Summary
The embryonic tentorial sinus regresses at the 60-80 mm embryologic stage and most of the deep venous channels constitute the basal vein of Rosenthal (BVR). Persisting remnants of the embryonic tentorial sinus can be seen in the adult configuration of the BVR. We tried to explain the anatomic representations of the BVR associated with the remnant embryonic tentorial sinus.
A total 41 patients and 82 hemispheres were included in this study. CT angiography was performed in all patients as screening for cerebrovascular disease or other intracranial disorders. A separate workstation and 3D software were used to evaluate the cranial deep venous systems with 3D volume rendering techniques, thin-slice MIP images, and MPR techniques for the analysis of complicated angioarchitecture. Variations of the BVR were classified according to the developmental alterations of efferent pathways into four groups: telencephalic group (A) including tributaries of the uncal vein, inferior frontal vein, anterior communicating vein, and inferior striatal vein; diencephalic group (B) of the interior ventricular vein and peduncular vein; tegmental bridging group (C) of the longitudinal LMV anastomosis; tectal group (D) of the superior vermian vein and internal occipital vein in relation to the Galenic connection. The BVR constituted from the embryonic tentorial sinus was also assessed and the developmental aspects reviewed.
Remnant embryonic tentorial sinus was visualized in 12% (10/82) of hemispheres, all of them invariably connected with the telencephalic (A) and diencephalic (B) groups. Most of those connections (9/10) to basal venous tributaries originated from the medial tentorial sinus except one case from the lateral tentorial sinus. No Galenic connections of the BVR were identified in 10% (8/82). Various tributaries of the BVR were classified as: Telencephalic group (A) 43% (35/82), Diencephalic group (B) 35% (29/82), Bridging group (C) 11% (9/82), and Tectal group (D) 6% (5/82). Four cases (5%) were unclassified and revealed only small basal tributaries of the BVR without connection to the great vein of Galen.
Anatomic variations of the BVR connected with persistent embryonic tentorial sinus could often be demonstrated in adult configurations considering the embryologic aspects of developmental regression and secondary cerebral venous adaptations.
Key words: basal vein of Rosenthal, embryology, tentorial sinus
Introduction
The basal vein of Rosenthal (BVR) originates below the anterior perforated substance and courses posteriorly between the midbrain and the temporal lobe towards and into the Galenic system of veins1.
In adult stage, the BVR is divided into a striate segment (first segment), a peduncular segment (second segment), and a mesencephalic segment (third segment)2. During fetal development, these segments may not develop fully or at all, or may fail to anastomose longitudinally with each other, resulting in a fragmented BVR with various patterns of venous drainage. The embryonic tentorial sinus is represented as venous drainage of the BVR in the adult and has been reported as anatomical variants on some angiographic studies3,4.
Anatomic visualization of cerebral venous systems can be obtained with 3D CT angiography5. Following volume acquisition of CT angiography, computerized manipulations of the data will readily allow us to simulate various anatomical dissections, without the need for strenuous cadaver dissections and also make it possible to stand inside or outside the space with virtual imaging tools.
We present our findings concerning the patterns of BVR as seen on 82 hemispheres of CT angiography, focusing on the embryologic anatomy of the embryonic regressed tentorial sinus as it may normally appear in routine clinical practice. The patterns of BVR associated with the remnant embryonic tentorial sinus will be discussed from an embryologic point of view.
Material and Methods
The drainage patterns of the BVR were prospectively evaluated on 50 consecutive diagnostic CT angiography studies. All the patients included were screened for cerebrovascular diseases or other intracranial diseases. A total of 41 patients and 82 hemispheres were included in this study protocol of cerebral CT angiography and 14 men and 27 women, aged from 30 to 77 years (mean age, 56 years) were examined. Among them, eight patients had intracranial atherosclerotic diseases with variable degrees of stenosis. Four patients with incidental small aneurysms were detected (two had mirror aneurysms of the MCA bifurcation). One patient with a small acute ischemic infarction in the basal ganglia was suggested, and one patient had suggestive findings of vertebrobasilar dissection. No other intracranial abnormalities were detected.
Three-dimensional CT angiography was initiated 22-28 seconds after the start of intravenous bolus administration of non-ionizing contrast material, injected at a rate of 4-5 mL/s for 20-25 seconds, for a total volume of 100 mL. The sections were 1.25 mm thick with 0.6 mm slice spacing. The FOV was 180 mm in each dimension and covered a brain region of 60 mm in a caudal to cranial scan direction. The angle of CT gantry axis was adjusted before the scan for visualization of the circle of Willis and cerebral venous system.
The raw image data of CT were transferred to PACS monitors and also to a separate PC workstation. We used the volume rendering method for 3D reconstruction with various tools including MPR, MIP, SSD, and virtual endoscopes in the software program (Rapidia 2.7, Infinitt, Seoul, Korea).
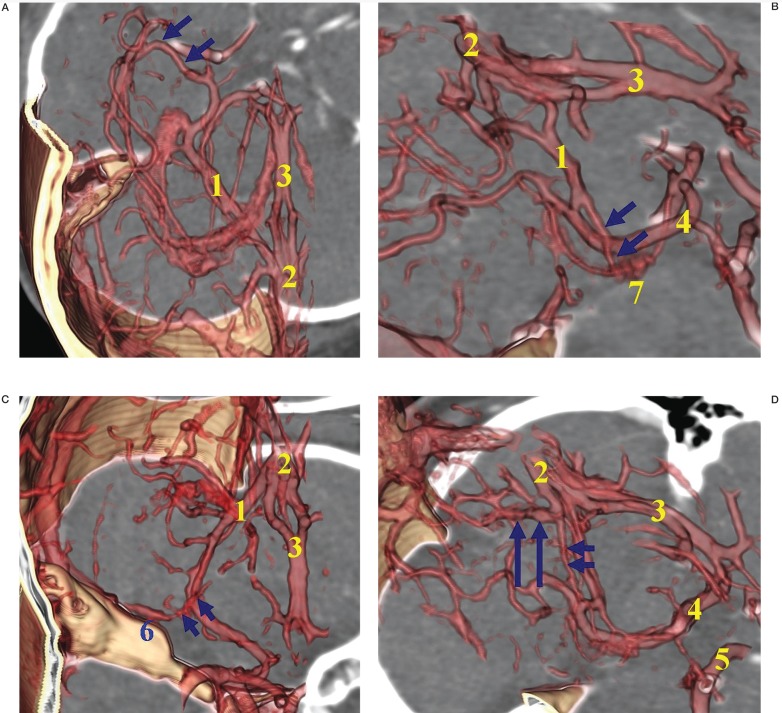
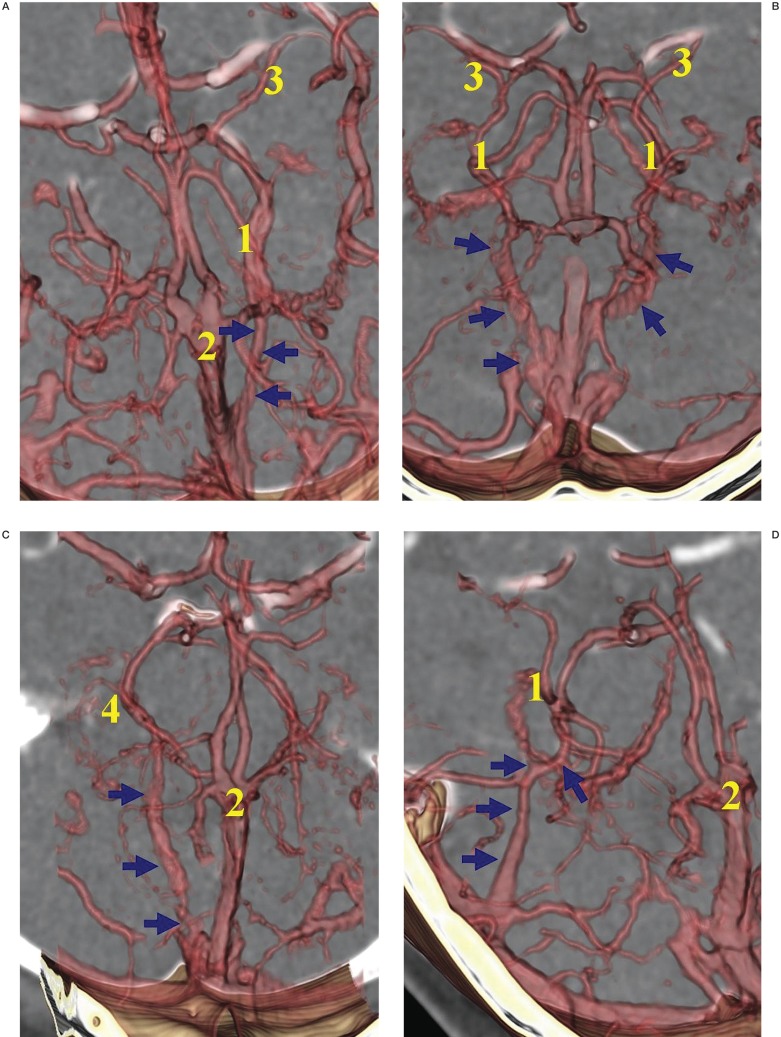
Variations of the BVR were classified according to the developmental alterations of efferent pathways into four groups (figure 1): Telencephalic group (A) including tributaries of the uncal vein, inferior frontal vein, anterior communicating vein, and inferior striatal vein; Diencephalic group (B) of the interior ventricular vein and peduncular vein; Tegmental bridging group (C) of the longitudinal lateromesencephalic vein (LMV) anastomosis; Tectal group (D) of the superior vermian vein and internal occipital vein in relation to the Galenic connection1. The BVR constituted into the regressed embryonic tentorial sinus was also evaluated and the developmental aspects reviewed.
Figure 1.
Classifications of the Basal vein of Rosenthal (BVR) according to the developmental alterations of efferent pathways:A) Telencephalic group, including tributaries of the inferior striatal vein, uncal vein, inferior frontal vein, anterior communicating vein,deep middle cerebral vein (arrows).B) Diencephalic group,including tributaries of the peduncular vein,inferior ventricular vein (arrows), posterior communicating vein. C) Tegmental bridging group of lateromesencephalic vein (LMV) anastomosis (arrows) with superior petrosal sinus. D) Tectal group of superior vermian vein. This figure shows the Galenic connection of the small BVR constituting the superior vermian vein (short arrows) and adjacent posterosuperior cerebellar vein (long arrows).
1, basal vein of Rosenthal (BVR); 2, vein of Galen; 3, deep middle cerebral vein (DMCV); 4, inferior striatal vein; 5, inferior ventricular vein; 6, superior petrosal sinus; 7, choroid plexus.
Results
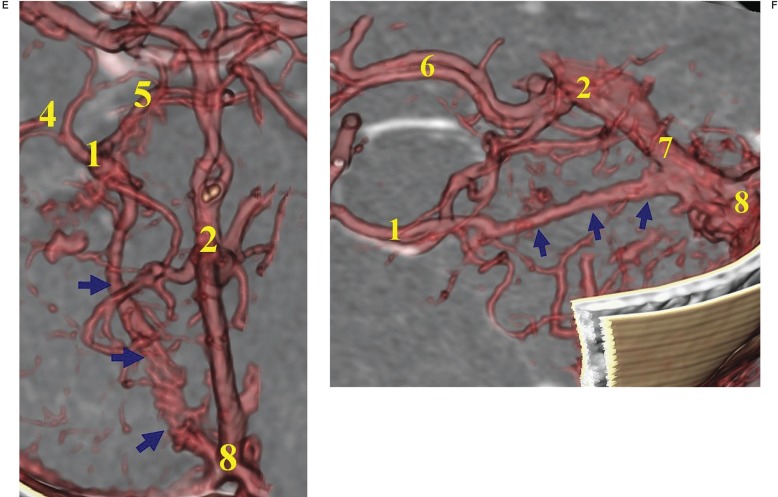
Remnant embryonic tentorial sinus was visualized in 12% (10/82) of hemispheres, all of them invariably connected with the telencephalic (8/10) and the diencephalic (2/10) groups (figure 2). The telencephalic terminations of the BVR from the remnant tentorial sinus consisted of one anterior communicating vein, five DMCVs and two uncal veins.
Figure 2.
Patterns of the remnant embryonic tentorial sinus constituting the BVR. A) Medial tentorial sinus (arrows) towards the telencephalic group. B) Bilateral tentorial sinuses (arrows) toward the telencephalic group. C) Medial tentorial sinus (arrows) towards the diencephalic group. D) Lateral tentorial sinus (arrows) towards the telencephalic group. E) Medial tentorial sinus (arrows) towards the diencephalic group, inferior ventricular and interpeduncular vein. F) Medial tentorial sinus (arrows) terminates into the straight sinus, viewed from the lateral direction.
1, basal vein of Rosenthal (BVR);
2, vein of Galen;
3, deep middle cerebral vein (DMCV);
4, inferior ventricular vein;
5, interpeduncular vein;
6, internal cerebral vein;
7, straight sinus;
8, torcular;
9, transverse sinus;
10, straight sinus.
The diencephalic group of the BVR from the remnant tentorial sinus terminated into one peduncular vein and inferior ventricular vein and one inferior ventricular vein. Most of those connections (9/10) to the BVR originated from the medial tentorial sinus except one hemisphere from the lateral tentorial sinus to the BVR. The distal connections of the BVR toward venous sinuses consisted of five straight sinuses, three straight sinus-torcular junctions, one torcular and one transverse sinus. The lateral tentorial sinus to the BVR terminated in the transverse sinus.
Various tributaries of the BVR were classified as: Telencephalic group (A) 43% (35/82), Diencephalic group (B) 35% (29/82), Bridging group (C) 11% (9/82), and Tectal group (D) 6% (5/82). Four hemispheres (5%) were unclassified (4/82) and revealed the small basal tributaries of the BVR without connection to the great vein of Galen.
No Galenic connections of the BVR were identified in 10% (8/82) of hemispheres.
Among them, four hemispheres (4/8) were unclassified and consisted only of small tributaries constituting the BVR.
Discussion
Embryologic aspects of regressed embryonic tentorial sinus with the BVR.
The tentorial sinus pattern in the adult has been reported as an anatomical variant by some angiographic studies 3,4. Those findings described the variations of the BVR and also mentioned the drainage of the BVR into the medial or lateral tentorial sinus 6. However, the relationships between the tentorial sinus of adults and that of the human embryo are not firmly established.
The embryonic tentorial sinus at the 24 mm stage drains the telencephalic and diencephalic territories and terminates in the straight sinus, torcular, or transverse sinus. Its tributaries are the superficial and deep telencephalic veins and the ventral and dorsal diencephalic veins 7. Dorsally, an anastomosis with the mesencephalic vein develops at the 40 mm stage, connecting to the future vein of Galen. At the 6080 mm stage, the connections between the ventral diencephalic system and mesencephalic vein become the lateral mesencephalic vein. The lateral mesencephalic vein anastomosing with the ventral metencephalic (great anterior cerebellar vein; future correspondence of superior petrosal sinus) vein sometimes becomes the only outlet of the BVR. The basal cerebral vein is formed by longitudinal anastomosis between several primitive pial veins: the deep (or the anterior) middle cerebral; the ventral and/or dorsal diencephalic veins; the mesencephalic vein; and a tributary of the primitive straight sinus 7.
In 60-80 mm stage, the embryonic tentorial sinus regresses and most of the venous channels constitute the embryonic segments of the BVR. Failing anastomosis at this stage, the diencephalic and mesencephalic veins will lead to different patterns, draining these venous territories supratentorially, infratentorially, or posteriorly.
Therefore, portions of the BVR may drain: 1) supratentorially and anteriorly toward veins of the middle cranial fossa, the superficial middle cerebral vein, or the dural sinuses of the middle cranial fossa; 2) infratentorially toward the anteromedian and anterolateral pontine and superior petrosal vein via the interpeduncular and lateral mesencephalic veins, respectively; and 3) posteriorly into an internal cerebral vein or the Galenic system of veins.
From a developmental standpoint, drainage of the BVR into an embryonic tentorial sinus illustrates the persistence of this early drainage pattern into the regressed remnant tentorial sinus of Padget (figure 3). Drainage of the BVR into a tentorial sinus could occur in the early stage of hemispheric expansion and represent another form of persistence of a portion of the regressed embryonic tentorial sinus constituting the posterior venous outflow into the straight sinus, torcular or transverse sinus. This particular anatomic configuration, that is, a tentorial venous sinus draining the BVR, is probably developmentally distinct from the frequently observed adult tentorial sinuses which receive cortical tributaries from the inferior aspects of the cerebral hemispheres and the cerebellar hemispheres4. In many cases, the posterior cerebellar veins are collected by a channel or a lake that constitutes a cerebellar tentorial sinus7,8. This lacuna, sometimes duplicated, lies in the tentorium near the torcular, and may be confluent with the caudal end of the cerebral tentorial sinus, prominent in prenatal life7. The morphologic characteristics of the remnant tentorial sinuses are quite specific to all of the cases illustrated in our study. The tentorium cerebelli, anatomically demarcated by cerebellar hemispheres, a space layering on top of the folia, exquisitely confines the remnant tentorial sinuses as multiple saw-tooth appearances on CT angiography. These descriptions are quite unique in our results and have not been described elsewhere.
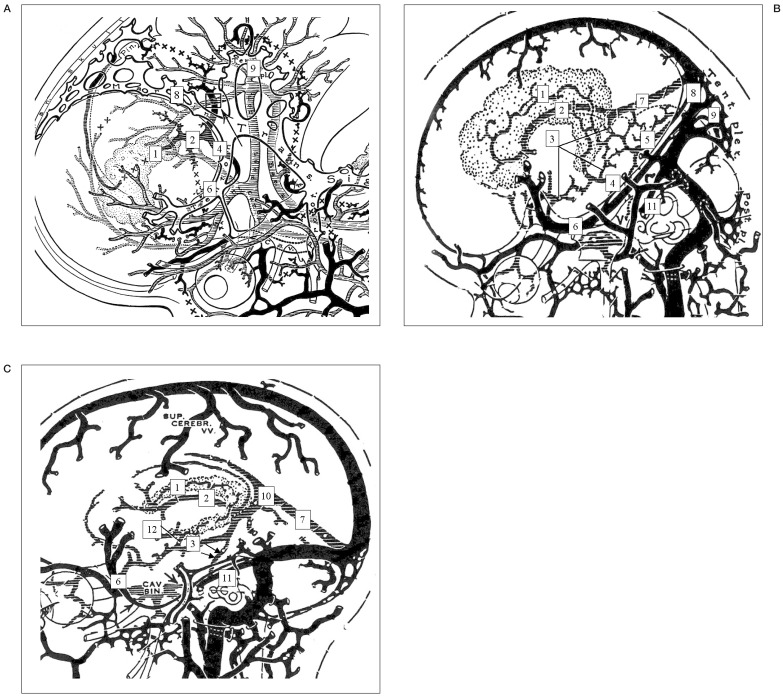
Figure 3.
Embryologic draining (anastomotic) patterns between the BVR and remnant embryonic tentorial sinus according to each stage of development by Padget DH7. A) Stage 6, 22-24 mm stage. Primarily the ventral diencephalic veins, supplemented by the primitive internal cerebral vein, drain the conspicuous choroid plexus of the lateral ventricle. B) Stage 7a, 60-80 mm stage. The internal cerebral vein is still primitive and constitutes the drainage of the large superior choroid vein into the straight sinus. The basal cerebral vein is formed by longitudinal anastomoses between several primitive pial veins, the deep middle cerebral, the ventral or dorsal diencephalic veins, the mesencephalic vein, and a tributary of the primitive straight sinus. C) Infant or adult stage. A lateral mesencephalic vein (LMV; arrows) anastomosing the basal veins with the ventral metencephalic veins sometimes become the only outlet of the basal veins.
1,superior choroidal vein;2,primitive internal cerebral vein; 3, primitive BVR; 4, ventral diencephalic vein; 5, mesencephalic vein, 6, embryonic tentorial sinus; 7, straight sinus; 8, marginal sinus (primitive transverse sinus); 9, tentorial plexus; 10, vein of Galen; 11, superior petrosal sinus (great anterior cerebellar vein); 12, inferior ventricular vein.
These findings can be explained according to the developments in 60-80 mm stage. The regressed remnant tentorial sinus could be directed toward the primitive transverse sinus before the tentorial plexus, which becomes the torcular. Then, the transverse sinus swung backward to become plexiform caudally as it is shifted toward the torcular. Although caudal remnants of the tentorial sinus traversing the free tent-portion of the tentorium are sometimes found, particularly at birth, it commonly ends in the transverse sinus just behind its junction with the sigmoid; or sometimes in the superior petrosal sinus, which it has previously crossed to reach the transverse sinus near the torcular 7. Therefore, the lateral tentorial sinus with the BVR should necessarily represent a typical remnant embryonic tentorial sinus.
The medial tentorial sinus with the BVR is not a continuation from the typical remnant tentorial sinus, but could be demonstrated as the patterns of anastomosis presenting between: 1) the lateral mesencephalic vein and 2) the mesencephalic vein to pial tributaries of the straight sinus.
The terminologies of the lateral and medial tentorial sinus with the BVR are not yet definitely determined and have been described as a misconception 6. However, the medial tentorial sinus constituting the BVR is a relatively frequent variation encountered in our series with consistent patterns: the BVR were drained by the telencephalic and diencephalic tributaries and emptied into the straight sinus and the confluences with the torcular which had been verified on previous angiographic study3.
Clinical Implications of BVR Variations
The anatomy and pathophysiology of the cerebral venous system have been underestimated due to theoretical preoccupations with the arterial blood supply and circulation of CSF9. The understanding of the development of the vascular system in relation to its territories and adjacent structures facilitates the anticipation of possible anatomic variations and clinical syndromes.
The importance of cerebral venous anatomy in interventional neuroradiology is still fundamental and should be reemphasized considering the potential understanding of various cerebral venous diseases.
The variations of BVR should be assessed especially in patients of remote cerebellar haemorrhages 10. Remote cerebellar haemorrhage (RCH) is an under-recognized complication following supratentorial craniotomies, specifically those involving opening of CSF cisterns or the ventricular system. Preoperative aspirin use and moderately elevated intraoperative blood pressures are potentially modifiable risk factors associated with the development of RCH10.
Cerebellar "sag" as a result of CSF volume loss, causing transient occlusion of superior bridging cerebellar veins and consequent haemorrhagic venous infarction, is the most likely cause of RCH. A pathophysiologic understanding of anatomic variations of those superior cerebellar veins coud help to identify the cause of haemorrhagic venous infarction in RCH. The medial or lateral tentorial sinuses that invariably receive the deep venous flow from telencephalic and diencephalic tributaries could result in certain types of illustrated cases.
From a neurosurgeon's perspective, preoperative understanding of the variant venous anatomy is critical to minimize complications resulting from venous outflow obstruction during various transtentorial approaches.
The importance of the medial or lateral tentorial sinuses constituting basal venous flow patterns has not yet been verified, but must be evaluated and a different operative approach should be applied to reduce the likelihood of venous complications. If the tentorial sinuses are the major outflow pathways of the basal cerebral tributaries without a route of collaterals, this could lead to potentially devastating outcomes.
Conclusions
Anatomic variations of SMCV can be clearly demonstrated with embryologic aspects of the tentorial sinus according to its developmental regression and postnatal secondary adaptations of cerebral venous drainage. Before any transtentorial approaches, both neuroradiologists and neurosurgeons must scrutinize the strategy of preserving normal venous drainage carefully using non-invasive imaging methods such as CT angiography.
References
- 1.Lasjaunias P, Berenstein A, ter Brugge KG. Clinical Vascular Anatomy and Variations. 1 2nd edition. Berlin: Springer-Verlag; 2001. Intracranial venous system. Surgical Neuroangiography. [Google Scholar]
- 2.Wolf BS, Huang YP, Newman CM. The lateral anastomotic mesencephalic vein and other variations in drainage of the basal cerebral vein. Am J Roentgenol Radium Ther Nucl Med. 1963;89:411–422. [PubMed] [Google Scholar]
- 3.terbrugge K, Lasjaunias P. Tentorial sinus. Radiologic and anatomic features of a case. Surg Radiol Anat. 1988;10:243–246. doi: 10.1007/BF02115244. [DOI] [PubMed] [Google Scholar]
- 4.San Millan Ruiz D, Fasel JH, et al. Bilateral tentorial sinus drainage of the basal vein (of Rosenthal) Clin Anat. 2003;16:264–268. doi: 10.1002/ca.10070. [DOI] [PubMed] [Google Scholar]
- 5.Casey SO, Alberico RA, et al. Cerebral CT venography. Radiology. 1996;198:163–170. doi: 10.1148/radiology.198.1.8539371. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki Y, Ikeda H, et al. Variations of the basal vein: identification using three-dimensional CT angiography. Am J Neuroradiol. 2001;22:670–676. [PMC free article] [PubMed] [Google Scholar]
- 7.Padget DH. The cranial venous system in man in reference to development, adult configuration, and relation to the arterie. Am J Anat 1956; 1956;98:307–355. doi: 10.1002/aja.1000980302. [DOI] [PubMed] [Google Scholar]
- 8.Natarajan M, Paramasivam P. Tentorial venous sinuses: an anatomic study anatomical report. Neurosurgery. 1998;42:363–371. doi: 10.1097/00006123-199802000-00097. [DOI] [PubMed] [Google Scholar]
- 9.Andeweg J. The anatomy of collateral venous flow from the brain and its value in aetiological interpretation of intracranial pathology. Neuroradiology. 1996;38:621–628. doi: 10.1007/s002340050321. [DOI] [PubMed] [Google Scholar]
- 10.Friedman JA, Piepgras DG, et al. Remote cerebellar haemorrhage after supratentorial surgery. Neurosurgery. 2001;49:1327–1340. doi: 10.1097/00006123-200112000-00008. [DOI] [PubMed] [Google Scholar]