdala. Our findings suggest that perceptual habituation associated with repeated stimulus delivery results both in an increase in top down modulation of attentional circuits, as well as in a reduction of arousal circuit-mediated interference with attentional mechanisms. Future studies using fMRI paradigms are needed to confirm this hypothesis.
Supplementary Material
Acknowledgments
We would like to acknowledge the invaluable support by the VA PET Center under the direction of Dr. Mark Mandelkern.
References
- Accarino AM, Azpiroz F, Malagelada JR. Attention and distraction: effects on gut perception. Gastroenterology. 1997;113:415–422. doi: 10.1053/gast.1997.v113.pm9247458. [DOI] [PubMed] [Google Scholar]
- Arbuckle JL. Amos™ 17.0 User's Guide. SPSS; 2005. [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff B, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller J, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. Journal of Neuroscience. 2008a;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, Ruby K, Mayer EA. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R268–276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008b;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry WD. Nonrecursive Causal Models. Sahe Publications, Inc; Newbury Park: 1984. [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Ann N Y Acad Sci. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cereb Cortex. 2007;17:1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Brown CA, Jones AK. A role for midcingulate cortex in the interruptive effects of pain anticipation on attention. Clin Neurophysiol. 2008;119:2370–2379. doi: 10.1016/j.clinph.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Coen SJ, Aziz Q, Yaguez L, Brammer M, Williams SC, Gregory LJ. Effects of attention on visceral stimulus intensity encoding in the male human brain. Gastroenterology. 2008;135:2065–2074. e2061. doi: 10.1053/j.gastro.2008.08.005. 2074. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig BAD. How do you feel — now? The anterior insula and human awareness. Nat Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crombez G, Eccleston C, Van den Broeck A, Goubert L, Van Houdenhove B. Hypervigilance to pain in fibromyalgia: the mediating role of pain intensity and catastrophic thinking about pain. Clin J Pain. 2004;20:98–102. doi: 10.1097/00002508-200403000-00006. [DOI] [PubMed] [Google Scholar]
- de Marco G, de Bonis M, Vrignaud P, Henry-Feugeas MC, Peretti I. Changes in effective connectivity during incidental and intentional perception of fearful faces. Neuroimage. 2006;30:1030–1037. doi: 10.1016/j.neuroimage.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Crombez G, Aldrich S, Stannard C. Attention and somatic awareness in chronic pain. Pain. 1997;72:209–215. doi: 10.1016/s0304-3959(97)00030-4. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fan J, Hof PR, Guise KG, Fossella JA, Posner MI. The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex. 2008;18:796–805. doi: 10.1093/cercor/bhm125. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fuster J. The Prefrontal Cortex—An Update: Time Is of the Essence Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Joreskog KG. Simultaneous factor analysis in several populations. Psychometrika. 1971;36:409–426. [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Kellow JE, Eckersley CM, Jones MP. Enhanced perception of physiological intestinal motility in the irritable bowel syndrome. Gastroenterology. 1991;101:1621–1627. doi: 10.1016/0016-5085(91)90400-f. [DOI] [PubMed] [Google Scholar]
- Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: Further validation of the Visceral Sensitivity Index. Psychosomatic Medicine. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Tracey I. Brain correlates of psychological amplification of pain. In: Mayer EA, Bushnell MC, editors. Functional Pain Syndromes. IASP Press; Seattle: 2008. [Google Scholar]
- Matthews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Research and Therapy. 1998;22:539–560. [Google Scholar]
- Mayer EA, Craske MG, Naliboff BD. Depression, anxiety and the gastrointestinal system. Journal of Clinical Psychiatry. 2001;62:28–36. [PubMed] [Google Scholar]
- Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 2004;23:764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23(Suppl 1):S250–263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb Cortex. 2008;18:2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata J, Naliboff B, Harraf F, Kodner A, Lembo T, Chang L, Silverman DH, Mayer EA. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;11:55–63. doi: 10.1016/s0016-5085(97)70219-1. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mandelkern MA, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006a;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mandelkern MA, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006b;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Chang L, Munakata J, Mayer EA. Towards an integrative model of irritable bowel syndrome. Prog Brain Res. 2000;122:413–423. doi: 10.1016/s0079-6123(08)62154-8. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997a;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997b;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Eigenraam L, Hoque T, McCaig RG, Troiano A, McKeown MJ. Levodopa-sensitive, dynamic changes in effective connectivity during simultaneous movements in Parkinson's disease. Neuroscience. 2009;158:693–704. doi: 10.1016/j.neuroscience.2008.06.053. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Posner MI. Measuring alertness. Ann N Y Acad Sci. 2008;1129:193–199. doi: 10.1196/annals.1417.011. [DOI] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. The development of attentional mechanisms. Nebr Symp Motiv. 1980;28:1–52. [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE. Attention genes. Dev Sci. 2007;10:24–29. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Posner MI, Sheese BE, Odludas Y, Tang Y. Analyzing and shaping human attentional networks. Neural Netw. 2006;19:1422–1429. doi: 10.1016/j.neunet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Posserud I, Syrous A, Lindstrom L, Tack J, Abrahamsson H, Simren M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113–1123. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Roelofs J, Peters ML, McCracken L, Vlaeyen JW. The pain vigilance and awareness questionnaire (PVAQ): further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain. 2003;101:299–306. doi: 10.1016/S0304-3959(02)00338-X. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Furey ML, Horwitz B, Grady CL. Altered connectivity among emotion-related brain regions during short-term memory in Alzheimer's disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb Cortex. 2005;15:85–95. doi: 10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem. 2003;80:245–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- SPSS 16.0 for Windows, Rel. 16.0.1, 2007. SPSS, Inc; Chicago: [Google Scholar]
- Thompson GW, Drossman DA, Richter J, Talley NJ, Thompson GW, Corazziari E, Whitehead WE. The Functional Gastrointestinal Disorders. Little,Brown; Boston: 1994. Functional bowel disorders and functional abdominal pain. pp. 115–174. [Google Scholar]
- Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: Pharmacological target for pelvic visceral dysfunction. Trends in Pharmacological Sciences. 1999;20:253–260. doi: 10.1016/s0165-6147(99)01332-2. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Vianna EPM, Labus JS, Berman SM, Suyenobu B, Jarcho J, Tillisch K, Naliboff BD, Mayer EA. Increased Allocation of Cognitive Resources for Selective Attention in IBS Patients. Gastroenterology. 2009;136((4) suppl.) [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology. 1998;115:1263–1271. doi: 10.1016/s0016-5085(98)70099-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.