Abstract
In Alzheimer’s disease (AD) there is a significant loss of locus coeruleus (LC) noradrenergic neurons. However, recent work has shown the surviving noradrenergic neurons to display many compensatory changes, including axonal sprouting to the hippocampus. The prefrontal cortex (PFC) is a forebrain region that is affected in dementia, and receives innervation from the LC noradrenergic neurons. Reduced PFC function can reduce cognition and disrupt behavior. Because the PFC is an important area in AD, we determined if noradrenergic innervation from the LC noradrenergic neurons is maintained and if adrenoreceptors are altered postsynaptically. Presynaptic PFC α2-adrenoreceptor (AR) binding site density, as determined by 3H-RX821002, suggests that axons from surviving noradrenergic neurons in the LC are sprouting to the PFC of subjects with dementia. Changes in postsynaptic α1-AR in the PFC of subjects with dementia indicate normal to elevated levels of binding sites. Expression of α1-AR subtypes (α1A- and α1D-AR) and α2C-AR subtype mRNA in the PFC of subjects with dementia is similar to what was observed in the hippocampus with one exception, the expression of α1A-AR mRNA. The expression of the α1A-AR mRNA subtype is significantly reduced in specific layers of the PFC in subjects with dementia. The loss of α1A-, α1D- and α2C-AR mRNA subtype expression in the PFC may be attributed to neuronal loss observed in dementia. These changes in postsynaptic AR would suggest a reduced function of the PFC. Consequence of this reduced function of the PFC in dementia is still unknown but it may affect memory and behavior.
Keywords: norepinephrine, RX 821002, prazosin, α1-adrenoreceptor, α2-adrenoreceptor, sprouting, Alzheimer’s disease
Alzheimer’s disease (AD), a neurodegenerative disorder, is characterized by cognitive impairment and the loss of neurons in the cortex and hippocampus. An area of the cortex that has shown the greatest amount of neuronal loss in AD is the prefrontal cortex (PFC) (Braak and Braak, 1991; Masliah et al., 1993; Salat et al., 2001). A change in PFC neuronal function can affect learning and memory (Brozoski et al., 1979; Carli et al., 1983; Arnsten, 1993) as well as behavior (Birnbaum et al., 2004; Arnsten and Li, 2005). The role of the PFC in behavior may be important in the expression of disruptive agitation that is commonly observed in AD.
Noradrenergic neurons in the locus coeruleus (LC) send projections to the PFC where they can modulate neuronal activity. There is a significant loss of noradrenergic neurons in the LC in AD (Mann et al., 1980; Tomlinson et al., 1981; Bondareff et al., 1982; Marcyniuk et al., 1986; Chan-Palay and Asan, 1989; German et al., 1992). However, the surviving neurons in the LC appear to be compensating for the neuronal loss (Adolfsson et al., 1979; Cross et al., 1981; Mann et al., 1981; Perry et al., 1981; Tomlinson et al., 1981; Gottfries et al., 1983; Raskind et al., 1984; Palmer et al., 1987; Reinikainen et al., 1988; Tohgi et al., 1992; Elrod et al., 1997; Russo-Neustadt et al., 1998; Hoogendijk et al., 1999; Szot et al., 2000). Recently, our laboratory showed that the remaining noradrenergic neurons in the LC of AD and a related dementing disorder, dementia with Lewy bodies (DLB), showed three different compensatory changes: (1) an increase in tyrosine hydroxylase mRNA expression in the remaining neurons; (2) sprouting of dendrites into the peri-LC dendritic zone, as determined by α2-adrenoreceptor (AR) and norepinephrine transporter binding sites; and (3) sprouting of axonal projections into the hippocampus as determined by α2-ARs (Szot et al., 2006). In AD and DLB subjects, the number of hippocampal postsynaptic α1-ARs was normal to elevated (Szot et al., 2006). Expression of α1A- and α2A-AR mRNA in the hippocampus of AD and DLB subjects was not altered, but expression of α1D- and α2C-AR mRNA was significantly reduced in the hippocampus of AD and DLB subjects (Szot et al., 2006). The focus of this work is to examine noradrenergic innervation to the PFC and postsynaptic AR status in the PFC, a region substantially affected in AD (Braak and Braak, 1991; Masliah et al., 1993; Salat et al., 2001) and important in cognition and behavior (Brozoski et al., 1979; Carli et al., 1983; Arnsten, 1993, Birnbaum et al., 2004; Arnsten and Li, 2005). To determine if noradrenergic axons are sprouting to the PFC in AD and DLB subjects, α2-AR binding sites were measured. To determine if postsynaptic ARs are affected in the PFC, α1-AR binding sites were measured as well as in situ mRNA expression of the different α1- and α2-AR subtypes.
Experimental Procedures
Subjects
All postmortem tissue was obtained from the University of Washington Alzheimer’s Disease Research Center, where permission for use of tissue in scientific experiments was obtained. AD is characterized by the insidious onset and gradual progression of impaired memory, language, and executive function. Psychosis, agitation, and other behavioral disturbances characteristically appear late in the disease course. DLB, which accounts for ~20% of patients with late-life dementia, presents early in its course with psychotic symptoms such as visual hallucinations and with fluctuating cognition and pronounced attentional deficits and often with bradykinesia and increased muscle tone (McKeith et al., 1996; Ballard et al., 1999; Barber et al., 2001). The subjects used in this study were the same subjects used in a previous study measuring changes in the noradrenergic nervous system in the LC and hippocampus and were described in detail in the previous publication (Szot et al., 2006). Briefly, PFC was studied in the following 17 nondemented age-comparable control subjects, with an age range of 38-90 years (mean ± SEM, 71.4 ± 3.5 years), seven males and 10 females with an average postmortem delay (PMD) of 8.5 ± 0.9 h; 15 AD subjects with an age range of 37-94 years (mean ± SEM, 68.6 ± 4.4 years), six males and nine females with an average PMD of 7.4 ± 0.9 h; and 22DLB subjects with an age range of 63-98 years (mean ± SEM, 79.5 ± 1.6 years), 16 males and six females with an average PMD of 8.0 ± 0.7 h. AD subjects met the National Institute on Aging Reagan criteria for AD (Braak stage IV/C or higher with no vascular dementia, frontotemporal dementia, or Lewy body pathology) (McKhann et al., 1984). DLB subjects met the same neuropathological diagnostic criteria for AD plus had the presence of Lewy body pathology in the brainstem and limbic regions, confirmed by α-synuclein immunohistochemistry.
Tissue
PFC tissue was accessed at autopsy using a protocol that provided a portion of the PFC in snap-frozen blocks. The region of the cortex where the PFC was obtained was consistent among all subjects. The size of the block was based on the dimensions of a standard slide that was used in the experiments. The fresh tissue block for each individual was dissected into 1-cm-thick coronal blocks, snap frozen in liquid nitrogen-cooled isopentane, and stored at −70°C. Serial coronal sections (20 μm) were cut on a cryostat, thaw mounted onto FisherSuper frost slides, and stored at −70°C for each individual.
3H-Prazosin and 3H-RX821002 binding sites
α1-AR binding sites were measured in the PFC with 3H-prazosin (PerkinElmer, Boston, MA) as previously described (Szot et al., 2005, 2006). Briefly, for each subject four consecutive slides, each containing a section of the PFC was run: three slides for total binding and the fourth for nonspecific binding. Nonspecific binding was defined in the presence of 10 μm phentolamine. Slides and 3H standards were apposed to Biomax MR film (Eastman Kodak, Rochester, NY) for 8 weeks. Films were developed and analyzed as described previously (Szot et al., 1997). Density measurements (microcuries per gram) were determined using MicroComputer Imaging Device system (MCID) (Imaging Research, St. Catherines, Ontario, Canada) in as many distinguishable layers as possible in the PFC (see Zilles, 2004 for extensive details on architecture of the human cortex), and the values are expressed as a mean (microcuries per gram) ± SEM for each subject group. Specific binding was obtained by taking the total average value minus nonspecific value in the same region. Specific binding for 3H-prazosin constituted ~90% of total binding. Data were analyzed in layers I/II, III/IV and V/VI with ANOVA, followed by a post hoc Fisher’s test; statistical significance was taken at p<0.05.
α2-AR binding sites were measured in the PFC with 3H-RX821002 (PerkinElmer) according to Happe et al., (2004). For each subject, four consecutive slides, each containing a section of the PFC, were run: three slides for total binding and the fourth for nonspecific binding. Nonspecific binding was defined in the presence of 10 μm rauwolscine. Slides and 3H standards were apposed to Biomax MR film (Eastman Kodak, Rochester, NY) for 8 weeks. Films were developed and analyzed as described previously (Szot et al., 1997). Density measurements (microcuries per gram) were determined using MCID (Imaging Research) in as many distinguishable layers as possible in the PFC, and the values are expressed as a mean (microcuries per gram) ± SEM for each subject group. Specific binding was obtained by taking the total average value minus nonspecific value in the same region. Specific binding for 3H-RX821002 constituted ~90% of total binding. Data were analyzed layers I/II, III, IV, V and VI as described above.
Oligonucleotides
α2C-, α1A- and α1D-AR mRNA expression were measured in the PFC as previously described (Szot et al., 2006). α2A-AR mRNA expression was not measured in the PFC because preliminary work showed α2A-AR mRNA levels to be undetectable (data not shown). Tissue preparation and labeling of α2C-, α1A- and α1D-AR oligonucleotide probes was performed as described previously for oligonucleotide labeling (Szot et al., 1997). Three consecutive slides in the PFC were used for each receptor subtype.
The α2C-AR probe consisted of a single oligonucleotide probe to the following nucleotides of the published human sequence (Lomasney et al., 1990): 875-925. The α2C-AR probe contained 0.16 × 106 cpm/50 μl for the PFC. Slides were apposed to film (Eastman Kodak) for 17 h at room temperature. The α1A-AR probe consisted of three separate oligonucleotide probes to the following nucleotides of the published human sequence (Schwinn et al., 1990; Hirasawa et al., 1993): 1-45, 1102-1156, and 1435-1483. The α1A-AR probe contained 1.4 × 106 cpm/50 μl for the PFC. Slides were apposed to film (Eastman Kodak) for 4 d at room temperature. The α1D-AR probe consisted of three separate oligonucleotide probes to the following nucleotides of the published human sequence (Weinberg et al., 1994; Schwinn et al., 1995): 587-635, 990-1038, and 1668-1716. The α1A-AR probe contained 0.4 × 106 cpm/50 μl for the PFC. Slides were apposed to film (Eastman Kodak) for 7 d at room temperature.
Film was developed and analyzed as described previously (Szot et al., 1997). α2C-,α1A- and α1D-AR mRNA expression in the PFC was measured as optical density (OD) using MCID as previously described (Szot et al., 2006). α2C-AR mRNA expression was analyzed in layers II, III/IV and V/VI. α1A- and α1D-AR mRNA expression was analyzed in layers I/II, III/IV and V/VI. The data are expressed as the average OD ± SEM for each group. Statistical analysis was performed as described above.
Results
α2-AR binding sites are reduced in dementia subjects but not to the degree of neuronal loss in LC
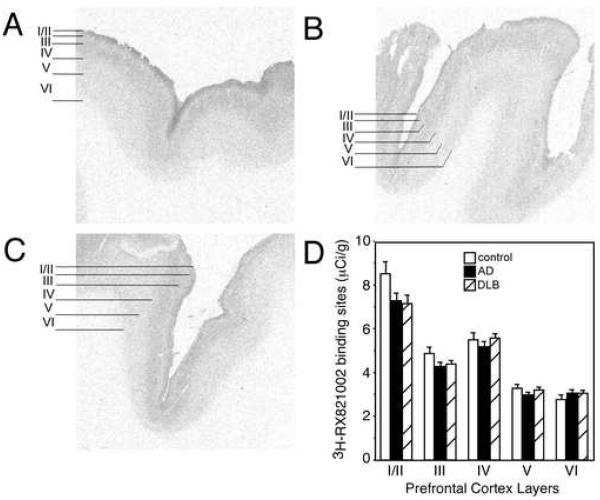
In control subjects, α2-AR binding sites have the greatest density in layer I/II, moderate levels in layers III and IV, and the lowest density in layers V and VI (Fig. 1 A). α2-AR binding sites are not statistically different between control, AD and DLB subjects in any layer of the PFC (Fig. 1), but there is a tendency for a reduction in binding sites in specific layers of the PFC in AD and DLB subjects. Since there are few differences between AD and DLB subjects as to cognitive loss, behavioral changes and expression of adrenergic receptors in the LC and hippocampus (this manuscript and Szot et al., 2006), combining data from AD and DLB subjects as a dementia group demonstrated a significant reduction in α2-AR binding sites in layers I/II (p<0.05) and III (p<0.05) from control subjects. No differences were observed in α2-AR binding sites between dementia subjects and controls in layers IV, V and VI. Because the α2A-AR subtype is not expressed in the PFC (data not shown) and 3H-RX821002 binds predominately to the α2A-AR (Ordway et al., 1993; Sastre and Garcia-Sevilla, 1994), the binding sites in the PFC represent presynaptic α2-AR binding sites from the LC. Therefore, there is a reduction in the presynaptic α2-AR binding sites in specific layers of the PFC in dementia, though the reduction in binding sites (~18%) isn’t near the reduction in noradrenergic cell bodies in the LC (~50-80%) (Szot et al., 2006). This data suggests that there is sprouting from the surviving LC noradrenergic neurons in the LC to the PFC, similar to what was observed in the hippocampus (Szot et al., 2006).
Figure 1. α-AR binding sites subjects with dementia tend to be reduced.
3H-RX 821002 labeling of α2-AR binding sites in the PFC of (A) control (n=14), (B) AD (n=15), and (C) DLB (n=19). The greatest amount of 3H-RX 821002 labeling is observed in layers I/II, with moderate levels observed in layers III and IV, and lowest levels of expression in layers V and VI. Definitions of PFC layers for data analysis are shown to the left of the autoradiograms for control, AD and DLB subjects. (D) Quantification of α2-AR binding sites in the different layers of the PFC in control, AD and DLB subjects. Data are represented as mean ± SEM.
Postsynaptic α2C-AR mRNA is reduced only in layer II of the PFC in dementia subjects
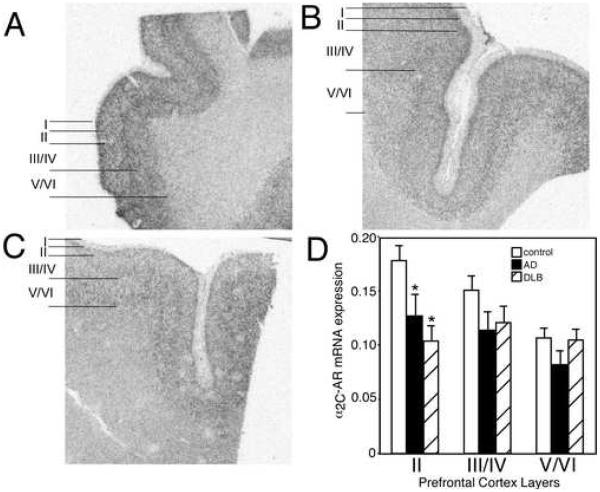
In control subjects, α2C-AR mRNA expression is greatest in layer II of the PFC with lower expression in layers III/IV and V/VI (Fig. 2 A). Quantitation of α2C-AR mRNA expression in AD and DLB subjects demonstrated a significant reduction only in layer II of the PFC, the layer with the greatest amount of expression in control subjects (Fig. 2). The reduction in α2C-AR mRNA expression in layer II of the PFC was comparable between AD and DLB subjects.
Figure 2. α2C-AR mRNA expression is reduced in the PFC of AD and DLB subjects.
α2C-AR mRNA expression in the PFC of (A) control (n=15), (B) AD (n=15), and (C) DLB (n=19). The greatest amount of α2C-AR mRNA expression in control subjects is observed in layer II, with less in layer III/IV and lowest expression in layer V/VI. Definition of PFC layers for data analysis are shown to the left of the autoradiograms for control, AD and DLB subjects. (D) Quantification of α2C-AR mRNA expression in the different layers of the PFC in control, AD and DLB subjects. * Significant difference (p<0.05) compared with control subjects. Data are represented as mean ± SEM.
Postsynaptic α1-AR binding sites tend to be elevated in layer I/II of PFC in subjects with dementia despite a reduction in α1A-AR mRNA
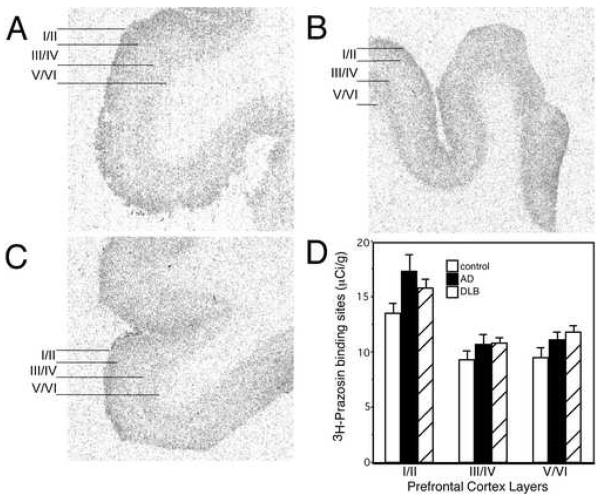
The α1-AR is solely a postsynaptic AR. 3H-Prazosin labels all layers of the PFC with the greatest density in layer I/II, and less expression in layers III/IV and V/VI (Fig. 3 A). Quantitation of 3H-prazosin binding sites in AD and DLB subjects are not statistically different from control subjects in any layer of the PFC, though there is a tendency for an increase in binding sites in layer I/II (Fig. 3). Combining α1-AR binding site values of AD and DLB subjects results in a significant increase in α1-AR binding sites in layer I/II as compared to control (p<0.05). These data indicate that the postsynaptic α1-AR is normal to elevated in subjects with dementia, similar to what has been observed in the hippocampus (Szot et al., 2006).
Figure 3. α1-AR binding sites tend to be elevated in subjects with dementia.
3H- prazosin labeling of α1-AR binding sites in the PFC of (A) control (n=17), (B) AD (n=15), and (C) DLB (n=22). The greatest amount of 3H-prazosin labeling in control subjects is observed in layers I/II, with moderate levels observed in layers III/IV and V/VI. Layers for data analysis are shown to the left of the autoradiograms for control, AD and DLB subjects. (D) Quantification of α1-AR binding sites in the different layers of the PFC in control, AD and DLB subjects. Data are represented as mean ± SEM.
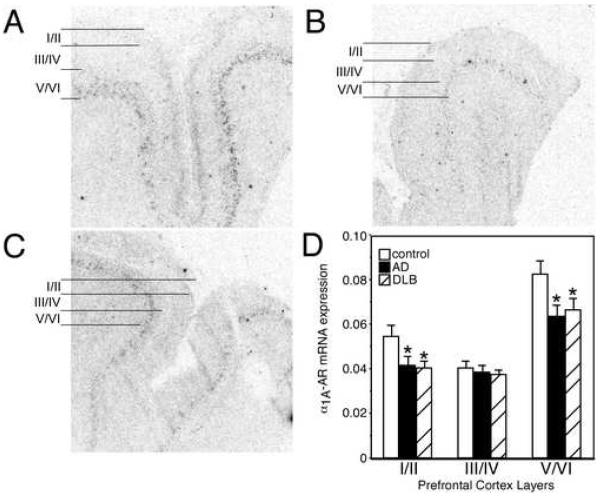
Similar to the α2-AR, the α1-AR is composed of several subtypes, the α1A-, α1B- and α1D-AR mRNA. 3 H-Prazosin binds mainly to the α1A- and α1B-AR subtype; however, only the α1A-(Fig 4. A-C) and α1D-AR (Fig 5. A-C) subtypes are expressed at detectable levels in the PFC. In control subjects, α1A-AR mRNA is expressed in all layers of the PFC with greatest amount of expression observed in layer V/VI, with lower levels in the other layers (Fig. 4 A). Quantitation of α1A-AR mRNA in AD and DLB subjects demonstrated a significant reduction in expression in layers I/II and V/VI (Fig. 4). The reduction in α1A-AR mRNA in the PFC was comparable between AD and DLB subjects.
Figure 4. α1A-AR mRNA expression is reduced in the PFC of AD and DLB subjects.
α1A-AR mRNA expression in the PFC of (A) control (n=16), (B) AD (n=15), and (C) DLB (n=21). The greatest amount of α2A-AR mRNA expression in control subjects is observed in layer V/VI with moderate levels in layer I/II and lowest expression in layer III/IV. Layers for data analysis are shown to the left of the autoradiograms for control, AD and DLB subjects. (D) Quantification of α1A-AR mRNA expression in the different layers of the PFC in control, AD and DLB subjects. * Significant difference (p<0.05) compared with control subjects. Data are represented as mean ± SEM.
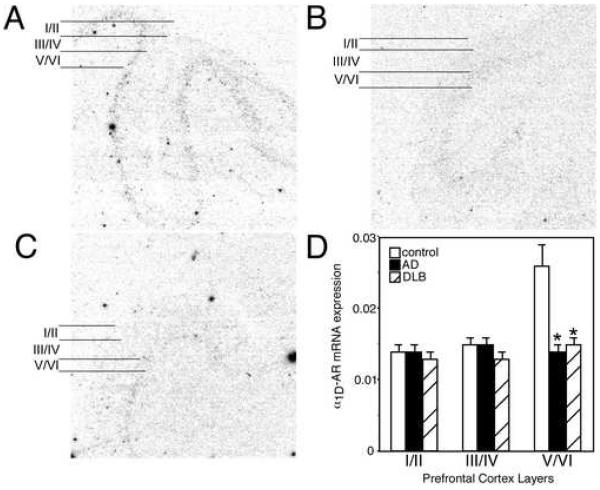
Figure 5. α1D-AR mRNA expression is reduced in the PFC of AD and DLB subjects.
α1D-AR mRNA expression in the PFC of (A) control (n=17), (B) AD (n=15), and (C) DLB (n=21). The greatest amount of α2A-AR mRNA expression in control subjects is observed in layer V/VI with lowest level of expression in layer I/II and III/IV. Layers for data analysis are shown to the left of the autoradiograms for control, AD and DLB subjects. (D) Quantification of α1D-AR mRNA expression in the different layers of the PFC in control, AD and DLB subjects. * Significant difference (p<0.05) compared with control subjects. Data are represented as mean ± SEM.
In control subjects, α1D-AR mRNA expression is mainly observed in layer V/VI, with the other layers having very low expression (Fig 5. A). Quantitation of α1D-AR mRNA in the PFC of AD and DLB subjects demonstrated a significant reduction of expression in layer V/VI (Fig 5). The reduction in α1D-AR mRNA in the PFC was comparable between AD and DLB subjects.
Discussion
In subjects with dementia, the surviving noradrenergic LC neurons are sprouting to the PFC
In subjects with dementia (AD and DLB subjects), the loss of noradrenergic neurons in the LC does not necessarily mean a loss of noradrenergic innervation to forebrain regions such as the PFC. Axonal sprouting of the surviving noradrenergic LC neurons to the PFC is occurring in subjects with dementia, though the sprouting does not result in normal noradrenergic innervation. In subjects with dementia, there is a reduction in presynaptic innervation to the PFC, but the reduction in presynaptic α2-AR binding is not as extensive as the loss of noradrenergic neurons in these subjects (Szot et al., 2006), indicating the presence of axonal sprouting. The data presented here support the lack of change in α2-AR binding in the PFC of AD subjects with other radiolabeled compounds (Shimohama et al., 1986; Leverenz et al., 2001; Matthews et al., 2002). α2-AR binding sites in the PFC represent presynaptic noradrenergic terminals because α2A-AR mRNA, the subtype 3H-RX821002 binds to (Ordway et al., 1993; Sastre and Garcia-Sevilla, 1994), is not expressed to detectable levels in the PFC. Although it would have been ideal to measure norepinephrine transporter (NET) binding sites in the PFC to confirm the data generated with 3H-RX821002, NET binding sites are not expressed at detectable levels in the PFC of humans (Szot, unpublished observation) or non-human primates (Smith et al., 2006).
The sprouting of noradrenergic innervation observed in the PFC of subjects with dementia is also observed in the hippocampus (Szot et al., 2006). However, it is unclear if in dementia noradrenergic sprouting is observed in all forebrain regions that receive noradrenergic innervation. Sprouting of noradrenergic neurons in the LC following neuronal loss has been observed in rats after the administration of the noradrenergic neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4), a selective noradrenergic neurotoxin which results in widespread degeneration of noradrenergic axon terminals in rodents. Shortly after the loss of terminals, a reduction in noradrenergic cell bodies is observed in the LC (Fritschy and Grzanna, 1991). However, with time the remaining noradrenergic neurons in the LC demonstrate sprouting (Fritschy et al., 1990; Fritschy and Grzanna, 1992), and this sprouting may account for the normal levels of α2-AR binding sites in the cortex and hippocampus seen in DSP-4 treated rats (Dooley et al., 1983; Hume et al., 1992; Heal et al., 1993; Wolfman et al., 1994). The similarity in noradrenergic sprouting following DSP-4 treatment in rats and the sprouting of noradrenergic neurons in AD and DLB subjects suggests the terminals of noradrenergic neurons in dementia may be destroyed first and then the cell bodies die later. This supports the hypothesis that the deposition of amyloid plaques in the forebrain may have degenerative properties in areas like the cortex and hippocampus. However, transgenic animal models of AD where plaques develop in the cortex and hippocampus fail to produce neuronal loss (Hock and Lamb, 2001; German and Eisch, 2004). Recent work in rats (injected with amyloid β) and amyloid precursor protein 23 (APP23) transgenic mice suggests that a loss of noradrenergic function, as observed with DSP-4 treatment, enhances plaque deposition, neuronal and memory loss (Heneka et al., 2002, 2006). This suggests that the loss of noradrenergic terminals in the cortex and hippocampus following DSP-4 treatment may result in the deposition of plaques and not that plaques causes the loss of terminals. If it is correct that a loss of noradrenergic function contributes to plaque deposition, then it still needs to be determined why noradrenergic neurons are destroyed in AD.
Another possible explanation for the preservation of α2-AR binding sites in the PFC is the presence of AR on astrocytes. α1-, α2- and β-AR binding sites have been observed in cultured astrocytes and these sites appear to be functional (McCarthy et al., 1995; Muyderman et al., 1998; Kilik et al., 1999; Kotter and Klein, 1999; Hosli and Hosli, 2000). The problem with these studies is that the work was done in cultures of just astrocytes. Electron microscope studies show the amount of β-AR and α2A-AR in astrocytes in the rat hippocampus to be significantly less than on neurons (Milner et al., 1998, 2000), and α1B-and α2C-AR are not even detected in astrocytes (Enkvist et al., 1996; Papay et al., 2004). Even though ARs are found on astrocytes, the level of these binding sites appears to be much lower than that of neurons; therefore, AR binding sites in astrocytes probable do not contribute to the α2-AR (or α1-AR) binding sites measured in the PFC of postmortem human subjects.
Postsynaptic α2C-AR mRNA is maintained in some layers of the PFC in subjects with dementia
Postsynaptic α2C-AR mRNA expression in the PFC of subjects with dementia is significantly reduced only in layer II of the PFC. Layers III/IV and V/VI, which have moderate levels of α2C-AR mRNA expression, remains unchanged in subjects with dementia. Expression of α2C-AR mRNA in the PFC is more preserved than in the hippocampus of subjects with dementia (Szot et al., 2006). This is important because the postsynaptic α2-AR in the PFC has been shown to increase cognitive performance with α2-AR agonists (Arnsten, 1993, 2003; Franowicz and Arnsten, 1999, 2002; Birnbaum et al., 2000; Franowicz et al., 2002). Preservation of postsynaptic α2-AR receptors in the PFC of AD subjects theoretically could be used to enhance cognitive function. The reduction in α2C-AR mRNA expression in the PFC in layer II may be attributed to the neuronal loss in this portion of the cortex in dementia (Braak and Braak, 1991; Masliah et al., 1993; Salat et al., 2001), because α1A-AR mRNA, which is also expressed in this layer, is also significantly reduced.
Postsynaptic α1-AR binding sites are elevated in subjects with dementia even though α1A- and α1D-AR mRNA expression is reduced
In subjects with dementia, α1-AR binding sites are normal to elevated in the PFC, supporting previously published work (Shimohama et al., 1986). These data also support the prior observation in the hippocampus of subjects with dementia where α1-AR binding sites were unchanged in the hilus, but statistically elevated in the molecular level of the dentate gyrus (Szot et al., 2006). These data indicate that this major postsynaptic AR is not altered in subjects with dementia despite the loss of neurons in this region in dementia (Braak and Braak, 1991; Masliah et al., 1993; Salat et al., 2001). The α1-AR binding sites are maintained despite the reduction in α1A- and α1D-AR mRNA, suggesting the remaining expressing neurons in the PFC of subjects with dementia are sprouting.
Stimulation of postsynaptic PFC α2-AR binding sites enhance cognitive function, while stimulation of PFC postsynaptic α1-AR binding sites can impair cognition and disrupt behavior (Birnbaum et al., 2004; Arnsten and Li, 2005). An enhanced stimulation of the PFC α1-AR can be observed under stressful conditions when an excess of norepinephrine is released, otherwise released norepinephrine will preferentially stimulate the α2-AR (Arnsten and Li, 2005). In dementia, PFC function may be shifted towards impaired cognition and disruptive behavior due to the normal to elevated levels of the postsynaptic α1-AR with the loss of postsynaptic α2-AR; this enhanced α1-AR would most likely be observed under stressful conditions. In dementia, a common behavior problem is increased agitation, and agitation is more frequently observed under stressful conditions. Interestingly, preliminary data from the Alzheimer’s Disease Research Center at the University of Washington (Murray Raskind; personnel communication) has found blocking the α1-AR with prazosin reduces disruptive agitation in subjects with dementia.
Conclusion
In AD and DLB subjects there is a significant loss of noradrenergic neurons in the LC (Mann et al., 1980; Tomlinson et al., 1981; Bondareff et al., 1982; Marcyniuk et al., 1986; Chan-Palay and Asan, 1989; German et al., 1992; Szot et al., 2006); however, in the hippocampus (Szot et al., 2006) and PFC, there is evidence of sprouting of the survivng noradrenergic neurons in the LC subjects with dementia. Sprouting of the surviving noradrenergic neurons in the LC of subjects with dementia has been suggested by a variety of other studies (Adolfsson et al., 1979; Cross et al., 1981; Mann et al., 1981; Perry et al., 1981; Tomlinson et al., 1981; Gottfries et al., 1983; Raskind et al., 1984; Palmer et al., 1987; Reinikainen et al., 1988; Tohgi et al., 1992; Elrod et al., 1997; Russo-Neustadt et al., 1998; Hoogendijk et al., 1999; Szot et al., 2000). The sprouting observed in postmortem dementia subjects is consistent with the sprouting of noradrenergic neurons in rodents after DSP-4 induced neuronal loss (Fritschy et al., 1990; Fritschy and Grzanna, 1992). What remains unknown at the present time is if these new terminals, due to sprouting, are replacing the old ones that have died or forming new connections in areas that have never received noradrenergic innervation before. A great deal of work is required to determine how noradrenergic neuronal loss and the subsequent sprouting contributes to pathophysiology and symptomatic expression of AD.
ACKNOWLEDGEMENT
These studies were supported by the Department of veterans Affairs Research and Developments Services, Northwest Network Mental Illness Research, Education and Clinical Center (MIRECC) and University of Washington Alzheimer’s Disease Research Center (ADRC) NIH P50 AGO05136 (MAR).
Abbreviations
- AD
Alzheimer’s disease
- Alpha
α
- AR
adrenoreceptors
- DLB
dementia with Lewy bodies
- LC
locus coeruleus
- PFC
prefrontal cortex
- NE
norepinephrine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnsten AF. Catecholamine mechanisms in age-related cognitive decline. Neurobiol Aging. 1993;14:639–641. doi: 10.1016/0197-4580(93)90054-f. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Adrenergic targets for the treatment of cognitive decline in schizophrenia. Psychopharmacology. 2003;174:25–31. doi: 10.1007/s00213-003-1724-3. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Li B-M. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Adolfsson R, Gottfries CG, Roos BE, Winblad B. Changes in the brain catecholamines in patients with dementia of Alzheimer’s type. Br J Psychiatry. 1979;135:216–223. doi: 10.1192/bjp.135.3.216. [DOI] [PubMed] [Google Scholar]
- Ballard C, Holmes C, McKeith I, Neill D, O’Brian J, Lantos P, Perry E, Ince P, Perry R. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer’s disease. Am J Psychiatry. 1999;156:1039–1045. doi: 10.1176/ajp.156.7.1039. [DOI] [PubMed] [Google Scholar]
- Barber R, Panikkar A, McKeith IG. Dementia with Lewy bodies: diagnosis and management. Int J Geriatr Psychiatry. 2001;16:S12–S18. doi: 10.1002/1099-1166(200112)16:1+<::aid-gps562>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Podell DM, Arnsten AFT. Noradrenergic alpha-2 receptor agonists reverse working memory deficits induced by the anxiogenic drug, FG7142, in rats. Pharmacol Biochem Behav. 2000;67:397–403. doi: 10.1016/s0091-3057(00)00306-3. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, Gobeske KT, Sweatt JD, Manji HK, Arnsten AFT. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Bondareff W, Mountjoy CQ, Roth M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology. 1982;32:164–168. doi: 10.1212/wnl.32.2.164. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurons on performance of a 5-choice serial reaction time task in rats: implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Sci. 9:361–380. doi: 10.1016/0166-4328(83)90138-9. 183. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V, Asan E. Alterations in the locus coeruleus in dementias of Alzheimer’s and Parkinson’s disease. Prog Brain Res. 1991;88:625–630. doi: 10.1016/s0079-6123(08)63839-x. [DOI] [PubMed] [Google Scholar]
- Cross AJ, Crow TJ, Perry EK, Perry RH, Blessed G, Tomlinson BE. Reduced dopamine-beta-hydroxylase activity in Alzheimer’s disease. Br Med J (Clin Res Ed) 1981;282:93–94. doi: 10.1136/bmj.282.6258.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley DJ, Bittiger H, Hauser KL, Bischoff SF, Waldmeier PC. Alteration of central alpha 2- and beta-adrenergic receptors in the rat after DSP-4, a selective noradrenergic neurotoxin. Neuroscience. 1983;9:889–898. doi: 10.1016/0306-4522(83)90277-4. [DOI] [PubMed] [Google Scholar]
- Elrod R, Peskind ER, DiGiacomo L, Brodkin KL, Veith RC, Raskind MA. Effects of Alzheimer’s disease severity on cerebrospinal fluid norepinephrine concentration. Am J Psychiatry. 1997;154:25–30. doi: 10.1176/ajp.154.1.25. [DOI] [PubMed] [Google Scholar]
- Enkvist MOK, Hamalainen H, Jansson CC, Kukkonen JP, Hautala R, Courtney MJ, Akerman KEO. Coupling of astroglial α2-adrenoreceptors to second messenger pathways. J Neurochem. 1996;66:2394–2401. doi: 10.1046/j.1471-4159.1996.66062394.x. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AFT. Treatment with the noradrenergic alpha-2 agonist clonidine, but not diazepam, improves spatial working memory in normal young rhesus monkeys. Neuropsychopharmacology. 1999;21:611–621. doi: 10.1016/S0893-133X(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AFT. Actions of α-2 noradrenergic agonists on spatial working memory and blood pressure in rhesus monkey appear to be mediated by the same receptor subtype. Psychopharmacology. 2002;162:304–312. doi: 10.1007/s00213-002-1110-6. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Kessler LE, Borja CMD, Kobilka BK, Limbrid LE, Arnsten AFT. Mutation of the α2A-adrenergic impairs working memory performance and annuls enhancement by guanfacine. J Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Experimentally-induced neuronal loss in the locus coeruleus of adult rats. Exp Neurol. 1991;111:123–127. doi: 10.1016/0014-4886(91)90058-k. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Restoration of ascending noradrenergic projections by residual locus coeruleus neurons: compensatory response to neurotoxin-induced cell death in the adult brain. J Comp Neurol. 1992;321:421–441. doi: 10.1002/cne.903210309. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Geffard M, Grzanna R. The response of noradrenergic axons to systemically administered DSP-4 in the rat: an immunohistochemical study using antibodies to noradrenaline and dopamine β-hydroxylase. J Chem Neuroanat. 1990;3:309–323. [PubMed] [Google Scholar]
- German DC, Eisch AJ. Mouse models of Alzheimer’s disease: insight into treatment. Reviews Neurosci. 2004;15:353–369. doi: 10.1515/revneuro.2004.15.5.353. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF, White CL, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–676. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- Gottfries CG, Adolfsson R, Aquilonius SM, Carlsson A, Eckernas SA, Nordberg A, Oreland L, Svennerholm L, Wiberg A, Winblad B. Biocehmical changes in dementia disorders of Alzheimer’s type (AD/SDAT) Neurobiol Aging. 1983;4:261–271. doi: 10.1016/0197-4580(83)90002-7. [DOI] [PubMed] [Google Scholar]
- Happe HK, Coulter CL, Gerety ME, Sanders JD, O’Rourke M, Bylund DB, Murrin LC. Alpha-2 adrenergic receptor development in rat CNS: an autoradiographic study. Neuroscience. 2004;123:167–178. doi: 10.1016/j.neuroscience.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Butler SA, Prow R, Buckett WR. Quantification of presynaptic α2-adrenoceptors in rat brain after short-term DSP-4 lesioning. Eur J Pharmacol. 1993;249:37–41. doi: 10.1016/0014-2999(93)90659-6. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Galea E, Gavriluyk V, Dumitreascu-Ozimek L, Daeschner J, O’Banion MK, Weinberg G, Klockgether T, Feinstein DL. Noradrenergic depletion potentiates β-amyloid-induced cortical inflammation: Implications for Alzheimer’s disease. J Neurosci. 2002;22:2434–2442. doi: 10.1523/JNEUROSCI.22-07-02434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Ramanathan M, Jacobs AH, Dumitrescu-Ozimek L, Bilkei-Gorzo A, Debeir T, Sastre M, Galldiks N, Zimmer A, Hoehn M, Heiss W-D, Klockgether T, Staufenbiel M. Locus ceruleus degeneration promotes Alzheimer’s pathogenesis in amyloid precursor protein 23 transgenic mice. J Neurosci. 2006;26:1343–1354. doi: 10.1523/JNEUROSCI.4236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa A, Horie K, Tanaka T, Takagaki K, Murai M, Yano J, Tsujimoto G. Cloning, functional expression and tissue distribution of human cDNA for the α1C-adrenergic receptor. Biochem Biophys Res Comm. 1993;195:902–909. doi: 10.1006/bbrc.1993.2130. T. [DOI] [PubMed] [Google Scholar]
- Hock BJ, Lamb BT. Transgenic mouse models of Alzheimer’s disease. Trends Genetics. 2001;17:S7–S11. doi: 10.1016/s0168-9525(01)02449-0. [DOI] [PubMed] [Google Scholar]
- Hoogendijk WJ, Freenstra MG, Botterblom MH, Gilhuis J, Sommer IE, Kamphorst W, Eikelenboom P, Swaab DF. Increased activity of surviving locus ceruleus neurons in Alzheimer’s disease. Ann Neurol. 1999;45:82–91. doi: 10.1002/1531-8249(199901)45:1<82::aid-art14>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Hosli E, Hosli L. Colocalization of neurotransmitter receptors on astrocytes in explant cultures of rat CNS. Neurochem Int. 2000;36:301–311. doi: 10.1016/s0197-0186(99)00138-2. [DOI] [PubMed] [Google Scholar]
- Hume SP, Lammertsma AA, Opacka-Juffry J, Ahier RG, Myers R, Cremer JE, Hudson AL, Nutt DJ, Pike VW. Quantification of in vivo binding of [3H]RX 821002 in rat brain: evaluation as a radioligand for central alpha 2-adrenoceptors. Int J Rad Appl Instrum B. 1992;19:841–849. doi: 10.1016/0883-2897(92)90170-4. [DOI] [PubMed] [Google Scholar]
- Kotter K, Klein J. Adrenergic modulation of astroglial phospholipase D activity and cell proliferation. Brain Res. 1999;830:138–145. doi: 10.1016/s0006-8993(99)01416-x. [DOI] [PubMed] [Google Scholar]
- Kulik A, Haentzsch A, Luckermann M, Reichelt W, Ballanyi K. Neuron-glia signaling via α1 adrenoceptor-mediated Ca2+ release in Bergmann glial cells in situ. J Neurosci. 1999;19:8401–8408. doi: 10.1523/JNEUROSCI.19-19-08401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverenz JB, Miller MA, Dobie DJ, Peskind ER, Raskind MA. Increased alpha 2-adrenergic receptor binding in locus coeruleus projection areas in dementia with Lewy bodies. Neurobiology Aging. 2001;22:555–561. doi: 10.1016/s0197-4580(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Lomasney JW, Lorenz W, Allen LF, King K, Regan JW, Yang-Feng TL, Caron MG, Lefkowitz RJ. Expansion of the α2-adrenergic receptor family: cloning and characterization of a human α2-adrenergic receptor subtypes, the gene for which is located on chromosome 2. Proc Natl Acad Sci USA. 1990;87:5094–5098. doi: 10.1073/pnas.87.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Lincoln J, Yates PO, Stamp JE, Toper S. Changes in the monoamine containing neurons of the human CNS in senile dementia. Br J Psychiatry. 1980;136:533–541. doi: 10.1192/bjp.136.6.533. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Stanley M, Neophytides A, deLeon MJ, Ferris SH, Gershon S. Central amine metabolism in Alzheimer’s disease: in vivo relationship to cognitive deficit. Neurobiol Aging. 1981;2:57–60. doi: 10.1016/0197-4580(81)90060-9. [DOI] [PubMed] [Google Scholar]
- Marcyniuk B, Mann DM, Yates PO. Loss of nerve cells from locus coeruleus in Alzheimer’s disease is topographically arranged. Neurosci Lett. 1986;64:247–252. doi: 10.1016/0304-3940(86)90336-8. [DOI] [PubMed] [Google Scholar]
- Masliah E, Miller A, Terry RD. The synaptic organization of the neocortex in Alzheimer’s disease. Med Hypotheses. 1993;41:334–340. doi: 10.1016/0306-9877(93)90078-5. [DOI] [PubMed] [Google Scholar]
- Matthews KL, Chen CPL-H, Esiri MM, Keene J, Minger SL, Francis PT. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatry. 2002;51:407–416. doi: 10.1016/s0006-3223(01)01235-5. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, Enkvist K, Shao Y. Astroglial adrenergic receptors: expression and function. In: Neuroglia, Kettenmann H, Ransom B, editors. Oxford Univ Press; Oxford, UK: 1995. pp. 354–366. [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne FJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, et al. Consensus guidlelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlam EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lee A, Aicher SA, Rosin DL. Hippocampal α2A-adrenergic receptors are located predominately presynaptic but are also found postsynaptically and in selective astrocytes. J Comp Neurol. 1998;395:310–327. [PubMed] [Google Scholar]
- Milner TA, Shah P, Pierce JP. β-adrenergic receptors primarily are located on the dendrites of granule cells and interneurons but also are found on astrocytes and a few presynaptic profiles in the rat dentate gyrus. Synapse. 2000;36:178–193. doi: 10.1002/(SICI)1098-2396(20000601)36:3<178::AID-SYN3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Muyderman H, Nilsson M, Blomstrand F, Khatibi S, Olsson T, Hansson E, Ronnback Modulation of mechanically induced calcium waves in hippocampal astroglial cells. Inhibitory effects of α1-adrenergic stimulation. Brain Res. 1998;793:127–135. doi: 10.1016/s0006-8993(98)00151-6. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Jaconetta SM, Halaris AE. Characterization of subtypes of alpha-2 adrenoreceptors in the human brain. J Pharmacol Expt Therap. 1993;264:967–976. [PubMed] [Google Scholar]
- Palmer AM, Wilcock GK, Esiri MM, Francis PT, Bowen DM. Monoaminergic innervation of the frontal and temporal lobes in Alzheimer’s disease. Brain Res. 1987;401:231–238. doi: 10.1016/0006-8993(87)91408-9. [DOI] [PubMed] [Google Scholar]
- Papay R, Gaivin R, McCune DF, Rorabaugh BR, Macklin WB, McGrath JC, Perez DM. Mouse α1B-adrnergic receptor is expressed in neurons and NG2 oligodendrocytes. J Comp Neurol. 2004;478:1–10. doi: 10.1002/cne.20215. [DOI] [PubMed] [Google Scholar]
- Perry EK, Blessed G, Tomlinson BE, Perry RH, Crow TJ, Cross AJ, Dockray GJ, Dimaline R, Arregui A. Neurochemical activities in human temporal lobe related to aging and Alzheimer’s-type changes. Neurobiol Aging. 1981;2:251–256. doi: 10.1016/0197-4580(81)90032-4. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Halter JB, Jimerson DC. Norepinephrine and MHPG levels in CSF and plasma in Alzheimer’s disease. Arch Gen Psychiatry. 1984;41:343–346. doi: 10.1001/archpsyc.1984.01790150033006. [DOI] [PubMed] [Google Scholar]
- Reinikainen KJ, Paljarvi L, Huuskonen M, Soininen H, Laakso M, Reikkinen P. A post-mortem study of noradrenergic, serotonergic and GABAergic neurons in Alzheimer’s disease. J Neurol Sci. 1988;84:101–116. doi: 10.1016/0022-510x(88)90179-7. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Zomorodian TJ, Cotman CW. Preserved cerebellar tyrosine hydroxylase-immunoreactive neuronal fibers in a behaviorally aggressive subgroup of Alzheimer’s disease patients. Neuroscience. 1998;87:55–61. doi: 10.1016/s0306-4522(98)00134-1. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer’s disease. Arch Neurol. 2001;58:1403–1408. doi: 10.1001/archneur.58.9.1403. [DOI] [PubMed] [Google Scholar]
- Sastre M, Garcia-Sevilla JA. Alpha 2-adrenoceptor subtypes identified by [3H]RX821002 binding in the human brain: the agonist guanoxabenz does not discriminate different forms of the predominant alpha 2A subtype. J Neurochem. 1994;63:1077–1085. doi: 10.1046/j.1471-4159.1994.63031077.x. [DOI] [PubMed] [Google Scholar]
- Schwinn DA, Lomasney JW, Lorenz W, Szklut PJ, Fremeau RT, Yang-Feng TL, Caron MG, Lefkowitz RJ, Cotecchis S. Molecular cloning and expression of the cDNA for a novel α1-adrenergic receptor subtype. J Biol Chem. 1990;265:8183–8189. [PubMed] [Google Scholar]
- Schwinn DA, Johnston GI, Page SO, Mosley MJ, Wilson KH, Worman NP, Campbell S, Fidock MD, Furness LM, Parry-Smith DJ, Peter B, Bailey DS. Cloning and pharmacological characterization of human alpha-1 adrenergic receptors: sequence corrections and direct comparison with other species homologues. J Pharmacol Exp Ther. 1995;272:134–142. [PubMed] [Google Scholar]
- Shimohama S, Taniguchi T, Fujiwara M, Kameyama M. Biochemical characterization of α-adrenergic receptors in human brain and changes in Alzheimer-type dementia. J Neurochem. 1986;47:1294–1301. [PubMed] [Google Scholar]
- Smith HR, Beveridge TJR, Porrino LJ. Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience. 2006;138:703–714. doi: 10.1016/j.neuroscience.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Szot P, Leverenz JB, Peskind ER, Kiyasu E, Rohde K, Miller MA, Raskind MA. Tyrosine hydroxylase and norepinephrine transporter mRNA expression in the locus coeruleus in Alzheimer’s disease. Mol Brain Res. 2000;84:135–140. doi: 10.1016/s0169-328x(00)00168-6. [DOI] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenrgic nervous system in the locus coeruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26:467–478. doi: 10.1523/JNEUROSCI.4265-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. α1-adrenoreceptor in human hippocampus: Binding and receptor subtype mRNA expression. Mol Brain Res. 2005;139:367–371. doi: 10.1016/j.molbrainres.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Szot P, White SS, Veith RC. Effect of pentylenetetrazol on the expression of tyrosine hydroxylase mRNA and norepinephrine and dopamine transporter mRNA. Mol Brain Res. 1997;44:46–54. doi: 10.1016/s0169-328x(96)00217-3. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Ueno M, Abe T, Takahashi S, Nozaki Y. Concentrations of monoamines and their metabolites in the cerebral spinal fluid from patients with senile dementia of the Alzheimer’s type and vascular dementia of the Binswanger’s type. J Neurol Transm Park Dis Dement Sect. 1992;4:69–77. doi: 10.1007/BF02257623. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Irving D, Blessed G. Cell loss in the locus coeruleus in senile dementia of the Alzheimer’s type. J Neurol Sci. 1981;49:419–428. doi: 10.1016/0022-510x(81)90031-9. [DOI] [PubMed] [Google Scholar]
- Wolfman C, Abo V, Calvo D, Medina J, Dajas F, Silveira R. Recovery of central noradrenergic neurons one year after the administration of the neurotoxin DSP-4. Neurochem Int. 1994;25:395–400. doi: 10.1016/0197-0186(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Weinberg DH, Trivedi P, Tan CP, Mitra S, Perkins-Barrow A, Borkowski D, Strader CD, Bayne M. Cloning, expression and characterization of human α adrenergic receptors α1A, α1B and α1C. Biochem Biophys Res Commun. 1994;201:1296–1304. doi: 10.1006/bbrc.1994.1845. [DOI] [PubMed] [Google Scholar]
- Zilles K. Architecture of the human cerebral cortex. In: Paxinos G, Mai JK, editors. The Human Nervous System. Academic Press; San Diego, CA: 2004. pp. 997–1055. 1990. [Google Scholar]