Abstract
Neuropetide galanin modulates a variety of central nervous system functions by signaling through three G-protein coupled receptor subtypes, GalR1 through GalR3. Galanin and its receptors are expressed at high levels in the limbic structures of the rodent brain. Intracerebroventricular injection of galanin has been shown to modulate depression and anxiety-like behaviors in the rat. We have previously shown that chronic antidepressant treatments increase the binding of a GalR2 preferring ligand, galanin (2–11), to the dorsal raphe nucleus (DRN) of the rat, which, along with the finding that intra-DRN infusion of galanin (2–11) increases the release of serotonin in the hippocampus, suggests that GalR2 signaling might exert antidepressant-like action by modulating ascending serotonergic outflow. Recently, two research groups reported their phenotypic analysis of a GalR2 knockout (GalR2KO) mouse line, produced by gene trapping method and maintained on a 129S1/SvImJ genetic background. The only positive finding in that GalR2KO mouse line was an anxiogenic-like phenotype specific to the elevated plus maze. Because it is known that genetic background can affect the outcome of behavioral tests, in the present study, we analyzed a separate GalR2KO line, which was produced by targeted deletion and maintained on a C57BL/6 backgroud, using a different set of depression and anxiety related tests. GalR2KO mice exhibited a more persistent depressive-like phenotype in the learned helplessness paradigm as well as increased immobility in the tail suspension test when results from the present studies were combined by fixed effect meta-analysis with that reported by Gottsch and colleagues. GalR2KO mutants showed anxiety-like behavior comparable to wild-type littermates in the elevated-plus maze, open field, and light-dark transfer tests. The present findings are consistent with a predicted antidepressant-like effect of GalR2 signaling, suggesting that GalR2 might be a valid drug target for depressive disorders.
Keywords: GalR2 knockout, depression, anxiety, desipramine
Introduction
Galanin, a neuropeptide first isolated from porcine intestine, is widely distributed in the central nervous system of the rodent brain, where it modulates a variety of physiological and pathological processes, including feeding, seizure, cognition, and endocrine function, through three G protein-coupled receptors, GalR1–3 (Bartfai et al., 1992; Crawley, 1995; Hokfelt et al., 1999; Branchek et al., 2000). A close link between galanin, monoamine and stress pathways has been established both morphologically and functionally (Melander et al., 1986; Xu and Hokfelt, 1997; Xu et al., 1998a), suggesting a possible role for galanin as a modulator of mood and anxiety. Regarding anxiety, intracerebroventricular injection of galanin in the rat has an anxiolytic-like effect in Vogel’s punished drinking test (Bing et al., 1993), while intra-amygdala injection of galanin produces anxiolytic-like effects in animals tested under heightened stress conditions in the elevated plus-maze (Khoshbouei et al., 2002b; Khoshbouei et al., 2002a; Morilak et al., 2003; Barrera et al., 2005). In mice, the α2 autoreceptor agonist, yohimbine, failed to produce an anxiogenic-like effect in galanin overexpressing mice (Holmes et al., 2002a), suggesting that galanin can modulate anxiety states induced by high levels of noradrenergic activation, but may be silent under less challenging situations (Karlsson et al., 2005).
With respect to depression-like behavior, the subtype non-selective galanin receptor agonists, galmic (Bartfai et al., 2004) and galnon (Lu et al., 2005), decreased immobility in the rat forced swim test. Conversely, a galanin receptor antagonist M40 blocked the antidepressant-like effect of chronic fluoxetine (Lu et al., 2005) in the same test, results which suggest that galanin receptor activation mediates antidepressant-like effects. In contrast, local infusion of galanin into the rat VTA reduced ambulation and rearing behaviors, and increased immobility in the forced swim test (Weiss et al., 1998), consistent with a depressant-like effect of galanin receptor activation in the VTA. However, the effect of galanin receptor signaling on depression is not as straightforward as a recent study failed to detect antidepressant-like effects of galnon in either the rat forced swim test or mouse tail suspension test (Rajarao et al., 2007). In rat, galanin icv was shown to increase immobility in the forced swim test (Kuteeva et al., 2007), whereas in mouse, galanin icv had no effect on immobility in the tail suspension test (Rajarao et al., 2007).
The regionally-specific effect of galanin receptor activation on forced swim immobility might be attributable to the fact that galanin effects are mediated by three distinct and differentially expressed receptor subtypes. GalR1 and GalR3 are predominately coupled to Gi and activation of GalR1 and GalR3 leads to a decrease in cAMP and the opening of potassium channels; GalR2 is coupled to Gq/11 and Gq and its activation, primarily leads to an increase in intracellular calcium (Smith et al., 1998; Wang et al., 1998). GalR1 is the most widely expressed galanin receptor subtype in the brain, followed by GalR2 and GalR3, which display successively more restricted expression patterns (O’Donnell et al., 1999). GalR1 receptors are thought to mediate some anxiolytic actions of galanin, because GalR1 knockout mice exhibit increased anxiety-like behavior in the elevated plus maze (Holmes et al., 2003). In contrast, antagonists of GalR3 are currently being tested in clinical trials for affective disorders as they produce antidepressant and anxiolytic-like effects in a variety of preclinical behavioral tests conducted in rat, mouse and guinea pig (Swanson et al., 2005). Several lines of evidence suggest that GalR2 receptors may also modulate mood and anxiety. GalR2 mRNA has been localized to the dentate gyrus of the hippocampus, hypothalamus, amygdala, locus coeruleus and dorsal raphe nucleus (O’Donnell et al., 1999). In the rat, chronic fluoxetine treatment increases binding of the GalR2-preferring ligand, galanin(2–11), in the dorsal raphe nucleus, which may correspond to an increase in GalR2 signaling (Lu et al., 2005). Thus, we have hypothesized that increased GalR2 signaling in the dorsal raphe may promote an antidepressant-like action. Accordingly, microinfusion of galanin (2–11), a GalR2-preferring agonist, but not galanin, a pan-GalR agonist, into rat dorsal raphe nucleus, increases 5-HT release in the ventral hippocampus (Mazarati et al., 2005).
Recently, two research groups have reported their independent phenotypic analysis of anxiety- and depression-related behaviors in GalR2 knockout mice. Gottsch et al reported no genotype effects in the tail suspension, light-dark transfer test, or stress induced hyperthermia (Gottsch et al., 2005) tests; Bailey et al recently reported increased anxiety-like behavior in the elevated plus-maze in the GalR2 knockout line (Bailey et al., 2007). Both groups studied the same GalR2KO mouse line, produced by retroviral gene trapping, on a 129S1/SvImJ genetic background. It is known that genetic background can affect the outcome of behavioral tests, and different methods of gene inactivation might also bring about different unexpected consequences (Phillips et al., 1999; Holmes et al., 2002b). For example, Holmes et al. found that behavior of serotonin transporter knockout mice in tests sensitive to antidepressant action differed on a 129S6, but not C57BL/6J background, as compared to wildtype littermates (Holmes et al., 2002b). Therefore, in the present study, we measured behavior of a different GalR2KO mouse line, which was produced by the gene targeting method (Shi et al., 2006), and maintained on the C57BL/6 genetic background, in several tests of depression- and anxiety-related behavior. Potential GalR2KO genotype moderation of antidepressant-like activity of desipramine in the forced swim test also was determined.
Material and Methods
The GalR2KO mice were generated on a 129/Sv genetic background and backcrossed into C57BL/6 mice (Charles River, MA) for six generations at Deltagen (San Carlos, CA) (Shi et al., 2006). Upon receipt at the Scripps Research Institute, mice were backcrossed for two additional generations with C57BL/6 mice of the Scripps in-house colony (descended four generations from Jackson Laboratories). Reverse transcriptase-PCR demonstrated the absence of the intact GalR2 transcript, as previously reported (Shi et al., 2006). Age-matched adult male mice (3 to 5 month old at the start of the experiments; 6 to 8 month old when the desipramine sensitivity test was performed) that were littermates from heterozygous matings were used in the study. Because N8 backcrossed offspring, on average would still retain ~0.2% 129/Sv genetic background, wildtype littermates were used as controls in all studies. The same cohort of animals was used in all the studies, per the following test sequence: elevated plus-maze->light-dark transfer-> forced swim test->tail suspension-> open field test->learned helplessness. Tests were spaced by at least 1 week, and the order of testing was chosen such that tests involving lower stress levels (elevated plus-maze, light-dark transfer), preceded those involving higher stress levels (forced swim, tail suspension, learned helplessness). The elevated plus-maze was performed first of all tests because it is especially sensitive to previous testing history (Bailey et al., 2007). The forced swim test was performed one week before the tail suspension test, because it has previously been shown that forced swim testing does not confound results in a tail suspension test performed 1 week later (Cryan et al., 2004). Six weeks after the learned helplessness test, mice were again tested in the forced swim to compare effects of GalR2KO genotype on sensitivity to desipramine treatment. Mice were allowed to habituate to the testing enviornment for 1 hr prior to all tests. All tests were videotaped and later scored by a trained observer who was unaware of both genotype and drug treatment. Slight differences in sample sizes (n) across tests are the result of ocassional recording failures or of animals that did not complete testing due to events such as falling off the apparatus and tail climbing.
All experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of the Scripps Research Institute. Animals were housed under standard conditions on a 12-h light/dark cycle with ad libitum access to food and water. Among the 32 mice used in the study, 28 were group-housed, 2 to 4 per cage, with same-sex littermates, and the remaining 4 mice, including 2 wildtypes and 2 knockout, were sigularly housed. As the number of singlularly housed animals were matched between the genotypes and the performance of sigularly housed animals didn’t seem to deviate from those of group housed mice in our experiments (within one standard deviation), they were included in the data analysis.
Elevated plus maze
The elevated plus maze test was performed as described (Holmes et al., 2002a). The plus-maze consists of two open arms (30 cm × 5 cm) and two closed arms of same size extending from a central area (5 cm × 5 cm). The arms and central area were elevated 30 cm above the ground. The closed arms were enclosed by walls made of clear Plexiglas (30 cm high), whereas the open arms only had a 0.5 cm clear Plexiglas lip around their edges. Lighting and background white noise on the open arms were ~2 lux and 52dB, respectively. Mice were placed in the central area facing an open arm and allowed to explore for 5 min. Open and closed arm entries (all 4 paws in the arm) and time spent in the open arms, closed arms, and center square were scored. The % open arm time (or entries), an inverse measure of anxiety-like behavior, was calculated as the % of total arm time (or entries) that was open arm time (or entries).
Light-dark transfer
The light-dark transfer test was performed according to previously described methods with minor modifications (Crawley and Goodwin, 1980; Anseloni et al., 1995; Bailey et al., 2007). The test apparatus was a Plexiglas rectangular box divided into two unequal compartments by a black Plexiglas partition with a small hole at the base (7.5 × 7.5 cm). The smaller compartment (14.5 × 27 × 26.5 cm) was dark (~0 lux) and the larger compartment (28.5 × 27 × 26.5 cm) was highly illuminated (900 lux) with a 75 light source located above it. Mice were placed in the center of the light compartment facing away from the partition to initiate the test session. The number of transitions between the two compartments and time spent in the light compartment during a 10-min test session were scored.
Tail suspension test
Mice were individually suspended by the tail from a horizontal ring-stand bar that was elevated 50 cm above a table top using Fisher Scientific adhesive tape affixed 2 cm from the tip of the tail. The time spent immobile during a 6-min test period was measured.
Forced swim test
The 6-min forced swim test (FST) was conducted using a larger diameter cylinder than that originally used in order to increase the sensitivity and specificity of detecting antidepressant-like effects in mice (Sunal et al., 1994). For testing, mice were placed in individual, clear polypropylene cylinders (30-cm-tall × 30-cm-diameter) containing 23–25°C water, 23 cm-deep to prevent the mouse’s tail from touching the cylinder bottom (Detke and Lucki, 1996). The water was changed between subjects. A time-sampling technique was used whereby the predominant behavior in each 5-s period of the 300-s test was recorded. Climbing behavior consisted of upward-directed movements of the forepaws, usually along the side of the swim chamber. Swimming behavior was defined as horizontal movement throughout the swim chamber, which usually included crossing into another quadrant. Immobility was assigned when no additional activity was observed other than that required to keep the mouse’s head above water. The immobility, swimming, and climbing counts were scored (Cryan and Lucki, 2000).
Open field test
The open field test apparatus was a square, walled arena (50 × 50 × 22 cm) with white Plexiglas walls and floor. The floor was marked into 16 equal squares, and the central four squares were defined as the center area. Mice were placed in the center of the open field and allowed to explore freely for 10 min. The test was performed under ambient room light (~450 lux) with a background white noise of 52 dB. Line-crossing behavior (defined as at least three paws in a square) and time spent in the center were quantified.
Learned helplessness paradigm
The learned helplessness paradigm was performed as described previously (Malberg and Duman, 2003; Chourbaji et al., 2005). Briefly, mice were subjected to unpredictable, inescapable foot shock sessions on two consecutive “induction” days and then on a third day tested for the presence of learned helplessness behavior, operationalized as failures to escape footshock in a shuttlebox. Sessions took place in a Gemini Active Avoidance apparatus (San Diego Instruments, San Diego, CA), which consisted of two chambers equipped with a stainless-steel grid floor. To induce learned helplessness, 360 footshocks were delivered on two consecutive training days (180 footshocks/day). A retractable metal door separating the two chambers was fixed in a closed position, such that shocks were inescapable, and footshocks (2 sec, 0.15 mA) were delivered on a pseudorandom, unpredictable schedule (1–15 sec intershock interval), over a total session duration of approximately 52 min.
Twenty four hours following training, mice were subjected to a 31-trial test for learned helplessness behavior. In each trial, mice were presented with a 5-s cue light plus overhead light (located on the side wall opposing the door and on the top of the test compartment, respectively), which would then be followed by a 10 s light + shock (0.150 mA, interval=30 s average, 25–35 s range) compound stimulus. The door between the two sides of the shuttle box opened with the onset of the cue light stimulus. If the animal avoided or escaped the shock by shuttling to the unshocked compartment, the light was turned off, and the gate was closed until the onset of the next trial. If the animal failed to escape, the shock was terminated at the end of the trial and the gate was closed. Behavioral outcomes of each trial were defined as follows: “avoidance,” shuttling to the other compartment following the onset of the cue light stimulus and before the onset of the shock; “escape,” shuttling to the other compartment after the onset of the electric shock, but before its termination, or “escape failures,” not shuttling to the other compartment before the end of the shock. The number of escape failures, escapes, and avoidances, as well as latencies of successful avoidances/escapes, were recorded.
Desipramine treatment and forced swim testing
Desipramine hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved freshly in 0.9% NaCl. Sixteen hours before drug treatment, mice were individually-housed, a strategy aimed to eliminate variations in the stress imposed on the mice due to sequential separation of group housed animals at the time of injection. Mice received desipramine administration (i.p., 8.5 mg/kg) in a volume of 5 ml/kg, 45 min prior to the forced swim test, which was performed as described above.
Statistics
Data on the effect of desipramine on forced swim behavior were analyzed by two-way factorial ANOVA with Genotype and Desipramine as between-subject factors. Bonferroni-corrected post hoc tests were used to interpret significant results. Data from learned helplessness testing were subject to two-way ANOVA with Genotype as a between-subject factor and Trial Block as a within-subject factor. Other data were analyzed using Student’s t-test.
Results
Elevated plus-maze
As summarized in Table 1, GalR2KO mice did not differ from wild type mice in anxiety-like behavior in the elevated plus-maze, as defined by absolute (open arm time or entries) or relative (% open arm time or entries) measures of open arm exploration. However, GalR2KO mice showed significantly fewer total arm entries as compared to wild type mice (p=0.03).
Table 1.
Elevated Plus Maze
| WT (mean ± SEM, n=14) | GalR2KO (mean ± SEM, n=15) | Student’s t test | |
|---|---|---|---|
| Open arm time | 102.8 ± 16.3 | 97.8 ± 13.6 | P=0.90 |
| Closed arm time | 152.1 ± 14.9 | 140.2 ± 12.8 | P=0.90 |
| Center time | 51.3 ± 14.9 | 62.0 ± 6.9 | P=0.74 |
| % open arm time | 39.6 ± 5.8 | 40.8 ± 5.6 | P=0.88 |
| Open arm entries | 11.1 ± 1.0 | 9.5 ± 1.1 | P=0.30 |
| Closed arm entries | 11.9 ± 1.1 | 9.2 ± 0.7 | P=0.05 |
| Total arm entries | 22.9 ± 1.4 | 18.7 ± 1.2 | *P=0.03 |
| % open arm entries | 48.6 ± 3.9 | 48.7 ± 4.5 | P=0.99 |
Light dark transfer
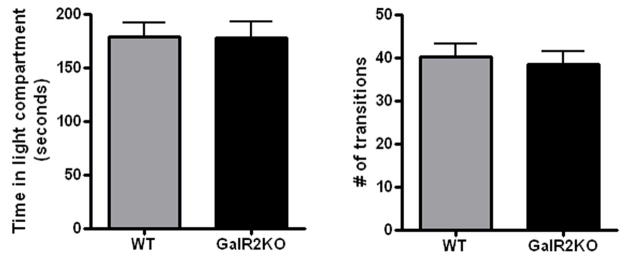
Wild type (n=16) and GalR2KO mice (n=16) did not differ in the time spent in the light compartment (mean ± SEM, 179.1 ± 12.7 vs. 170.3 ± 14.2 s, p=0.92) or the total number of transitions (40.1 ± 3.5 vs. 38.4 ± 3.2, p=0.69) made in the light-dark box test (Figure 1).
Figure 1.
Light dark transfer.
There was no significant difference between the wild type and GalR2KO in the time spent in the light compartment or the total number of transitions in the light dark transfer test (n=16 wild type, 16 GalR2KO). All bars represent mean values with vertical lines indicating 1 SEM.
Tail suspension test
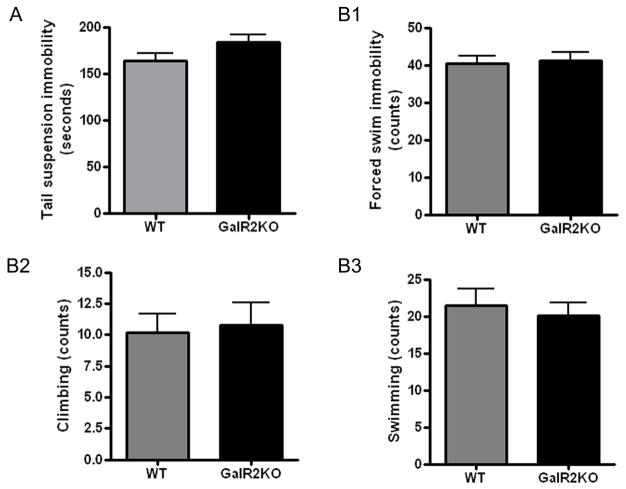
Wild type (n=15) and GalR2 knockout mice (n=14) showed similar total immobility time in the tail suspension test (164.2 ± 8.4 vs. 183.9 ± 8.08, p=0.16) (Figure 2A). Immobility time also did not differ per genotype if the test session was subdivided into the first 2 min vs. last 4 min of observation (data not shown).
Figure 2.
Tail suspension (A) and forced swim test (B). There was no significant difference between the wild type and GalR2KO in immobility in the tail suspension test (left) and in the forced swim test (right) (n=15 wild type, 14 GalR2KO). All bars represent mean values with vertical lines indicating 1 SEM.
Forced swim test
Untreated wild type (n=16) and GalR2 (n=16) knockout mice did not differ in counts of immobility (mean ± SEM, 40.36 ± 0.20 vs 41.13 ± 2.40, p=0.63), swimming (21.43 ± 0.22 vs 20.07 ± 1.73, p=0.80) or climbing (10.21 ± 0.15 vs 10.80 ± 1.77, p=0.81), during the 6-min forced swim test (Figure 2B). Again, similar performance was observed between genotypes if the test session was subdivided into the first 2 min vs. last 4 min of observation (not shown).
Open field test
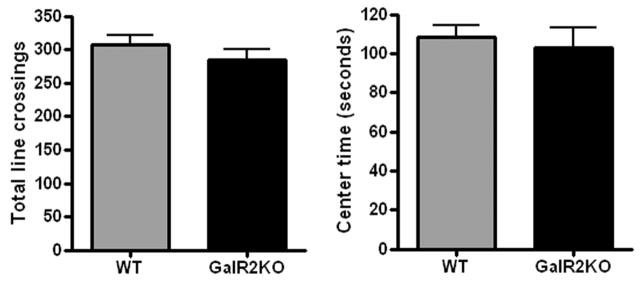
There was no significant difference between wild type (n=16) and GalR2 knockout mice (n=16) in locomotor activity during the open-field test as measured by total line crossings (mean ± SEM, 298.4 ± 14.7 vs. 275.6 ± 15.7, p=0.29) (Figure 3). Genotypes also did not differ in the time spent in the center area (106.6 ± 6.8 vs. 97.4 ± 11.5 s, p=0.49).
Figure 3.
Open field test. There was no significant difference between the wild type and GalR2KO in general locomoter activity as measured by total line crossings or in center time (n=16 wild type, 16 GalR2KO). All bars represent mean values with vertical lines indicating 1 SEM.
Learned helplessness
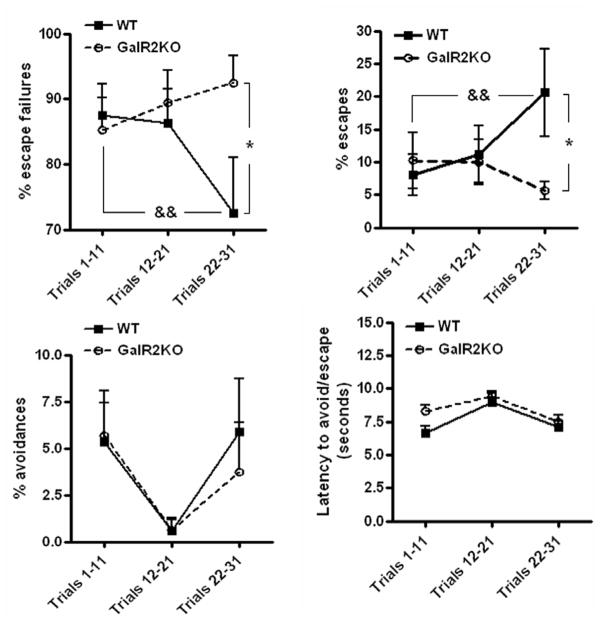
There were significant Trial Block [within-subject] x Genotype [between-subject] interactions for % escape failure [F(2, 60)=5.89, p=0.004] and for % escape [F(2,60)=5.0, p=0.01]. These interactions reflected that wild type (n=16) and GalR2KO mice (n=16) showed similarly high levels of escape failures during the initial 11 trials of learned helplessness testing (Figure 4). However, with continued testing, performance of wild type mice had improved significantly by the 3rd trial block of testing (trials 22–31) (% escape failures ± SEM, 1st trial block vs. 3rd trial block, 87.5 ± 4.7, 72.5 ± 8.4, p<0.01, Bonferroni t test; Figure 4). In contrast, performance of GalR2 KO mice had not improved, leading to significantly more escape failures in GalR2 KO mice than wild type mice during the final trial block (GalR2KO vs. WT, 92.5 ± 4.1 %, 72.5 ± 8.5 %, p<0.05; Figure 4). Inspection of Figure 4 also suggested greater variability in the performance of wildtype controls than of GalR2KO mutants during block 3 of learned helplessness testing, which was confirmed by Bartlett’s test (p=0.0158). Visual inspection of frequency histograms and Kolmogorov-Smirnov tests indicated that the greater variability in wildtype performance still conformed to a normal or log-normal, rather than bimodal, distribution. Differential escape performance did not appear to relate to variability in earlier forced swim or tail suspension performance either within or across genotypes.
Figure 4.
The learned helplessness data were analyzed in 3 blocks that consisted of 10 to 11 trials each. All bars represent mean values with vertical lines indicating 1 SEM. There were significant Trial Block [within-subject] x Genotype [between-subject] interactions for % escape failure [F(2, 60)=5.89, p=0.004] and for % escape [F(2,60)=5.0, p=0.01]. There was significant differences between genotypes in percent escape failures and percent escapes in the last 10 trial block (n=16 wild type, 16 GalR2KO) and significant differences in wild type mice between the last 10 trial block and first 11 trial block in percent escape failures and percent escapes. *, p<0.05, &&, p<0.01, Bonferroni t test.
Further analysis of differences in escape deficits showed that wildtype mice made more successful escapes, but not avoidances, across trial blocks (% escape ± SEM, 1st trial block vs. 3rd trial block, 8.0 ± 3.1, 20.6 ± 6.7, p<0.01; Figure 4), whereas performance of GalR2KO did not improve across trial blocks, leading to significantly fewer escapes, but not avoidances, by GalR2 KO mice as compared to wildtype mice within the final trial block (GalR2KO vs WT, 5.6 ± 3.8 %, 20.6 ± 6.7 %, p<0.05) (Figure 4). There was no difference between wildtype and GalR2KO in the latency to avoid/escape in any of the 3 blocks.
Effect of desipramine in forced swim test
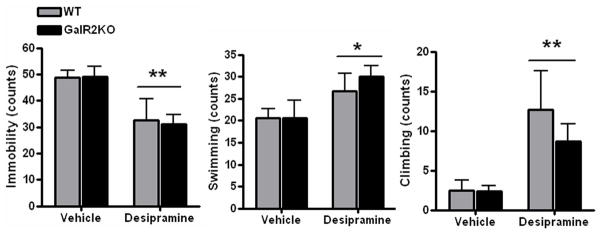
Desipramine drug treatment had a significant main effect on forced swim immobility [F(1,27)=14.87, P=0.0006], with no significant effects of Genotype or Drug Treatment x Genotype interactions observed (Figure 5). Likewise, there was a main effect of Drug Treatment on swimming [F(1,27)=5.9, p=0.023] and climbing behaviors [F(1,27)=11.7, p=0.002] (Figure 5). There was no significant Genotype or Drug Treatment × Genotype effects observed for these two behaviors.
Figure 5.
Effect of desipramine treatment on active behaviors in the forced swim test. There was a significant main effect of drug treatment [F(1,27)=14.87, P=0.0006], but not genotype, and no significant drug treatment x genotype interaction, on immobility. Likewise, there was a main effect of drug treatment on swimming F(1,27)=5.9, p=0.023] and climbing behaviors [F(1,27)=11.7, p=0.002], but no significant effect of genotype or significant drug treatment × genotype interaction was observed in these two behaviors. All bars represent mean values with vertical lines indicating 1 SEM. *, p<0.05; **, p<0.01.
Discussion
In the initial characterization of the present GalR2KO mutant line, mice with targeted deletion of the GalR2 gene did not differ from their wildtype littermates in general health, performance in sensory and neurological screens, or baseline pain thresholds (Shi et al., 2006). In the present study, GalR2KO mice also were found not to differ from their wildtype littermates in three assays of anxiety-like behavior (elevated plus-maze, light dark transfer, open field center time) or two tests of depression-related behavior (tail suspension, forced swim test). The present negative findings are generally consistent with results obtained in previous studies of another GalR2KO mouse line, obtained via gene-trapping and studied on a 129S rather than C57BL/6 genetic background (Gottsch et al., 2005; Bailey et al., 2007). Previous studies of GalR2KO mice examined subjects in the tail suspension, light-dark transfer, elevated plus-maze and stress-induced hyperthermia tests. Most of these assays were included in the present studies, in which potential genotype differences in the forced swim, learned helplessness, and open field tests also were measured.
A possible positive finding from the present studies is that GalR2 KO mice did not show the reduction of escape deficits that was seen across trials of learned helplessness testing in wildtype mice. Thus, although GalR2KO mice developed a similarly high initial degree of escape deficits following inescapable shock as did wildtype mice, GalR2 KO mice thereafter failed to begin to show reversal of escape deficits across repeated trials in which escape was possible. It would be of interest to determine whether GalR2 deficiency similarly attenuates restoration of escape behavior in the “straw suspension” modified forced swim test, in which a potential escape opportunity is introduced following acquisition of immobility (Nishimura et al., 1988). On the other hand, perhaps GalR2 deficiency impairs reversal learning. It is worth noting that GalR2KO mice on a 129S background did not show deficits in acqusition of spatial navigation in the Morris water maze and performed comparably to their wildtype littermates in fear conditioning (Gottsch et al., 2005; Bailey et al., 2007), so they are not generally cognitively impaired. Another alternative is that the persistent escape deficits seen in GalR2KO mice might reflect a phenotypic difference in stress-induced analgesia, a possible mediator of learned helpless behavior (Snow et al., 1982; Maier et al., 1983; Hunziker, 1992; Teixeira et al., 1997). Indeed, because serotonergic systems in the dorsal raphe subserve stress-induced analgesia (Snow et al., 1982), a role for GalR2 systems in modulating stress-induced analgesia via serotonin is possible. Directly measuring nociceptive thresholds or comparing actions of opioid receptor ligands between genotypes on the expression of escape deficits could help test this hypothesis.
Like Gottsch and colleagues, we observed a nonsignificant trend for GalR2 KO mice to exhibit increased immobility in the tail suspension test, an apparent replication of a negative finding. However, if the similar effect sizes for immobility seen in the present study (r=0.249) and that of Gottsch et al (r=0.221) are combined by fixed effect meta-analysis (Rosenthal and DiMatteo, 2001; Zorrilla et al., 2001), then GalR2 KO mice are actually found to exhibit slightly, but significantly, greater immobility in the tail suspension test than wildtype littermates (r[53]=0.235, p<0.05). This positive meta-analytic finding, combined with the more persistent depressive-like phenotype of GalR2KO mice in the learned helplessness test, leaves open the possibility that GalR2 signaling may have some antidepressant-like action (Lu et al., 2005). Also consistent with this hypothesis, chronic treatment (14 days) with fluoxetine (10 mg/kg i.p.; or desipramine, 15 mg/kg, ip, Lu and Bartfai, unpublished observation) previously was observed to increase binding of a GalR2-preferring ligand, galanin (2–11), to the dorsal raphe nucleus. Perhaps GalR2 activity in the dorsal raphe nucleus serves to maintain the function of ascending serotonergic efferents, because GalR2, as a Gq-coupled receptor, is excitatory in many cases (Kerekes et al., 2003; Mazarati et al., 2005).
Unlike Bailey et al., we did not observe GalR2KO mice to show increased anxiety-like behavior in the elevated plus maze. Rather, a decrease in plus-maze locomotor activity was observed, as represented in total arm entries. To facilitate comparison across studies and because the elevated plus-maze test is sensitive to previous testing history (Bailey et al., 2007), we here performed the elevated plus-maze test before all other tests and used a test apparatus resembling that used by Bailey and colleagues. Potential differences between studies which might account for the different results include the genetic background of GalR2KO mutants (C57BL/6 vs. 129S1/SvImJ; see also Holmes et al., 2002b), the method of GalR2KO inactivation (gene-targeting vs. gene-trapping), and the amount of illumination on the plus-maze (2 vs. 20 lux). If genes that differ between the C57BL/6 and 129S genetic backgrounds moderate the anxiety- or depressive-like phenotype associated with GalR1 deficiency, it would be of interest to identify them. On the other hand, both studies similarly found that GalR2 KO mice did not show altered anxiety-like behavior in the light-dark box or open-field tests, and Bailey and colleagues likewise found no genotype difference in elevated zero-maze performance (Bailey et al., 2007). Collectively, the results indicate that mice constitutively deficient in GalR2 do not show broad changes in anxiety-like behavior.
A limitation of the current studies is that the same mice were tested in a series of tests. Thus, it is possible that earlier tests affected subsequent performance in a manner that obscured genotype effects. To mitigate this possibility, behavioral measures were ordered such that those tests that are more sensitive to testing history and which are less stressful or invasive were generally performed earlier in the series. Furthermore, the inclusion of desipramine administration in the forced swim test at the conclusion of testing confirmed that mice remained sensitive to antidepressant action despite their previous testing history. The history of testing with uncontrollable stressors may have contributed to the higher baseline forced immobility of the second, as compared to first, forced swim test session.
The stressful history also may have elevated the baseline rate of escape deficits seen during learned helplessness testing to levels higher than those seen by Chourbaji and colleagues employing similar inescapable footshock schedules with C57BL/6N mice (Chourbaji et al., 2005) or by Shanks and Anisman (Shanks and Anisman, 1993) studying inbred descendants of C57BL/6ByJ mice (mean escape latency: ~4 sec). Still, Shanks and Anisman (1988) in an earlier study that used the same test conditions as their subsequent study with C57BL/6ByJ mice (Shanks and Anisman, 1988) reported that C57BL/6J mice exhibited mean shock escape latencies of ~8 sec, performance similar to that of wildtype controls in the present study. Thus, it is possible that cohort or subtle substrain characteristics contributed to the higher rates of escape failures seen here. The present methodology also differed from previously cited studies in that the inescapable shock induction here was administered within the same apparatus that was used for subsequent escape testing. Under such conditions, Calderone and colleagues (2000) observed a mean escape latency of ~12 sec, even longer than that seen in the present study, in male C57BL/6J mice during 24-sec escape trials. Thus, perhaps the high rate of escape failures observed in the present study also partly reflected the use of 10-sec (Chourbaji et al., 2005), rather than 24-sec, escape trials (Shanks and Anisman, 1988, 1993; Caldarone et al., 2000).
Regarding negative genotype findings in forced swim testing, GalR2KO mice did not differ from wildtype mice in their spontaneous forced swim performance nor in the ability of the positive control desipramine to decrease immobility. Testing was performed using a modified forced swim test paradigm, in which an apparatus with a wider diameter was used to increase the sensitivity and specificity of the test (Sunal et al., 1994), and behavior was scored with a robust time-sampling method (Cryan and Lucki, 2000). The tricyclic antidepressant desipramine is considered primarily an inhibitor of the norepinephrine transporter. While it has been observed in rats that norepinephrine reuptake inhibitors primarily reduce forced swim immobility by increasing climbing, rather than swimming behavior (Detke et al., 1995; Lucki, 1997), desipramine administration to mice was here found to significantly increase both climbing and swimming behavior when data from both genotypes were analyzed together (the amplitude of effect was more pronouced on climbing than on swimming). GalR2KO mice did not differ from wildtype mice in behavioral responses to desipramine in the forced swim test (Figure 5). It will be of future interest to determine the sensitivity of GalR2 KO mice to a selective serotonin reuptake inhibitor, given known functional and anatomical relations between GalR2 and 5-HT systems (Melander et al., 1986; Xu et al., 1998b; Mazarati et al., 2005), and the reported ability of a subtype-non-selective galanin receptor antagonist to attenuate fluoxetine’s antidepressant action (Lu et al., 2005). In this context, Holmes et al. observed that GalR1KO mice did not have altered sensitivity to fluoxetine in the tail suspension test (Holmes et al., 2005).
In summary, our data showed that GalR2KO mice may exhibit a more persistent depressive-like phenotype in the learned helplessness paradigm as well as increased immobility in the tail suspension test, when the present data are combined by fixed effect meta-analysis with that reported previously by Gottsch and colleagues (Gottsch et al., 2005). These data are consistent with a predicted antidepressant-like effect of GalR2 signaling (Lu et al., 2005), though several alternative explanations remain to be evaluated. The limited amplitude of effect seen in our study suggests that GalR2 signaling may mediate a more subtle modulatory, rather than major mediating, effect on brain functions relevant to mood regulation. It is also possible that the limited depressive-like phenotypes observed here are due to developmental compensations. Future development of conditional GalR2 knockout mice will provide additional information on the role of GalR2 in depression and anxiety.
Acknowledgments
We thank Dr. Amanda Roberts for helpful discussions and comments. This study was supported by MH074055 and MH063080 (T.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anseloni VZ, Motta V, Lima G, Brandao ML. Behavioral and pharmacological validation of the elevated plus maze constructed with transparent walls. Braz J Med Biol Res. 1995;28:597–601. [PubMed] [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera G, Echevarria DJ, Poulin JF, Laforest S, Drolet G, Morilak DA. One for all or one for one: does co-transmission unify the concept of a brain galanin “system” or clarify any consistent role in anxiety? Neuropeptides. 2005;39:289–292. doi: 10.1016/j.npep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Bartfai T, Fisone G, Langel U. Galanin and galanin antagonists: molecular and biochemical perspectives. Trends Pharmacol Sci. 1992;13:312–317. doi: 10.1016/0165-6147(92)90098-q. [DOI] [PubMed] [Google Scholar]
- Bartfai T, Lu X, Badie-Mahdavi H, Barr AM, Mazarati A, Hua XY, Yaksh T, Haberhauer G, Ceide SC, Trembleau L, Somogyi L, Krock L, Rebek J., Jr Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. Proc Natl Acad Sci U S A. 2004;101:10470–10475. doi: 10.1073/pnas.0403802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing O, Moller C, Engel JA, Soderpalm B, Heilig M. Anxiolytic-like action of centrally administered galanin. Neurosci Lett. 1993;164:17–20. doi: 10.1016/0304-3940(93)90846-d. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, George TP, Zachariou V, Picciotto MR. Gender differences in learned helplessness behavior are influenced by genetic background. Pharmacol Biochem Behav. 2000;66:811–817. doi: 10.1016/s0091-3057(00)00271-9. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, Gass P. Learned helplessness: validity and reliability of depressive-like states in mice. Brain Res Brain Res Protoc. 2005;16:70–78. doi: 10.1016/j.brainresprot.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Biological actions of galanin. Regul Pept. 1995;59:1–16. doi: 10.1016/0167-0115(95)00083-n. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther. 2000;295:1120–1126. [PubMed] [Google Scholar]
- Cryan JF, O’Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, Page ME, Dalvi A, Thomas SA, Lucki I. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci U S A. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Zeng H, Hohmann JG, Weinshenker D, Clifton DK, Steiner RA. Phenotypic analysis of mice deficient in the type 2 galanin receptor (GALR2) Mol Cell Biol. 2005;25:4804–4811. doi: 10.1128/MCB.25.11.4804-4811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Broberger C, Diez M, Xu ZQ, Shi T, Kopp J, Zhang X, Holmberg K, Landry M, Koistinaho J. Galanin and NPY, two peptides with multiple putative roles in the nervous system. Horm Metab Res. 1999;31:330–334. doi: 10.1055/s-2007-978748. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Crawley JN. Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. J Mol Neurosci. 2002a;18:151–165. doi: 10.1385/JMN:18:1-2:151. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002b;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Holmes A, Li Q, Koenig EA, Gold E, Stephenson D, Yang RJ, Dreiling J, Sullivan T, Crawley JN. Phenotypic assessment of galanin overexpressing and galanin receptor R1 knockout mice in the tail suspension test for depression-related behavior. Psychopharmacology (Berl) 2005;178:276–285. doi: 10.1007/s00213-004-1997-1. [DOI] [PubMed] [Google Scholar]
- Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L, Vishwanath J, Saavedra MC, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Crawley JN. Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology. 2003;28:1031–1044. doi: 10.1038/sj.npp.1300164. [DOI] [PubMed] [Google Scholar]
- Hunziker MH. Opioid nature of learned helplessness and stress induced analgesia observed without re-exposure to shock. Behav Pharmacol. 1992;3:117–121. [PubMed] [Google Scholar]
- Karlsson RM, Holmes A, Heilig M, Crawley JN. Anxiolytic-like actions of centrally-administered neuropeptide Y, but not galanin, in C57BL/6J mice. Pharmacol Biochem Behav. 2005;80:427–436. doi: 10.1016/j.pbb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Kerekes N, Mennicken F, O’Donnell D, Hokfelt T, Hill RH. Galanin increases membrane excitability and enhances Ca(2+) currents in adult, acutely dissociated dorsal root ganglion neurons. Eur J Neurosci. 2003;18:2957–2966. doi: 10.1111/j.1460-9568.2003.03057.x. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Morilak DA. Modulatory effects of galanin in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuropsychopharmacology. 2002a;27:25–34. doi: 10.1016/S0893-133X(01)00424-9. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA. Behavioral reactivity to stress: amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol Biochem Behav. 2002b;71:407–417. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Wardi T, Hokfelt T, Ogren SO. Galanin enhances and a galanin antagonist attenuates depression-like behaviour in the rat. Eur Neuropsychopharmacol. 2007;17:64–69. doi: 10.1016/j.euroneuro.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Lu X, Barr AM, Kinney JW, Sanna P, Conti B, Behrens MM, Bartfai T. A role for galanin in antidepressant actions with a focus on the dorsal raphe nucleus. Proc Natl Acad Sci U S A. 2005;102:874–879. doi: 10.1073/pnas.0408891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Maier SF, Sherman JE, Lewis JW, Terman GW, Liebeskind JC. The opioid/nonopioid nature of stress-induced analgesia and learned helplessness. J Exp Psychol Anim Behav Process. 1983;9:80–90. [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Baldwin RA, Shinmei S, Sankar R. In vivo interaction between serotonin and galanin receptors types 1 and 2 in the dorsal raphe: implication for limbic seizures. J Neurochem. 2005;95:1495–1503. doi: 10.1111/j.1471-4159.2005.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander T, Hokfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A, Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci. 1986;6:3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Cecchi M, Khoshbouei H. Interactions of norepinephrine and galanin in the central amygdala and lateral bed nucleus of the stria terminalis modulate the behavioral response to acute stress. Life Sci. 2003;73:715–726. doi: 10.1016/s0024-3205(03)00392-8. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Tsuda A, Ida Y, Tanaka M. The modified forced-swim test in rats: influence of rope- or straw-suspension on climbing behavior. Physiol Behav. 1988;43:665–668. doi: 10.1016/0031-9384(88)90223-5. [DOI] [PubMed] [Google Scholar]
- O’Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- Phillips TJ, Hen R, Crabbe JC. Complications associated with genetic background effects in research using knockout mice. Psychopharmacology (Berl) 1999;147:5–7. doi: 10.1007/s002130051128. [DOI] [PubMed] [Google Scholar]
- Rajarao SJ, Platt B, Sukoff SJ, Lin Q, Bender CN, Nieuwenhuijsen BW, Ring RH, Schechter LE, Rosenzweig-Lipson S, Beyer CE. Anxiolytic-like activity of the non-selective galanin receptor agonist, galnon. Neuropeptides. 2007;41:307–320. doi: 10.1016/j.npep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, DiMatteo MR. Meta-analysis: recent developments in quantitative methods for literature reviews. Annu Rev Psychol. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- Shanks N, Anisman H. Stressor-provoked behavioral changes in six strains of mice. Behav Neurosci. 1988;102:894–905. doi: 10.1037//0735-7044.102.6.894. [DOI] [PubMed] [Google Scholar]
- Shanks N, Anisman H. Escape deficits induced by uncontrollable foot-shock in recombinant inbred strains of mice. Pharmacol Biochem Behav. 1993;46:511–517. doi: 10.1016/0091-3057(93)90538-5. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Hua XY, Lu X, Malkmus S, Kinney J, Holmberg K, Wirz S, Ceccatelli S, Yaksh T, Bartfai T, Hokfelt T. Sensory neuronal phenotype in galanin receptor 2 knockout mice: focus on dorsal root ganglion neurone development and pain behaviour. Eur J Neurosci. 2006;23:627–636. doi: 10.1111/j.1460-9568.2006.04593.x. [DOI] [PubMed] [Google Scholar]
- Smith KE, Walker MW, Artymyshyn R, Bard J, Borowsky B, Tamm JA, Yao WJ, Vaysse PJ, Branchek TA, Gerald C, Jones KA. Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem. 1998;273:23321–23326. doi: 10.1074/jbc.273.36.23321. [DOI] [PubMed] [Google Scholar]
- Snow AE, Tucker SM, Dewey WL. The role of neurotransmitters in stress-induced antinociception (SIA) Pharmacol Biochem Behav. 1982;16:47–50. doi: 10.1016/0091-3057(82)90011-9. [DOI] [PubMed] [Google Scholar]
- Sunal R, Gumusel B, Kayaalp SO. Effect of changes in swimming area on results of “behavioral despair test”. Pharmacol Biochem Behav. 1994;49:891–896. doi: 10.1016/0091-3057(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Blackburn TP, Zhang X, Zheng K, Xu ZQ, Hokfelt T, Wolinsky TD, Konkel MJ, Chen H, Zhong H, Walker MW, Craig DA, Gerald CP, Branchek TA. Anxiolytic- and antidepressant-like profiles of the galanin-3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299. Proc Natl Acad Sci U S A. 2005;102:17489–17494. doi: 10.1073/pnas.0508970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira NA, Pereira DG, Hermini AH. Effects of naltrexone and cross-tolerance to morphine in a learned helplessness paradigm. Braz J Med Biol Res. 1997;30:775–782. doi: 10.1590/s0100-879x1997000600012. [DOI] [PubMed] [Google Scholar]
- Wang S, Hashemi T, Fried S, Clemmons AL, Hawes BE. Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry. 1998;37:6711–6717. doi: 10.1021/bi9728405. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Bonsall RW, Demetrikopoulos MK, Emery MS, West CH. Galanin: a significant role in depression? Ann N Y Acad Sci. 1998;863:364–382. doi: 10.1111/j.1749-6632.1998.tb10707.x. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Hokfelt T. Expression of galanin and nitric oxide synthase in subpopulations of serotonin neurons of the rat dorsal raphe nucleus. J Chem Neuroanat. 1997;13:169–187. doi: 10.1016/s0891-0618(97)00043-4. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Shi TJ, Hokfelt T. Galanin/GMAP- and NPY-like immunoreactivities in locus coeruleus and noradrenergic nerve terminals in the hippocampal formation and cortex with notes on the galanin-R1 and -R2 receptors. J Comp Neurol. 1998a;392:227–251. doi: 10.1002/(sici)1096-9861(19980309)392:2<227::aid-cne6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Zhang X, Pieribone VA, Grillner S, Hokfelt T. Galanin-5-hydroxytryptamine interactions: electrophysiological, immunohistochemical and in situ hybridization studies on rat dorsal raphe neurons with a note on galanin R1 and R2 receptors. Neuroscience. 1998b;87:79–94. doi: 10.1016/s0306-4522(98)00151-1. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]