INTRODUCTION
In the mature animal, a variety of stressors activate the hypothalamic-pituitary-adrenal (HPA) axis:1–3 Increased secretion of corticotropin-releasing hormone (CRH) from the hypothalamic paraventricular nucleus (PVN) induces ACTH release from the pituitary and increases plasma corticosterone (CORT). The depletion of hypothalamic CRH is followed by a “compensatory” up-regulation of CRH gene expression.2–4 Limbic input from hippocampus and amygdala, mainly the central nucleus (ACE), further modulates stress-induced alteration of CRH gene expression.5,6
The presence of most of these components of the “stress response” in the neonatal (first postnatal week) and infant (second postnatal week) rat has been debated.7,8 Elevation of plasma glucocorticoids in response to stressors and the relative roles of CRH and vasopressin have been subjects of investigation.4,7–10 A “stress-hypo-responsive” period with diminished or immature hormonal responses has been described during the first two postnatal weeks.
The goal of this report is to present data confirming the presence of robust stress-induced elevation of plasma glucocorticoids (GC) and the involvement of CRH in this response. Evidence for immature regulation of CRH gene expression by GC and stress will be presented, as well as the role of the cyclic AMP cascade in the regulation of the CRH gene promoter.
MATERIALS AND METHODS
Animals
Pups were products of time-pregnant Sprague-Dawley rats (Zivic-Miller, Zeliehople, PA), kept on a 12-h light/dark cycle and given access to unlimited lab chow and water. Delivery was verified at 12-h intervals, and the date of birth was considered day 0. Litters were culled to 12 pups, and undisturbed for 48 h prior to experiments. All experiments were started at 9 a.m. to avoid circadian variation in HPA axis components and tone.1,11
Materials
Dibutyryl-cyclic-AMP (db-cAMP), isobutyl methylxanthine (IBMX), dexa-methasone (DEX), forskolin, and GTP were obtained from Sigma Chemical Co. (St. Louis, MO). DEX was dissolved in 100% ethanol, and forskolin was dissolved in DMSO. Stock solutions of both drugs were diluted with 102 to 106 volumes of buffer immediately prior to use. CRH antiserum (CRH-AS) produced in sheep was a gracious gift of Dr. W. W. Vale (pool No. 238-293). Normal sheep serum (NSS) was purchased from Calbiochem (San Diego, CA). CRH-antagonist, (9-41)-alpha-helical CRH, was purchased from BACHEM (Torrance, CA).
Stress Paradigm and Hormonal Studies
Acute cold-separation was chosen as a potent, age-specific and painless stress.4,10 Pups were separated from their mothers and placed individually in glass jars in a cold room (4 °C). Preliminary experiments defined maximally tolerated cold exposure, as defined by the development of rigor and little response to tactile stimulus (core temperature averaged 9.8 °C). Thus, cold exposure lasted an average of 30 min in 4–6-day-old pups and 60 min in 11–16-day-old pups. Stressed pups were rewarmed on a heating pad under a heat lamp as a group. Rats were sacrificed by decapitation and trunk blood was collected before the onset of stress and at 0, 20, 40 (or 30), 60, 150, and 240 min after its termination. Control rats were left in home cages and sacrificed within 45 s of disturbance. Plasma CORT levels were determined by radioimmunoassay (ICN, Irvine, CA). Assay sensitivity was 0.5 μg/dL; interassay variability was determined by two dilutions of adult rat plasma, and averaged 15%.4,10
CRH-Antiserum Effect on Stress-Induced Plasma CORT Elevation
Rat pups were divided into the following experimental groups: undisturbed controls, injection controls, cold exposure, cold and NSS-injection and cold and CRH-AS injection. Rats were injected with CRH-AS or NSS (100 μL, ip) at 9:30 a.m. One hour later, rats were exposed to cold for 25 min (6-day-old) or 40 min (9-10-day-old), rewarmed for 50 min and sacrificed. Undisturbed controls were sacrificed at 9 a.m. and injection controls (not exposed to cold) immediately prior to the corresponding cold-exposed groups.
Time-Course of and Age Dependence of Stress-Induced Up-Regulation of CRH-mRNA Abundance
In a preliminary time-course experiment, 16-day-old-rats were divided into six groups (n = 4). Groups 1–3 were subjected to cold-separation stress as above, and sacrificed at 1, 4, or 28 h after termination of stress. Groups 4–6 served as controls for both separation-cold stress and diurnal variability in CRH-mRNA.11 Controls were undisturbed in home cages until sacrifice at the corresponding hours. Based on the results of this experiment,4 6-, 9- and 16-day-old rats were subjected to cold-separation stress as above, and decapitated 4 or 28 h after the onset of cold-stress (the 28-h group was returned to home cages at 4 h and sacrificed 24 h later).
Effect of db-cAMP on Stress-Induced CRH Synthesis in the 6-Day-Old Rat
Six-day-old rats were assigned to one of the following groups: (1) Undisturbed controls; (2) cold-stressed controls; (3) cannulated, saline-infused, stressed controls; (4) cannulated, db-cAMP-infused, stressed; and (5) cannulated, stressed, with db-cAMP infused into the striatum. Cold was used as both stressor and anesthetic. Rats were placed in an ice-bath and transferred to a stereotaxic apparatus when motionless. Stainless steel cannulae were implanted unilaterally, with the tip directed immediately above the PVN.12,13 We have previously shown that in the neonatal rat, a unilateral infusion of a number of compounds results in diffusion to the contralateral PVN (refs. 12 and 13 and unpublished observations). Coordinates for striatal cannulae were A: 0.95, V: 4.0, L: 3.4 in reference to bregma. Saline or db-cAMP (1 μmole in 1 μL) was infused using a Hamilton syringe. Cannula placement was verified in each animal using 20-μm coronal sections stained with cresyl violet.
Methodology of cAMP Analysis
Rats were sacrificed at 9 a.m., and brains were removed within 45 s of initial disturbance. Anterior hypothalami were dissected on ice as follows: The brain was placed, hypothalamus up, on an ice-chilled petri dish. With a razor blade, a coronal cut was made 2 mm (for immature) or 2.5 mm (for adult rats) anterior to the mammillary bodies. Diagonal cuts separated the hypothalamus, including the medial preoptic area, from the telencephalon. A horizontal cut, 3 mm above the inferior surface of the hypothalamus separated it from the thalamus. The resultant tissue-block, as determined in preliminary experiments, consisted of the PVN, periventricular and medial preoptic nuclei, and the anterior hypothalamic area. Dissection was achieved in 20 s or less, and the weight of the excised block averaged 19.8 ± 0.8 mg. Dissected tissues were immediately transferred to ice-chilled buffer, pooled, and minced to a thickness of 300–400 μm. Minced tissue was suspended in 50 vol of freshly oxygenated Krebs buffer (NaCl; 119, KC1; 2.5, MgSO4; 1.3, CaCl2; 2.5, KH2P04; 1.0, NaHC03; 26.2, glucose; 11 raM, pH 7.4) containing 0.5 mM IBMX and 0.1 μM GTP, and incubated for 30 min at 37 °C. During preincubation, medium was replaced with equal volume of freshly oxygenated buffer. Tissue aliquots were portioned into test tubes containing fresh buffer with various concentrations of DEX for an initial incubation of 20 min at 37 °C, and then pipetted into chambers containing fresh buffer and desired concentrations of forskolin and DEX for a final 15 min. Incubation was terminated by transferring chambers onto ice and pipetting the tissue into test tubes containing 500 μL of ice-cold 10% trichloroacetic acid. Homogenization (on ice) and centrifu-gation (1,000 × g for 10 min) were followed by extraction of supernatants with 5 vol of diethyl ether (3x). Supernatants were lyophilized and stored at −20 °C until used for cAMP assay. Pellets were resuspended in 50 mM sodium acetate buffer and assayed for protein. cAMP radioimmunoassay was performed using commercial cAMP tracer (Biomedical Technologies Inc., Stoughton, MA) and cAMP antibody obtained from the National Institutes of Health. Assay sensitivity was 0.05 pmol/mL.
Tissue Processing and in Situ Hybridization
In all experiments, brains were rapidly removed onto powdered dry ice and stored at −80 °C. Brains were cut into 20-μm coronal sections in a cryostat and mounted on gelatin-coated slides.14,15 Preparation of oligonucleotides for CRH and CRF1, and details of ISH and image analysis have been described.4,10,12,14,15 Briefly, prior to in situ hybridization (ISH), slides were brought to room temperature, air-dried and fixed in buffered paraformaldehyde. Following a graded ethanol treatment, sections were exposed to acetic anhydride-triethanolamine, then dehydrated through 100% ethanol. Sections were prehybridized for 1 h, then hybridized for 20 h at 40 °C in a humidity chamber. Serial washes (2× SSC for 15 min x4 at 40 °C; lx and 0.3× SSC for 30 min each at room temperature) were followed by dehydration and apposition to film (Hyperfilm B-Max, Amersham). Selected sections were subsequently dipped in emulsion (NTB-2; Kodak), and developed as previously described, with the exception that the emulsion was not diluted.
Analysis
Quantitation and statistical analysis were described previously.4,12,14,15 Briefly, optical density (OD) was determined over areas of interest, using the MCID software image analysis system (Imaging Research, Ont., Canada). Each point was derived from 6–12 sections from a minimum of four individual rats. Statistical significance between groups was determined using two-way analysis of variance, followed by Duncan’s multiple range tests.
RESULTS
Cold-Separation Stress Elevates Plasma CORT throughout the First Two Postnatal Weeks
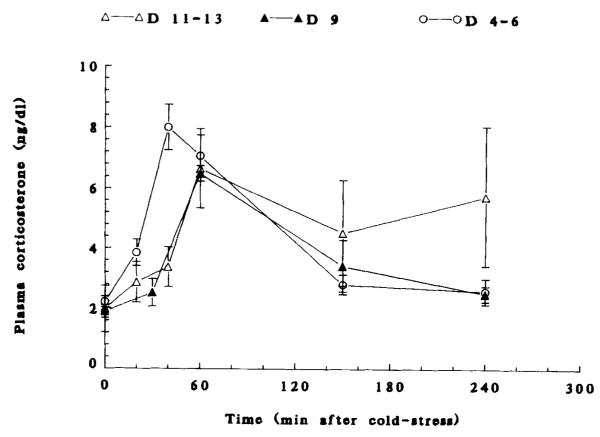
The time course and magnitude of cold-induced CORT elevation are shown in Figure 1. Clear, stress-induced peaks of plasma CORT were evident on postnatal days (PND) 4–6, 9, and 11–13. Maximal cold–stress induced plasma CORT values were 7.57 ± 0.86, 6.48 ± 0.26, and 6.64 ± 1.31 (μg/dL on PND 6, 9, and 11–13, respectively.
FIGURE 1.
Plasma CORT time courses in response to coid-separation stress in 4- to 6-, 9-, and 11- to 13-day-old rats. Values are mean ± SEM of 6–8 rats/group. For all age groups, 60-min values are significantly different from prestress ones (p < 0.05). On postnatal day 6, the 40-min value is significantly elevated as well (p < 0.05).
Cold-Stress-Induced Elevation of Plasma CORT Is Abolished by Antiserum to CRH
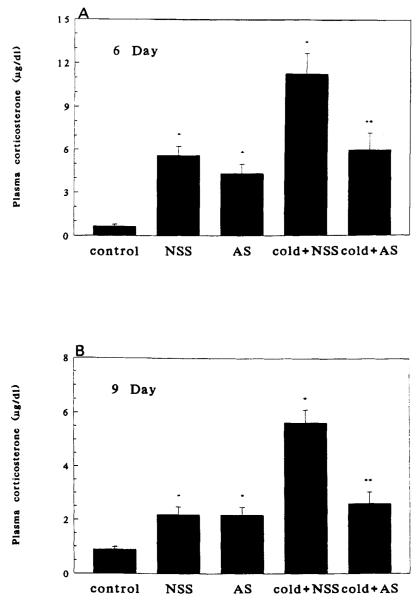
Injection of either AS or NSS resulted in increased plasma CORT, presumably because of handling and pain. Cold-separation stress induced a significant (p < 0.01) further increase in plasma CORT levels at both days 6 and 9 (Fig. 2A and 2B). At both ages, administration of CRH-AS abolished cold-induced plasma CORT (p < 0.01, cold + NSS vs. cold + AS).
FIGURE 2.
Effect of passive immunization against CRH on cold-separation stress-induced plasma CORT. Panel A: On postnatal day 6. Panel B: On postnatal day 9. NSS, normal sheep serum; AS, antiserum directed against CRH. n = 6 per group. Bars indicate standard errors. *Significantly different from control (p < 0.05); **significantly different from cold + NSS (p < 0.05).
Stress Induces CRH Gene Expression Only in 9-Day, or Older, Rats
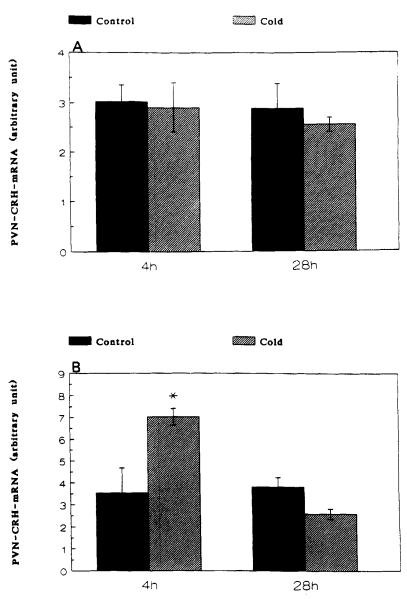
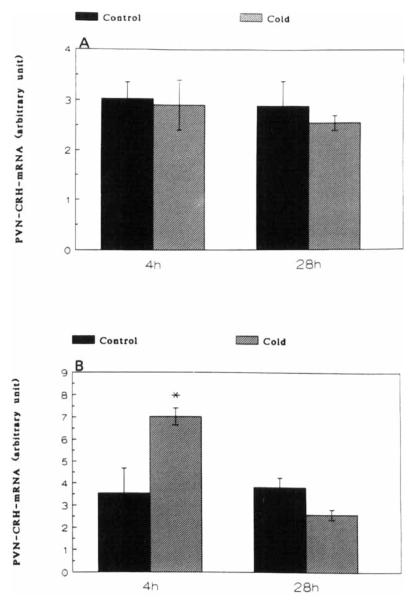
A significant increase of CRH-mRNA abundance in the PVN of 16-day-old rats was observed at 4 h (but not at 1 h), and persisted at 28 h subsequent to cold-separation stress. Therefore, these time points were studied in younger rats. In 9-day-old rats, cold-separation stress enhanced CRH-mRNA abundance at 4 h, but not at 28 h. No change in the CRH-message was found in 6-day-old rats (Fig. 3).
FIGURE 3.
Effect of cold-separation stress on CRH-mRNA in the paraventricular nucleus of 6-(panel A) and 9-(panel B) day-old rats. Pups were subjected to age-appropriate maximal tolerated cold-stress. CRH-mRNA was determined using in situ hybridization. Values (mean ± SEM) were derived as detailed in the Methods section. *Significantly different from control (p < 0.05).
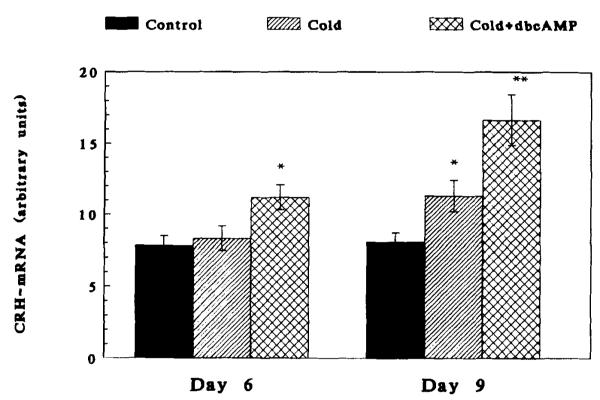
Infusion of a Cyclic AMP Analogue Enables the Up-Regulation of CRH Synthesis by Stress in the 6-Day-Old Rat
As is evident from Figure 4, infusion of db-cAMP permitted cold stress to up-regulate CRH gene expression in response to cold stress. This was evident upon infusion of the cAMP analogue to the PVN. Striatal infusion of db-cAMP was ineffective (not shown). db-cAMP was thus able to “reconstitute” a missing regulatory factor(s) acting on the CRH gene promoter.
FIGURE 4.
The effect of db-cAMP infusion, combined with cold + surgical stress, on CRH-mRNA abundance in the paraventricular nucleus of the 6- and 9-day-old rats. In the 6-day-old, stress alone did not result in “compensatory” increase of CRH-mRNA abundance at 4 h. db-cAMP enabled this effect, seen normally in the 9-day-old. In the 9-day-old rat, db-cAMP further increased stress-induced elevation of steady-state CRH-mRNA levels. *Significantly different from control (p < 0.05); **different from cold stress.
The Regulation of cAMP by Glucocorticoids Is Deficient in Immature Rat Hypothalamus
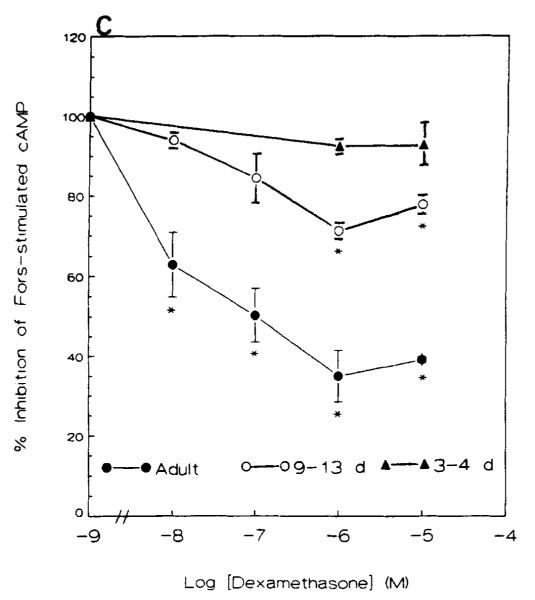
Anterior hypothalami derived from adult rats contained less basal cAMP (pmole/mg protein) than those of neonatal rats (Fig. 5A). This was probably due to higher protein concentrations in the adult rat. DEX alone (0.01 μM to 10 μM) did not significantly affect cAMP content at any age (Fig. 5A). Forskolin (10 μM) stimulated adenylate cyclase in the anterior hypothalamus significantly in all age groups (Fig. 5B). A 10-fold increase in cAMP production was found in adult rats, and a 3.2- to 6-fold increase in neonatal rats (3- to 13-day-old, p < 0.05).
FIGURE 5.
(A) Basal cAMP content (pmole/mg protein) in the anterior hypothalamus of neonatal and adult rats. Dexamethasone alone did not significantly affect cAMP accumulation at any age. Values represent the mean ± SEM of 6–7 experimental pools (10–12 neonatal or 2 adult hypothalami/pool. (B) Forskolin-stimulated adenylate cyclase in the anterior hypothalamus of neonatal and adult rats. Forskolin (2-20 μM) caused a robust increase at all ages. Values are the mean ± SEM of 5–6 pooled experiments. (C) Effect of dexamethasone on forskolin-induced increase in adenylate cyclase in anterior hypothalamus of neonatal and adult rats. *p < 0.05 vs. forskolin alone. Values obtained from 5–6 pooled experiments. Fors, forskolin; Dex, dexamethasone.
DEX inhibited the stimulatory effect of forskolin on adenylate cyclase in the anterior hypothalamus of adult rats in a concentration-dependent manner, with maximal effect (a 60% reduction) observed with 1 μM. This inhibitory effect of dex was considerably reduced in the anterior hypothalamus of 9- to 13-day-old rats, and was minimal in 3- to 4-day-old rats (Fig. 5C). Data analysis revealed a significant effect of age (p < 0.05), IC50’s of the inhibition curves for the adult and 9- to 13-day-old rats were 1.87 ± 0.4 × 10−8 M and 1.68 ± 0.32 × 10−7 M, respectively.
CRH Receptor Is Highly Abundant in Limbic Structures of the Immature Rat
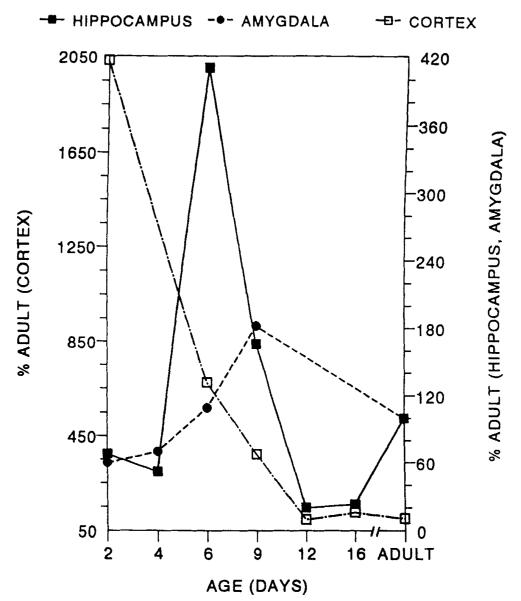
Figure 6 is a schematic representation of the CRH receptor (CRF1) messenger RNA distribution in hippocampus, amygdala, and cortex of the developing rat. Hippocampal values are a composite of CA1–CA2 and CA3 values.16 Amygdala values represent primarily the basolateral and central nuclei. Cortex was sampled at the frontoparietal convexity. Maximal receptor levels in the hippocampus are evident on PND 6 and peak in amygdala on PND 9.
FIGURE 6.
Schematic of the developmental profile of corticotropin-releasing hormone receptor (CRF1) in the amygdala, hippocampus, and cortex of the rat. See reference 16 for methodology.
DISCUSSION
The hormonal response to stressful cues is complex. The final common pathway consists of a transient, CRH-induced elevation of plasma ACTH and CORT. Negative feedback effects of glucocorticoids, which help terminate the response, up-regulation of CRH gene expression after stress-induced “depletion” of hypothalamic stores, and limbic input impinging on the hypothalamic PVN are only some of the molecular events contributing to this complex adaptive mechanism.1–6
During the neonatal and infant developmental periods in the rat, “immaturity” of this hormonal stress response implies quantitative or qualitative alterations of one or more components of this chain of events.4,7–10 The specific control points at which the immature animal differs from the adult are unclear. Obviously, perturbation of regulation of CRH gene expression, for example, should result in significant alteration of “downstream” components such as plasma CORT.
The studies described affirm that cold, a defined, age-specific acute stress, induces plasma CORT throughout the neonatal and infant period. Further, this elevation of plasma CORT depends on CRH in that it is abolished by antiserum to the peptide. As such, no major qualitative difference exists between the neonatal and adult rat in the “effector” arm of the CRH-CORT cascade.
Stress-induced up-regulation of CRH synthesis does not occur to any significant degree prior to the second postnatal week. This is consistent with previous findings regarding “insensitivity” of the CRH gene promoter, in vivo, in the neonatal rat.12 Neither the elimination of negative glucocorticoid feedback, by blocking of GC receptors in the PVN, nor the chronic stress of surgical cannula implantation resulted in increased steady-state CRH-mRNA prior to the ninth day of life.12 Both maneuvers increased CRH gene expression in the PVN of older rats.12
Candidate mechanisms for this insensitivity of the CRH gene promoter in the neonatal rat in vivo are suggested in Figures 4 and 5. First, infusion of a cAMP analogue, dibutyryl-cAMP, into the PVN during cold-surgery stress on PND 6, permitted enhancement of CRH-mRNA abundance, an effect normally observed on PND 9 or later. The effect was specific to db-cAMP and was not observed upon saline infusion. Further, neither db-cAMP nor saline was effective upon infusion into the striatum, suggesting anatomic specificity to the hypothalamus. A similar approach, implicating cAMP in the regulation of neuropeptide Y has recently been reported.17 The mechanism by which db-cAMP, in conjunction with acute stress, enhanced CRH-mRNA abundance on PND 6 remains speculative. The rationale for infusing the cAMP analogue derives from the fact that the CRH gene promoter contains a cAMP response element (CRE).18–20 Forskolin enhances the human CRH gene promoter, and mutation of the CRH-CRE sequence completely abolishes the stimulation by cAMP.19 We speculated that the cAMP-dependent signal transduction pathway might not be fully functional on PND 6. An excess of exogenous cAMP enabled this “mature response” of the CRH promoter on PND 6, consistent with a role for cAMP in the activation of the CRH gene promoter.
Additionally, the interaction between GC and the cAMP second messenger cascade is altered in the developing hypothalamus (Fig. 5A–C).21 A consensus GC response element sequence is not found at, or upstream from, the CRH gene promoter site, suggesting other mechanisms for the observed down-regulation of CRH synthesis by GC in the adult.20 We find that GC decreases cAMP production in mature hypothalamus.21 The response curve is shifted markedly to the right in the neonatal or infant rat (Fig. 5C).
An additional determinant of the magnitude of the CRH effect is the presence and abundance of receptors mediating the peptide’s effects.22–24 In the immature rat, distinct temporal and spatial profiles of one of these receptor types, CRF1, is clearly evident. For example, on the sixth postnatal day, the hippocampal/amygdala receptor ratio is higher than on the ninth postnatal day or in the adult. Whether this may result in augmented inhibitory input from hippocampus to PVN at this age remains speculative.16
SUMMARY
The ability to respond to adverse environmental cues is present in the neonatal and infant rat, although in an immature form: A number of laboratories have demonstrated stress-induced elevations of plasma glucocorticoids during the first two postnatal weeks. The limbic and hypothalamic mechanisms controlling the hormonal stress-response during this period are not fully understood and are, therefore, the focus of this report.
Both hypothalamic corticotropin-releasing hormone (CRH) and vasopressin contribute to the release of ACTH from the pituitary in the adult. The relative roles of these two peptides during the neonatal (first week) and infant (second week) developmental period, are controversial. Evidence is presented that argues strongly for a major role for CRH.
Up-regulation of hypothalamic CRH synthesis is a major component in the mature stress response. CRH-mRNA levels in the hypothalamic PVN are increased with cold stress by the ninth postnatal day, but not during the first postnatal week. Further, down-regulation of CRH gene expression by glucocorticoids (GC) constitutes a critical “shut-down” mechanism for the hormonal stress response. In vivo and in vitro experiments supporting the “immaturity” of GC feedback on CRH synthesis during the first postnatal week are described.
CRH-mediated neurotransmission, in both the endocrine and neuronal effector arms of the response to stress may be modeulated via alteration of receptor number. The first member of the CRH receptor family, CRF1, probably mediates the neuroendocrine effects of CRH. The developmental profile of CRF1-mRNA reveals several distinctive spatial and temporal patterns. In the hippocampal CA1, CA2, and CA3a, peak (300–600% adult values) CRF1-mRNA is found on postnatal day 6. In the amygdala, CRH receptor mRNA levels are maximal on the ninth postnatal day (at 180% of adult values). In cortex, a steady decline from high postnatal day 2 levels results in adult levels by day 12. These findings demonstrate distinct, regional, age-specific control of the synthesis of CRF1. Receptor expression profile may provide important information regarding modulation of the age-specific roles of CRH in different regions. For example, a high ratio of hippocampus/amygdala receptors may preferentially activate negative hippocampal input to the hypothalamus during the neonatal period. Additionally, increased CRH receptor mRNA in the infant compared with the adult provides a mechanism for enhanced excitatory effect of the peptide at this age.
In conclusion, increasing evidence exists for multiple control points of the early postnatal response and adaptation to stress. CRH synthesis in hypothalamus and amygdala, its sensitivity to GC feedback, and the abundance and distribution of at least two distinct CRH receptors in the limbic central nervous system and the pituitary are developmentally regulated. All serve as control points permitting an effective endocrine, autonomic, and behavioral response to stressful environmental cues.
Footnotes
This work was supported in part by NINDS NS 28912 (T.Z.B.) and by USC-CHLA BRSG awards (S.J.Y. and S.A.E.).
REFERENCES
- 1.Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH: Variations on a theme of B. Recent Prog. Horm. Res. 1987;43:113–131. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- 2.Lightman SL, Harbuz MS. Expression of corticotropin releasing factor mRNA in response to stress. Corticotropin Releasing Factor; CIBA Symposium; Chichester: Wiley; 1993. pp. 173–198. [DOI] [PubMed] [Google Scholar]
- 3.Harbuz MS, Lightman SL. Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J. Endocrinol. 1989;122:705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- 4.Yi S-J, Baram TZ. Corticotropin releasing factor mediates the response to cold stress in the neonatal rat, without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the HPA axis. Endocr. Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 6.Gray TS, Carney C, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic PVN: Possible role in stress-induced ACTH release. Neuroendocrinology. 1989;50:433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- 7.Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical systems of neonatal rats is responsive to stress throughout development in a time dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 8.Sucheki D, Mozaffarian D, Graziella G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology. 1993;57:204–212. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- 9.Paulmyer-Lacroix O, Anglade G, Grino M. Stress regulates differently the AVP-containing and AVP-deficient CRF synthesizing cell bodies in the hypothalamic paraventricular nucleus of the developing rat. Endocrine. 1994;2:1037–1043. [Google Scholar]
- 10.Avishai-Eliner S, Yi SJ, Newth CL, Baram TZ. Effects of maternal and sibling deprivation on basal and stress-induced HPA components in the infant rat. Neurosci. Lett. 1995;192:1–4. doi: 10.1016/0304-3940(95)11606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watts AG, Swanson LW. Diurnal variation in the content of preprocorti-cotropin releasing hormone mRNA in the hypothalamic paraventricular nucleus of rats of both sexes as measured by in situ hybridization. Endocrinology. 1989;125:1734–1738. doi: 10.1210/endo-125-3-1734. [DOI] [PubMed] [Google Scholar]
- 12.Yi S-J, Masters JN, Baram TZ. The effect of RU-38486 on CRH gene expression in the neonatal rat hypothalamus. Dev. Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]
- 13.Yi S-J, Baram TZ. Methods for implanting steroid-containing cannulae into the paraventricular nucleus of neonatal rats. J. Pharmacol. Toxicol. Methods. 1993;30:97–102. doi: 10.1016/1056-8719(93)90012-4. [DOI] [PubMed] [Google Scholar]
- 14.Baram TZ, Lerner SP. Corticotropin releasing hormone: Ontogeny of gene expression in rat hypothalamus. Int. J. Dev. Neurosci. 1991;9:473–478. doi: 10.1016/0736-5748(91)90033-i. [DOI] [PubMed] [Google Scholar]
- 15.Baram TZ, Schultz L. CRH gene expression in the fetal rat is not increased after pharmacological adrenalectomy. Neurosci. Lett. 1992;142:215–218. doi: 10.1016/0304-3940(92)90376-i. [DOI] [PubMed] [Google Scholar]
- 16.Avishai-Euner S, Yi SJ, Baram TZ. Developmental profile of CRH-receptor messenger RNA in the rat limbic system. Dev. Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akabayashi A, Zaia CTBV, Gabriel SM, Silva I, Cheung WK, Leibowitz SF. Intracerebroventricular injection of dibutyryl cyclic adenosine 3′5′-monophosphate increases hypothalamic levels of neuropeptide Y. Brain Res. 1994;660(2):323–328. doi: 10.1016/0006-8993(94)91306-4. [DOI] [PubMed] [Google Scholar]
- 18.Seasholtz AF, Thompson RC, Douglass IO. Identification of a cyclic AMP-responsive element in the rat corticotropin-releasing hormone gene. Mol. Endocrinol. 1988;2:1311–1318. doi: 10.1210/mend-2-12-1311. [DOI] [PubMed] [Google Scholar]
- 19.Spengler D, Rupprecht R, Van LP, Holsboer F. Identification and characterization of 3′,5′-cyclic adenosine monophosphate responsive element in the human corticotropin releasing hormone gene promoter. Mol. Endocrinol. 1992;6:1931–1941. doi: 10.1210/mend.6.11.1480179. [DOI] [PubMed] [Google Scholar]
- 20.Majzoub JA, Emanuel R, Adler GK, Martinez C, Robinson B, Wittert G. Second messenger regulation of mRNA for CRF. Corticotropin Releasing Factor; CIBA Symposium; Chichester: Wiley; 1993. pp. 30–43. [DOI] [PubMed] [Google Scholar]
- 21.Yi S-J, Baram TZ. Ontogeny of forskolin-stimulated cAMP-dependent protein kinase and of dexamethasone suppression of forskolin-stimulated cAMP production in rat hypothalamus. Soc. Neurosci. Abstr. 1993;23:1186. [Google Scholar]
- 22.De Souza EB. Corticotropin-releasing factor receptors in the rat central nervous system: Characterization and regional distribution. J. Neurosci. 1987;7:88–100. doi: 10.1523/JNEUROSCI.07-01-00088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang C-P, Pearse RV, O’Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- 24.Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin-factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- 25.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]