Renal failure is a common and serious complication of longstanding diabetes mellitus. Diabetes is now the most common cause of end-stage renal failure requiring dialysis in the United States, accounting for almost 40% of all new dialysis patients (1). Moreover, the incidence of renal failure caused by diabetes, particularly type II diabetes, is rising dramatically worldwide (2). Compounding the tragedy of the explosive growth in the incidence renal failure caused by diabetes is the grim reality that the survival of patients with renal failure caused by diabetes is much worse than that of patients with renal failure resulting from other causes. In Germany, for example, Koch et al. (3) reported a 5-year survival of only 5% among patients with type II diabetes undergoing dialysis. Fortunately, progress is being made in our understanding of the pathogenesis of diabetic renal disease and in our ability to delay, or even prevent, this devastating complication. Two studies appearing in this issue of PNAS (4, 5) are illustrative of this progress. Diabetic nephropathy refers to a characteristic set of structural and functional kidney abnormalities that occur in patients with diabetes. Although best described in patients with type I diabetes (6), similar findings are now known to occur in the more common type II diabetic patient (7). Structural abnormalities include hypertrophy of the kidney, an increase in the thickness of glomerular basement membranes, accumulation of extracellular matrix components in the glomerulus (nodular and diffuse glomerulosclerosis), tubular atrophy, and interstitial fibrosis (6, 7). Functional alterations include an early increase in the glomerular filtration rate with intraglomerular hypertension, subsequent proteinuria, systemic hypertension, and eventual loss of renal function (8).
Clinicopathologic studies of diabetic nephropathy have established that the extent of matrix accumulation in both the glomeruli and the interstitium correlate strongly with the degree of renal insufficiency and proteinuria (9). Accordingly, the factors responsible for the deposition and accumulation of extracellular matrix material within the kidney are of considerable interest. A host of mediators, such as hyperglycemia, glycosylated proteins, intracellular polyols, vasoactive hormones, systemic and glomerular hypertension, proteinuria, growth factors, and cytokines have been implicated in the pathogenesis of diabetic nephropathy (10, 11). Of these, the cytokine transforming growth factor (TGF-β) has emerged as having a key role in the development of renal hypertrophy and accumulation of extracellular matrix in diabetes (10, 12). TGF-β is known to have powerful fibrogenic actions resulting from both stimulation of matrix synthesis and inhibition of matrix degradation (12). In humans and animal models, TGF-β mRNA and protein levels are significantly increased in the glomeruli and tubulointerstitium in diabetes (13–17). Moreover, short-term administration of TGF-β neutralizing antibodies to rats with chemically induced diabetes prevented glomerular enlargement and suppressed the expression of genes encoding extracellular matrix components (18). The study reported by Ziyadeh et al. (4) in this issue of PNAS extends these observations and provides the strongest evidence to date for the causal role of TGF-β in the structural and functional abnormalities of diabetic nephropathy.
Specifically, Ziyadeh et al. (4) examined the effects of long-term administration of a neutralizing TGF-β antibody on the renal function and renal histology of diabetic mice. These mice, the db/db strain, lack the hypothalamic leptin receptor and develop obesity caused by overeating, insulin resistance, and hyperglycemia. In these respects, the dbldb mouse is a model of type II diabetes mellitus, which accounts for about 70–80% of all diabetic end-stage renal failure (1). Kidneys from untreated db/db mice exhibited an increase in TGF-β mRNA expression in glomeruli and an increase in TGF-β receptor expression when compared with nondiabetic db/m littermates. Similarly, there was an increase in kidney type IV collagen α1 and fibronectin mRNA expression and histologic evidence of mesangial matrix expansion in the dbldb mice. Functional abnormalities of diabetic nephropathy included a 40% fall in the glomerular filtration rate and a large increase in urinary albumin excretion. Remarkably, treatment with the neutralizing antibody almost completely prevented the increase in collagen and fibronectin expression and the mesangial matrix expansion in the diabetic mice. These results are quite consistent with previous in vitro studies demonstrating the effects of TGF-β on production of extracellular matrix components (19, 20). The most significant finding, though, was that neutralization of TGF-β prevented the fall in glomerular filtration rate. Thus, these results provide clear evidence that TGF-β activity contributes to the development of diabetic glomerulosclerosis and, ultimately, to renal failure.
A curious observation in this study was the failure of the TGF-β neutralizing antibody to reduce proteinuria in the diabetic mice. Proteinuria is an early manifestation of diabetic nephropathy and is a powerful predictor of subsequent renal dysfunction (21). Indeed, it has been suggested that proteinuria itself plays a pathogenic role in diabetic nephropathy and other renal diseases (22). That Ziyadeh et al. (4) were able to dissociate proteinuria from the development of glomerulosclerosis and renal insufficiency permits two conclusions. First, TGF-β is apparently not a key factor in the glomerular hemodynamic and permeability changes that result in proteinuria. Second, the deleterious effects of proteinuria on the progression of renal disease may themselves be mediated via TGF-β.
The study by Lang et al. (5), also in this issue of PNAS, addresses the possible role of a serine/threonine kinase, hSGK, in the dysregulation of sodium transport in the diabetic kidney. hSGK, found in most tissues (23) is known to be up-regulated by TGF-β (24). Lang et al. (5) found that increasing the extracellular glucose concentration increased hSGK expression in cultured fibroblasts and endothelial cells. Moreover, the effect of glucose was mediated through a pathway involving TGF-β. They also examined levels of hSGK expression in the human kidney by in situ hybridization. hSGK mRNA was markedly increased in kidney biopsies obtained from diabetic patients and was localized predominantly to the mesangium, the distal tubules, and the thick ascending limb of Henle's loop. These latter two sites are important for the regulated reabsorption of sodium from the glomerular filtrate, and hence, for regulation of extracellular fluid volume. Two major transporters for sodium in the late distal tubule (and collecting duct) and the thick ascending limb of Henle are the epithelial sodium channel, ENaC, and the sodium, potassium, chloride cotransporter, BSC-1, respectively (25). It recently had been shown that SGK from Xenopus increases the activity of ENaC channels (26, 27). Lang et al. (5) confirmed that human SGK also increased ENaC activity and also demonstrated that hSGK stimulated the activity of BSC-1. The physiologic significance of these observations remains to be established because it is not known whether sodium transport mediated by either ENaC or BSC-1 is increased in diabetes. However, it is certainly possible that stimulation of ENaC and BSC-1 activity could account for the increased incidence of hypertension in diabetic nephropathy. Likewise, Lang et al. (5) speculate that an increase in sodium reabsorption in the thick ascending limb could, via the phenomenon of tubuloglomerular feedback, result in the glomerular hyperfiltration that characterizes early diabetic nephropathy. A reduction in NaCl delivery to the macula densa caused by increased thick limb sodium reabsorption also could stimulate the renin-angiotensin axis, resulting in additional TGF-β production (see below). Finally, although hSGK expression in cultured cells was stimulated by TGF-β, it is not certain that TGF-β was responsible for the increase in hSGK seen in diabetic kidneys. Studies of the sort reported by Ziyadeh et al. (4) may answer this question.
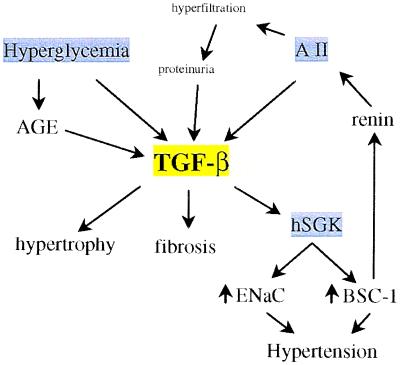
As illustrated by these studies, TGF-β appears to play a central role in the development and progression of diabetic nephropathy (Fig. 1). It is interesting to consider how certain clinical interventions that slow the progression of diabetic nephropathy might relate to alteration in either the synthesis or activity of TGF-β in the kidney. Hyperglycemia is the sine qua non of diabetes. Prospective clinical trials have established that good glycemic control reduces the risk for the development and progression of diabetic nephropathy (28, 29). Hyperglycemia itself stimulates the production of TGF-β via activation of protein kinase C in cultured renal tubular epithelial cells, glomerular cells, and interstitial fibroblasts (19).
Figure 1.
Schematic view of the possible roles of TGF-β in diabetic nephropathy. AGE, advanced glycation end products; AII, angiotensin II; ENaC, epithelial sodium channel; BSC-1, sodium, potassium, chloride cotransporter; hSGK, serine/threonine kinase.
In addition, advanced glycation end products (AGE), produced by nonenzymatic reactions with glucose, stimulate TGF-β production by kidney cells (20, 30). These observations provide a rationale for the beneficial effects of lowering blood glucose concentrations on diabetic nephropathy. Certainly, glucose may have other deleterious effects that are not related to TGF-β production. However, the results of Ziyadeh et al. (4), in which diabetic nephropathy was almost completely arrested by the TGF-β antibody in the face of persistent hyperglycemia (table 1 of ref. 4), argues that the impact of these other effects must be relatively small compared with TGF-β. Angiotensin converting enzyme inhibitors (ACE-I) slow the progression of kidney disease in both type I and type II diabetes (31, 32). Early work attributed the beneficial effect of ACE-I to their ability to attenuate angiotensin II (AII) induced vasoconstriction of the glomerular efferent arteriole. However, AII also is a potent stimulus for TGF-β production by kidney cells (33) and acts in synergy with elevated glucose concentrations to stimulate matrix production by renal epithelial cells (10). Thus, it is quite likely that some of the beneficial effects of ACE-I in diabetic nephropathy (and other kidney diseases) are related to suppression of TGF-β production. In fact, in patients with type I diabetes treated with either a placebo or the ACE-I captopril, the reduction in circulating TGF-β levels was inversely correlated with the rate of decline of the glomerular filtration rate (34). The inability of ACE-I treatment to completely halt the progression of diabetic nephropathy may relate to the incomplete suppression of TGF-β production by these agents. In a review of this subject, Border and Noble (35) found that ACE-I or AII receptor antagonists reduced TGF-β production by only about 50%. Likewise, in the captopril study mentioned above (34), serum TGF-β levels were reduced only 14% by captopril. Thus, it will be important to develop more effective approaches for suppressing TGF-β production or activity and to determine whether these approaches are more effective in halting the progression of kidney disease (33). In this regard, the following paragraphs present a brief overview of TGF-β synthesis and signaling.
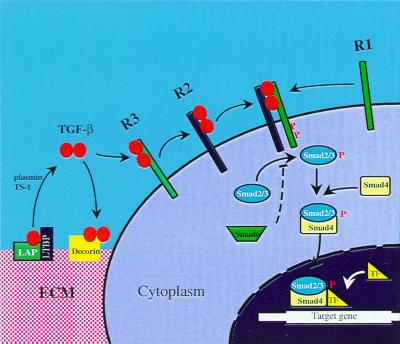
There are three highly similar isoforms of TGF-β in mammals: TGF-β1, TGF-β2, and TGF-β3. Of the three, TGF-β1 may be the most important in fibrosis (12). TGF-β is synthesized as an inactive precursor containing an amino terminal prodomain. The prodomain, also termed latency-associated protein (LAP), is cleaved within the Golgi but remains noncovalently bound to TGF-β, thereby preventing TGF-β from binding to its receptors (36). This latent complex of TGF-β and LAP also associates with latent TGF-β binding proteins (LTBPs) before secretion from the cell (36). After secretion, most TGF-β is stored in the extracellular matrix as this inactive complex with LAP and LTBP (Fig. 2). The latent TGF-β complex can be activated into mature, biologically active TGF-β through the actions of proteases, such as plasmin, or by thrombospondin-1. After release from the latent complex, TGF-β dimers initiate their cellular effects by binding to cell membrane receptors. Three TGF-β receptors are involved in TGF-β signal transduction (36, 37). TGF-β first binds to the type III TGF-β receptor, which then presents the ligand to the type II receptor. The type I receptor subsequently is recruited to form a heterotetramer consisting of two type I and two type II receptors. Upon formation of the complex, the type II receptor phosphorylates certain serine and threonine residues on the type I receptor, thereby activating its kinase activity. Activation of the type I receptor leads to the recruitment and phosphorylation of certain members of the Smad family of signal transducing proteins (38). Smad2 and Smad3 are phosphorylated by the type I receptor, homodimerize, and subsequently bind to Smad4. The heteromeric complex of Smad2 or Smad3 and Smad4 translocates to the nucleus where it regulates the transcription of target genes. The regulation of gene transcription by Smad complexes likely involves the interaction of other transcription factors, e.g., AP-1, FAST, and Sp1, and interactions with other signaling cascades, e.g., mitogen-activated protein kinases (36).
Figure 2.
Mechanism of activation and signal transduction mediated by TGF-β. LAP, latency-associated protein; LTBP, latent TGF-β binding protein; ECM, extracellular matrix; TF, transcription factor; P, phosphorylated amino acid; TS-1, thrombospondin-1.
Such a complex system provides numerous levels of regulation and numerous targets for intervention. For example, the ability of TGF-β to bind with its receptor could be reduced by inhibiting its proteolytic activation or by scavenging active TGF-β with excess latency-associated protein (39), with neutralizing antibodies, as demonstrated by Ziyadeh et al. (4) and Border et al. (40), or with other binding proteins, such as decorin. In fact, overexpression of decorin, an extracellular matrix glycoprotein that binds and inactivates TGF-β (41), markedly reduced glomerular damage and proteinuria in an in vivo model of glomerulonephritis (42). Likewise, activity of the TGF-β receptor and signal transduction could be inhibited at several levels. A number of cytoplasmic proteins, e.g., FKBP12, STRAP, and TRAP-1, can inhibit the kinase activity of the TGF-β receptor (36). Smad6 and Smad7 interfere with the phosphorylation of Smad2 and Smad3, thereby inhibiting TGF-β signal transduction (37). Inhibition of mitogen-activated protein kinases could alter the transcriptional activity of Smads or other interacting cofactors, e.g., c-Jun. It remains to be seen whether any of these steps can be manipulated in a clinically meaningful way to reduce the progression of diabetic nephropathy and other chronic renal diseases.
Finally, genetic factors influence the risk of developing diabetic nephropathy (43). A genetic predisposition to diabetic nephropathy is suggested by the observation that the diabetic sibling of a patient with diabetic nephropathy has a 3-fold greater risk of developing nephropathy than the diabetic sibling of a diabetic without nephropathy. Likewise, certain populations, e.g., American Indians, Hispanics and Blacks, are at greater risk for developing diabetic nephropathy than Caucasian populations. The identification of genes that account for an increased risk of diabetic nephropathy is under investigation. It will be interesting to see whether any of the genes identified are involved in TGF-β production or action.
Footnotes
References
- 1.National Institutes of Health. U.S. Renal Data System. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 1998. [Google Scholar]
- 2.Ritz E, Rychlik I, Locatelli F, Halimi S. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 3.Koch M, Kutkuhn B, Grabensee B, Ritz E. Nephrol Dial Transplant. 1997;12:2603–2611. doi: 10.1093/ndt/12.12.2603. [DOI] [PubMed] [Google Scholar]
- 4.Ziyadeh F N, Hoffman B B, Han D C, Iglesias-de la Cruz M C, Hong S W, Isono M, Chen S, McGowan T A, Sharma K. Proc Natl Acad Sci USA. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang F, Klingel K, Wagner C A, Stegen C, Wärntges S, Friedrich B, Lanzendörfer M, Melzig J, Moschen I, Steuer S, et al. Proc Natl Acad Sci USA. 2000;97:8157–8162. doi: 10.1073/pnas.97.14.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mauer S, Steffes M, Ellis E, Sutherland D, Brown D, Goetz F. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueno M, Kawashima S, Nihsi S, Shimada S, Shimada N, Karasawa R, Suzuki Y, Muruyama Y, Arakawa M. Kidney Int. 1997;52:S191–S194. [PubMed] [Google Scholar]
- 8.Hostetter T H, Troy J L, Brenner B M. Kidney Int. 1981;19:410–415. doi: 10.1038/ki.1981.33. [DOI] [PubMed] [Google Scholar]
- 9.Lane P H, Steffes M W, Fioretto P, Mauer S M. Kidney Int. 1993;43:661–667. doi: 10.1038/ki.1993.95. [DOI] [PubMed] [Google Scholar]
- 10.Wolf G, Ziyadeh F N. Kidney Int. 1999;56:393–405. doi: 10.1046/j.1523-1755.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert R E, Cooper M E. Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 12.Border W A, Noble N A. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Nakamura T, Noble N A, Ruosiahti E, Border W A. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma K, Ziyadeh F N, Alzahabi B, McGowen T A, Kapoor S, Kurnick B R C, Weisberg L S. Diabetes. 1997;46:854–859. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- 15.Park S, Kiyomoto H, Abboud S L, Abboud H E. Diabetes. 1997;46:473–480. doi: 10.2337/diab.46.3.473. [DOI] [PubMed] [Google Scholar]
- 16.Pankewycz O G, Guan J X, Bolton W K, Gomez A, Benedict J F. Kidney Int. 1994;46:748–758. doi: 10.1038/ki.1994.330. [DOI] [PubMed] [Google Scholar]
- 17.Iwano M, Kubo A, Nishino T, Sato H, Nishioka H, Akai Y, Kurioka H, Fujii Y, Kanauchi M, Shiiki H, Dohi K. Kidney Int. 1996;49:1120–1126. doi: 10.1038/ki.1996.162. [DOI] [PubMed] [Google Scholar]
- 18.Sharma K, Jin Y, Guo J, Ziyadeh F N. Diabetes. 1996;45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 19.Ziyadeh F N, Sharma K, Ericksen M, Wolf G. J Clin Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Throckmorton D C, Brodgen A P, Min B, Rasmussen H, Kashgarian M. Kidney Int. 1995;48:111–117. doi: 10.1038/ki.1995.274. [DOI] [PubMed] [Google Scholar]
- 21.Mogensen C E. N Engl J Med. 1984;310:356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 22.Remuzzi G. Kidney Int. 1997;51:2–15. doi: 10.1038/ki.1997.2. [DOI] [PubMed] [Google Scholar]
- 23.Waldegger S, Barth P, Raber G, Lang F. Proc Natl Acad Sci USA. 1997;94:4440–4445. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldegger S, Klingel K, Barth P, Sauter M, Lanzendorfer M, Kandolf R, Lang F. Gastroenterology. 1999;116:1081–1088. doi: 10.1016/s0016-5085(99)70011-9. [DOI] [PubMed] [Google Scholar]
- 25.Reeves W B, Andreoli T E. In: Diseases of the Kidney. Schrier R W, Gottschalk C W, editors. Boston: Little Brown; 1997. pp. 127–162. [Google Scholar]
- 26.Chen S Y, Bhargava A, Mastroberardino L, Meijer O C, Wang J, Buse P, Firestone G L, Verrey F, Pearce D. Proc Natl Acad Sci USA. 1999;96:2514–2519. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naray-Fejes-Toth A, Canessa C, Cleaveland E S, Aldrich G, Fejes-Toth G. J Biol Chem. 1999;274:16973–16978. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- 28.The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 29.The United Kingdom Prospective Diabetes Study Group. Lancet. 1998;352:837–853. doi: 10.1016/s0140-6736(98)22937-0. [DOI] [PubMed] [Google Scholar]
- 30.Ziyadeh F N, Han D C, Cohen J A, Guo J, Cohen M P. Kidney Int. 1998;53:631–638. doi: 10.1046/j.1523-1755.1998.00815.x. [DOI] [PubMed] [Google Scholar]
- 31.Lewis E J, Hunsicker L G, Bain R P, Rohde R D. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 32.Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R. Ann Int Med. 1998;128:982–988. doi: 10.7326/0003-4819-128-12_part_1-199806150-00004. [DOI] [PubMed] [Google Scholar]
- 33.Border W A, Noble N A. Kidney Int. 1997;51:1388–1396. doi: 10.1038/ki.1997.190. [DOI] [PubMed] [Google Scholar]
- 34.Sharma K, Eltayeb B O, McGowan T A, Dunn S R, Alzahabi B, Rohde R, Ziyadeh F N, Lewis E J. Am J Kidney Dis. 1999;34:818–823. doi: 10.1016/s0272-6386(99)70037-5. [DOI] [PubMed] [Google Scholar]
- 35.Border W A, Noble N A. Hypertension. 1998;31:181–188. doi: 10.1161/01.hyp.31.1.181. [DOI] [PubMed] [Google Scholar]
- 36.Piek E, Heldin C-H, Ten Dijke P. FASEB J. 1999;13:2105–2124. [PubMed] [Google Scholar]
- 37.Blobe G C, Schiemann W P, Lodish H F. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 38.Zhou S, Kinzler K W, Vogelstein B. N Engl J Med. 1999;341:1144–1145. doi: 10.1056/NEJM199910073411510. [DOI] [PubMed] [Google Scholar]
- 39.Bottinger E P, Factor V M, Tsang M L-S, Weatherbee J A, Kopp J B, Oian S W, Wakefield L M, Roberts A B, Thorgeirsson S S, Sporn M B. Proc Natl Acad Sci USA. 1996;93:5877–5882. doi: 10.1073/pnas.93.12.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Border W A, Okuda S, Languino L R, Sporn M B, Ruoslahti E. Nature (London) 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 41.Hildebrand A, Romaris M, Rasmussen L M, Heinegard D, Twardzik D R, Border W A, Ruoslahti E. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaka Y, Brees D K, Ikegaya K, Kaneda Y, Imai E, Noble N A, Border W A. Nat Med. 1996;2:418–423. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 43.Krolewski A S. Kidney Int. 1999;55:1582–1596. doi: 10.1046/j.1523-1755.1999.00371.x. [DOI] [PubMed] [Google Scholar]