Summary
Adjacent to the arterial circle of Willis at the base of the brain, there is an anastomotic circle of veins linking the right and left halves of the cerebral deep venous system. This venous circle is formed by anterior and posterior transverse anastomotic channels (the anterior and posterior communicating veins), and paramedian longitudinal vessels (the basal veins of Rosenthal). This collateral venous network has received considerably less attention than its arterial counterpart, but is its functional homologue. Although inconstant, it can be seen readily with current neuroimaging techniques including three-dimensional digital subtraction venographic phase 3D arteriography (3D-DSV) and CT venography (CTV). The venous circle represents a route of contralateral venous drainage that may become important, particularly when segments of the basal vein are absent (with or without complex DVA), or in high flow states including arteriovenous shunts that access the deep venous system. We review the gross anatomy and provide examples of the radiologic imaging of this venous circle.
Key words: venous anastomoses, cerebral veins, basal vein of Rosenthal, communicating veins
Introduction
The arterial circle of Willis, although often incomplete, is the primary intracranial source of collateral arterial supply to the brain. Adjacent to this arterial circle, there is a functionally homologous anastomotic venous circle formed by the anterior and posterior communicating veins and the basal veins (of Rosenthal) (figure 1). Like the arterial circle, direction of flow may be bidirectional, and may reverse in the presence of pathology. Also like the arterial circle, the venous circle provides a route of collateral blood flow, linking in this case the right and left halves of the deep venous system across the midline at the level of the optic chiasm and cerebral peduncles. Although inconstant in its complete form, it is readily identified when present with current neuroimaging techniques, including computed tomographic venography (CTV) and three-dimensional digital subtraction venography (3D-DSV).
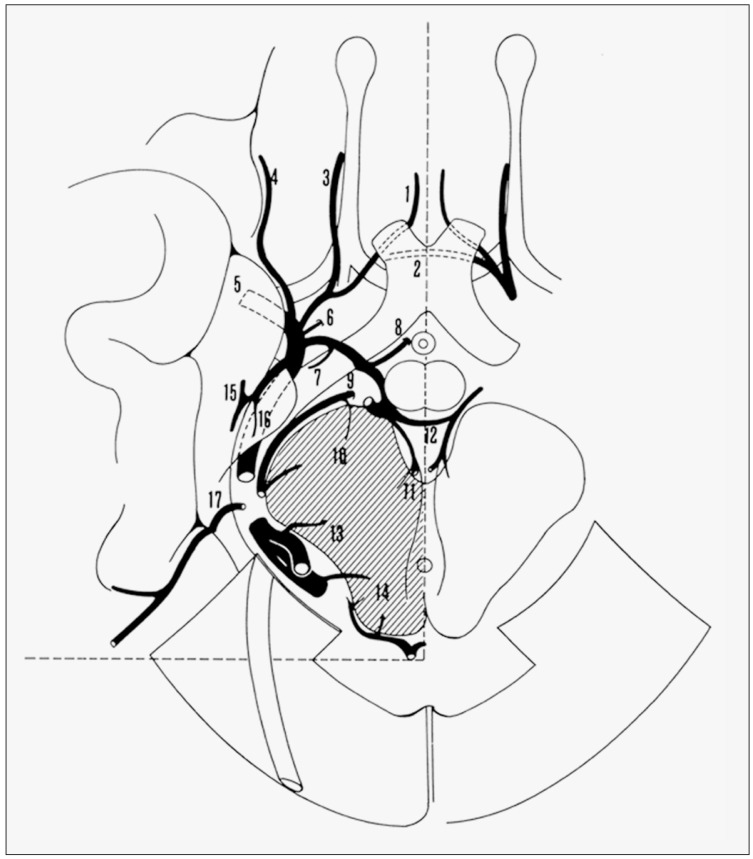
Figure 1.
The venous circle (reprinted from 6). 1, anterior cerebral vein;2, anterior communicating vein; 3, olfactory vein;4, inferior frontal vein;5, deep middle cerebral vein;6, inferior striate vein;7, vein of the optic tract;8, hypothalamic vein;9, peduncular vein;10, peduncular perforating vein; 11, transmesencephalic vein; 12, posterior communicating vein; 13, tegmental perforating vein; 14, tectal perforating vein; 15, hippocampal and medial temporal veins;16, uncal vein;17, infratemporal vein.
Gross Anatomy of the Venous Circle
The circle connects the anterior afferents to the basal vein system in the chiasmatic region and the posterior portion of the same system ventral to the cerebral peduncles. An anterior channel connects the anterior cerebral veins and is termed the anterior communicating vein (figure 2). This vein is present in a little more than 50% of patients1. It courses along the lamina terminalis and above the optic chiasm, parallel to the anterior communicating artery, which is located inferiorly and anteriorly. Its diameter is generally the size of the anterior cerebral veins, or a little smaller. As is the case with the anterior communicating artery and the A1 segments, discrepancy in the size of one anterior cerebral vein or a basal vein will generally correspond to a larger communicating vein. It may also exist as multiple, smaller channels. Afferent branches contributing to the anterior venous circle include the septal veins, callosal veins, anterior cerebral veins, chiasmatic veins and olfactory veins near the midline, and the inferior striate veins, insular veins and uncal veins more laterally.
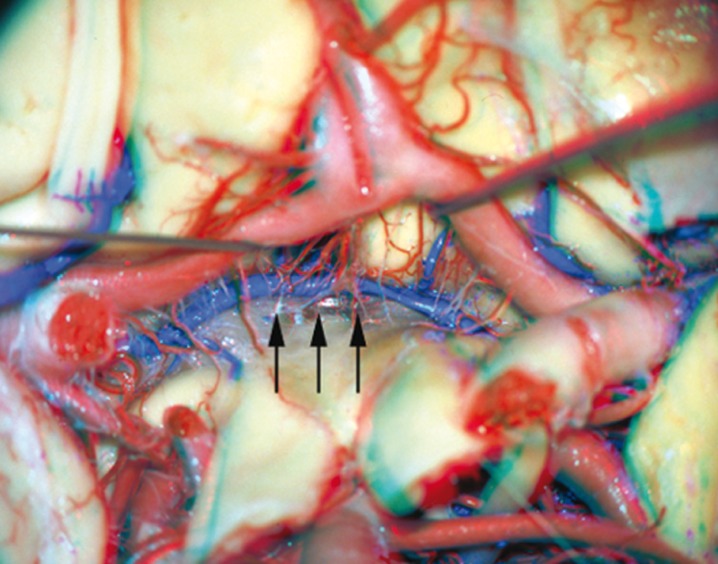
Figure 2.
Anterior communicating vein (arrows) - gross specimen.
Posteriorly, a transverse connection can be identified coursing in the interpeduncular fossa posterior to the basilar artery (figure 3). This vein, called the posterior communicating vein, connects interpeduncular veins and links the caudal portion of the basal veins. This vein is almost always present, and is generally larger than the anterior communicating vein. Its diameter will correspond to the caliber of the interpeduncular veins which are its tributaries.
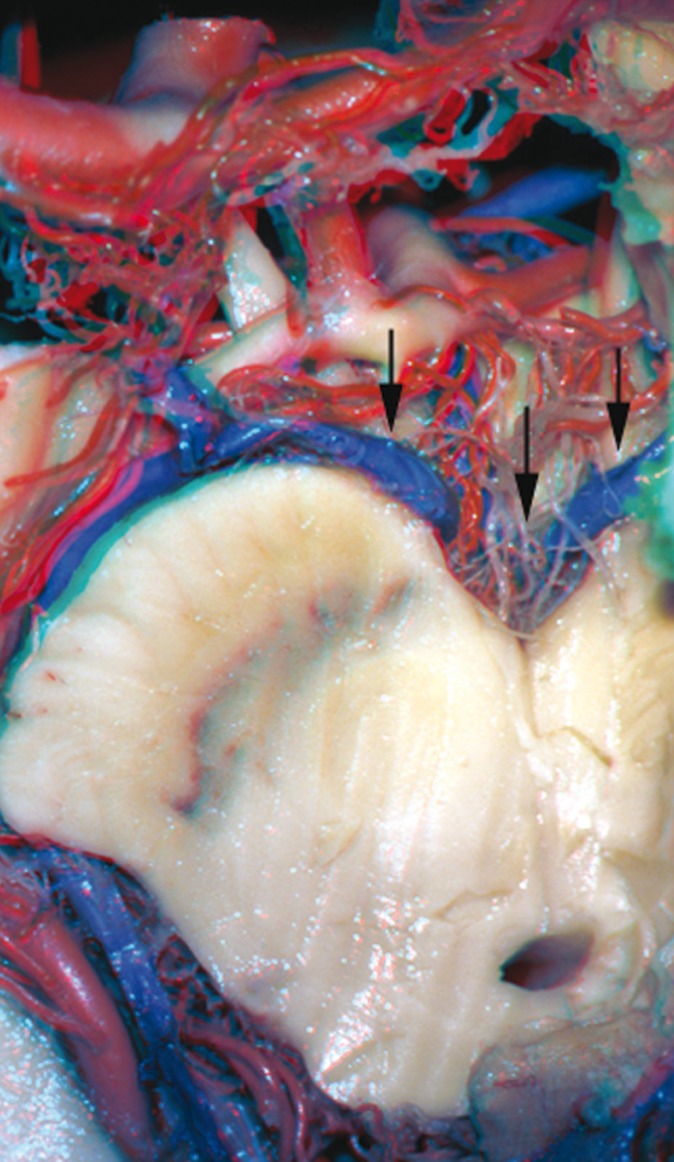
Figure 3.
Posterior communicating vein (arrows) - gross specimen.
Afferent branches contributing to the posterior venous circle include the hypothalamic and ventral mesencephalic veins medially and laterally.
Egress from the venous circle is via the deep sylvian veins to the cavernous plexus anteriorly, and via the lateral mesencephalic, lateral and medial pontine veins to the petrous vein and superior petrosal sinus posteriorly.
Radiologic Imaging of the Venous Circle
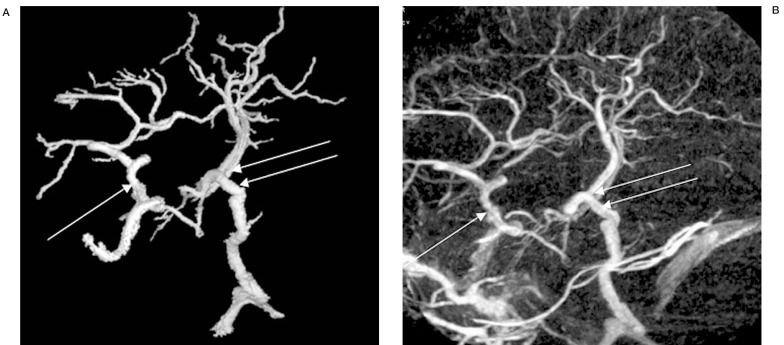
The anterior communicating vein can be difficult to visualize on conventional DSA due to its inconstant opacification, small diameter and short length. Veins are often difficult to see on angiography as only those carrying opacified blood are visible; the circle involves too many non-opacified systems to be reliably visualized in a selective injection unless associated with a variant. When seen on the midline, it is located superior and slightly posterior to the anterior communicating artery, and at the angle of the anterior cerebral vein and at the anterior extent of the basal vein. Wash-out of contrast from the controlateral anterior cerebral vein or basal vein will delimit its transverse extent. 3D-DSA will generally improve the conspicuity of this structure, especially when the venogram is fused to an MRI (figure 4).
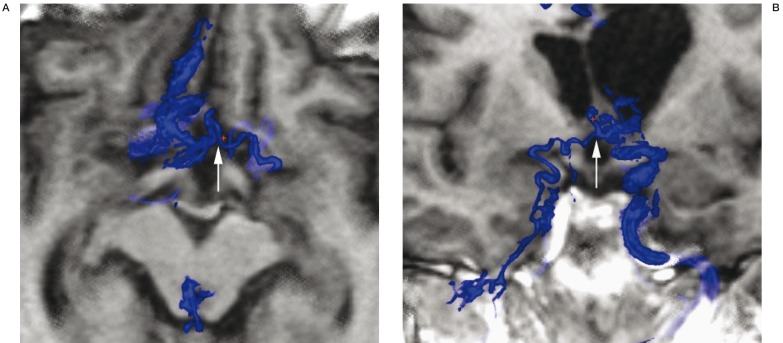
Figure 4.
Axial (A) and coronal (B) 3D-DSV MR fusion image of anterior communicating vein (arrows) draining an arteriovenous malformation.
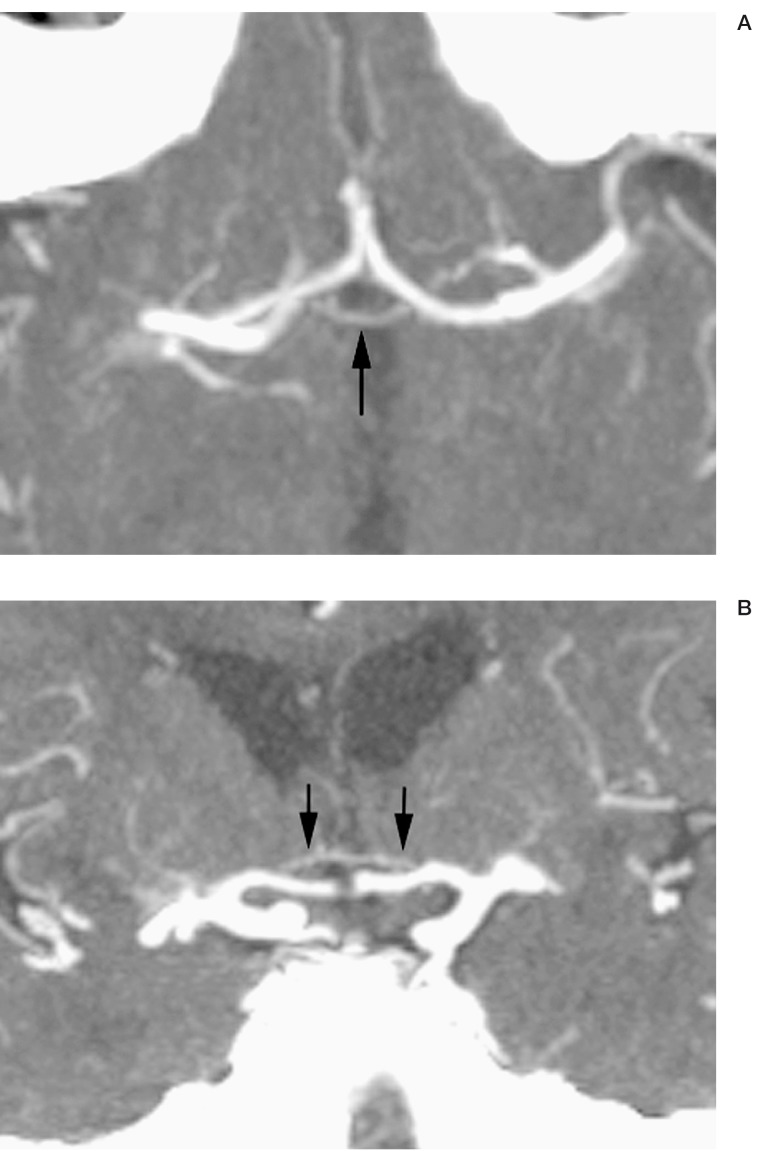
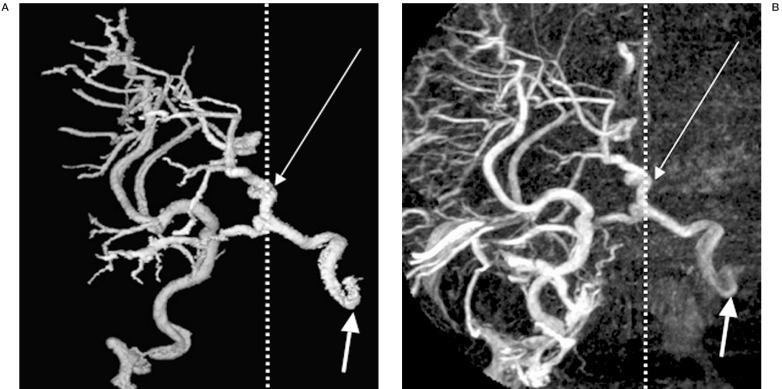
CTV will more consistently depict the anterior communicating vein when present, as opacification of the entire venous system is achieved (figure 5). The anterior communicating vein is seen most easily in the axial or coronal plane, located just superior to the optic chiasm at the convergence of the anterior cerebral veins and the basal veins.
Figure 5.
Axial (A) and coronal (B) CT venographam (CTV) images of anterior communicating vein (arrows).
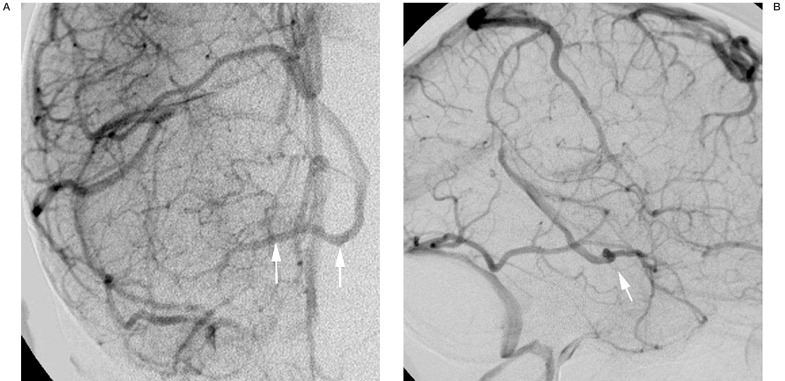
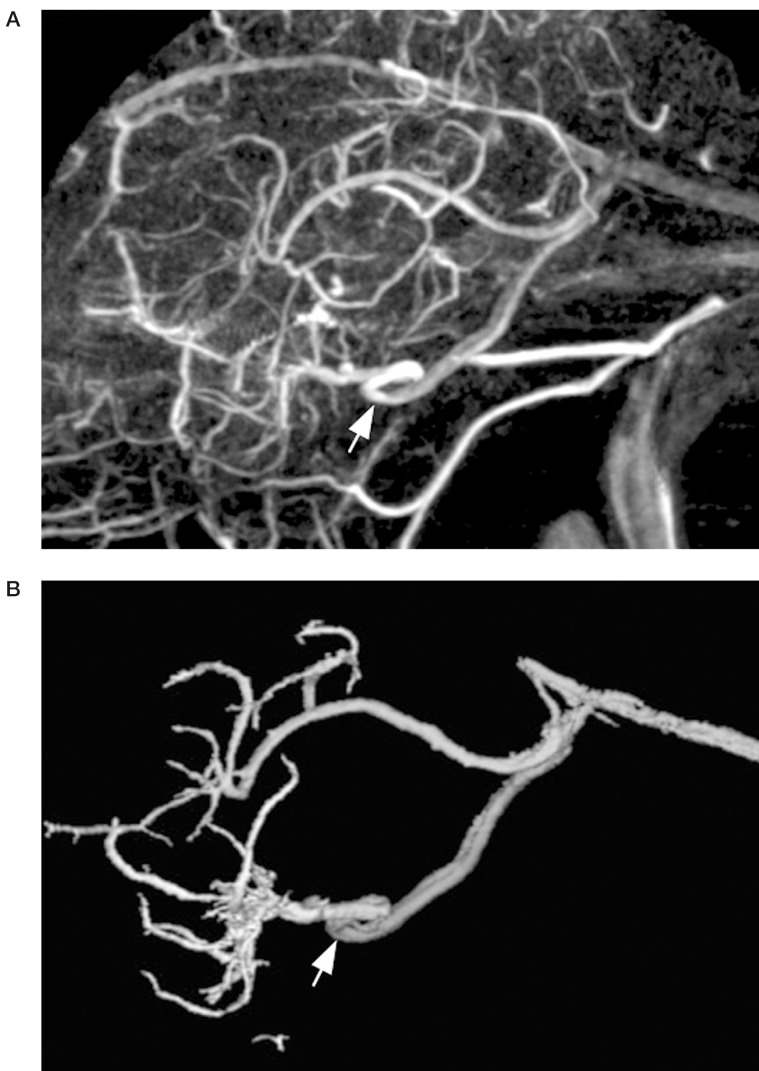
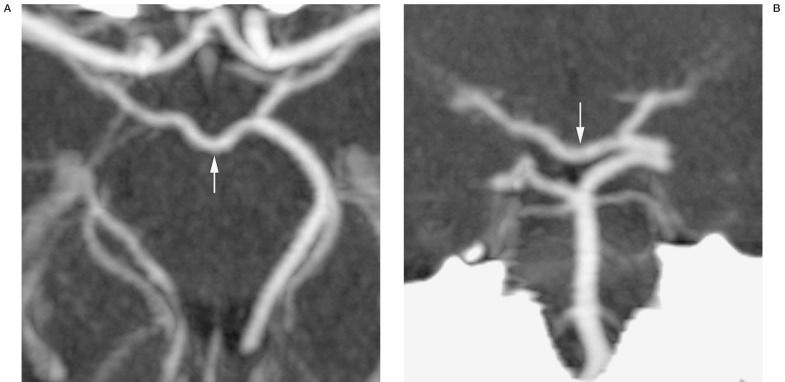
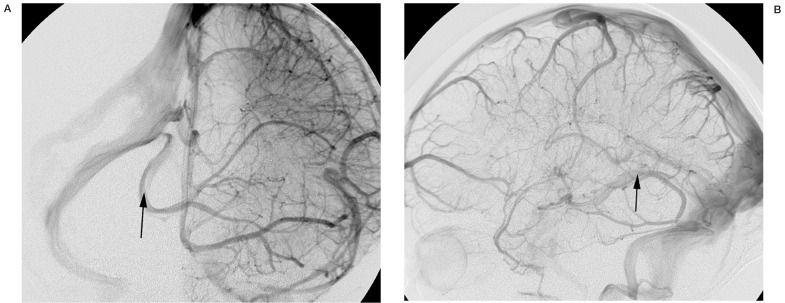
The posterior communicating vein is larger, more constant, and thus more readily identified on imaging studies. Occasionally, drainage in the posterior basal vein on DSA will be noted to cross the midline and opacify the contralateral basal vein before entering the great cerebral vein (figure 6). On lateral projections, a "notch" seen in the midline will correspond to the posterior communicating vein as it traverses in the depression of the interpeduncular fossa (figure 7). This is also apparent on 3D-DSV. CTV, similarly, shows the posterior communicating vein as it links the basal veins posteriorly (figure 8).
Figure 6.
Anteroposterior (A) and lateral (B) late phase of an internal carotid angiogram showing a large posterior communicating vein, with controlateral venous drainage (arrows).
Figure 7.
Lateral view 3D DSV of posterior communicating vein, showing "notch" of interpeduncular fossa (arrows).
Figure 8.
CTV axial (A) and coronal (B) images of posterior communicating vein (arrows).
The components of the venous circle are often more apparent in the presence of variant patterns of deep venous drainage. In the socalled split deep venous drainage, with agenesis of the vein of Galen and/or in the presence of a developmental venous anomaly (DVA) involving the deep venous system, the anterior communicating vein can be particularly prominent (figures 9,10). A mesencephalic AVM may access the posterior communicating vein to drain across midline into the venous circle (figure 11). Inflow from a tentorial sinus may be revealed by controlateral drainage via the posterior communicating vein (figure 12).
Figure 9.
Lateral views of 3D DSV showing agenesis of vein of Galen with complex developmental venous anomaly (DVA) with controlateral venous drainage via a large anterior communicating vein. Septal vein (single arrow). Epsilon-shaped drainage (double arrow).
Figure 10.
Anteroposterior views of 3D DSV showing agenesis of vein of Galen with complex developmental venous anomaly (DVA) with controlateral venous drainage via a large anterior communicating vein. Septal vein (single small arrow). Left cavernous plexus drainage (single large arrow).
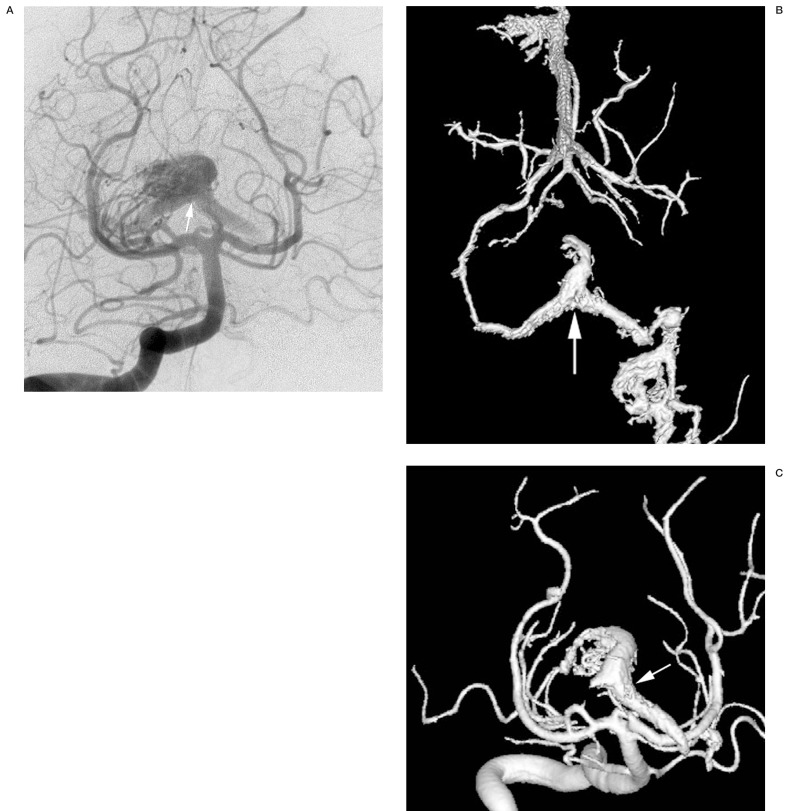
Figure 11.
Two-dimensional (A) and 3D angiographic images (B,C) of arteriovenous malformation with controlateral venous drainage via the posterior communicating vein (arrows).
Figure 12.
Late phase of an internal carotid angiogram showing large posterior communicating vein with wash-in from tentorial sinus (arrows).
Discussion
There is an anastomotic venous circle at the base of the brain. This is formed by the anterior and posterior communicating veins, which link the anterior and posterior segments of the basal veins of Rosenthal across the midline. This circle is functionally homologous to the arterial circle of Willis and provides a route of collateral venous drainage from the optic chiasm to the cerebral peduncles and between the right and left halves of the cerebral deep venous system. Vascular pathology such as arteriovenous shunts may access this circle for controlateral venous drainage (figure 10). The elements of this venous circle were described by Rosenthal in his treatise on the basal vein in 18242. Padget identified the components of the circle in her articles on the developing human venous circulation 3,4. Braun et Al provided gross anatomical and angiographic documentation of the anterior and posterior communicating veins 5. Current imaging techniques including 3D-DSV and CTV readily identify portions or all of this venous circle. The present study provides gross anatomical examples of the venous circle with current neuroimaging correlates.
The existence of a venous homologue to the arterial circle points to similarities between the arterial and venous systems that have not been stressed in the literature. In general, although the functional similarities between arteries and veins are immediately apparent, traditionally the differences between these two segments of the circulatory tree have been highlighted. These differences have until recently largely been ascribed to physiologic and flow-related phenomena. In addition, no doubt because of its variability and inherent complexity, the venous system and its role in disease states has not for the most part commanded the attention of the arterial circulation.
Padget pointed out that the development of the cranial venous system and its adult arrangement, despite their complexity and variability, are just as orderly as the arterial system 3. That order, or patterning, can be seen to reflect a series of modifications of the general plan of the spinal circulation, which is highly conserved throughout vertebrate evolution6. This principle can be expected to apply to arteries as well as veins (with the exception of the dural venous sinuses, which are a novel structure, not present in the spine). The fusion of the caudal divisions of the internal carotid artery to form the posttrigeminal basilar artery, for example, is analogous in this sense to the fusion of longitudinal paramedian components of the anterior spinal axis into a midline anterior spinal artery. While venous patterning in the spine is more complex, superficial transverse and transmedullary veins are present which connect the dominant longitudinal anterior and posterior median spinal veins7. The presence of a duplicated ventral spinal vein or a diamond shape disposition ventral to the cord can hardly be compared to the venous circle of the brain as the former location is subpial while the latter is subarachnoid. Comparisons with the more caudal dispositions of the ventral veins seems less fruitful than the same approach with the arteries.
Two important concepts have emerged from recent investigations regarding arterial and venous differentiation and vessel patterning on the molecular level. First, although many details remain to be worked out, it has become clear that artery-vein differentiation is not exclusively the consequence of circulatory dynamics, as was previously generally thought. Rather, through complex molecular pathways involving vegf, notch, and ephrin/Eph signaling, the molecular identity of arteries and veins becomes distinct before the vascular cords would allow flow to further characterize them 8. Second, many of the same molecular signaling pathways that play crucial roles in the specification, patterning and differentiation of the nervous system are also important in the differentiation and patterning of blood vessels9. The presence of a venous circle in frequent instances reveals that venous vasculogenesis, as a basic matrix allowing for its development, is present there in most cases.
It appears likely that these signaling pathways act locally very early in embryogenesis, during vasculogenesis, to produce an arterial - and a venous - collateral circle in close proximity to one another and capable of bidirectional flow. Yet they have very few relationships in terms of variation: asymmetry of variation of either system is never associated with mirror variation of the other. Transverse connections - relatively rare in the cerebral circulation - often occur at sites of neuronal network convergence: the optic chiasm, the anterior commissure, the merging of the cerebral peduncles in the mesencephalon. Other vessels crossing themidline, the bihemispheric anterior cerebral artery, the limbic arch system and bihemispheric posterior inferior cerebellar artery, can also be noted to use the adjacent brain as a "bridge"10 or use the midline seated leptomeningeal cisterns to cross the midline and contribute to the pial source of the supply to the brain. Much further work is needed to elucidate the links between neural, leptomeningeal and vascular patterning in the "neuro-pialvascular unit."
Conclusions
Adjacent to the arterial circle of Willis is a venous homologue, a collateral circle linking the anterior and posterior and right and left portions of the cerebral deep venous system. This anastomotic circle consists of the anterior and posterior communicating veins, and the basal veins. This circle may become important when segments of the basal vein are absent, or in high-flow states such as arteriovenous shunts that access the deep venous system. The existence of a homologous venous structure in close proximity to its better known arterial counterpart raises interesting - and as yet unanswered - questions regarding arterial and venous identity and vessel patterning in the cerebral circulation.
References
- 1.Duvernoy H. The superficial veins of the human brain. Berlin, Heidelberg, New York: Springer; 1975. [Google Scholar]
- 2.Rosenthal F. De intimis cerebri venis seu de venae magnae galena ramis. Nova acta physico-medica Academie Caesareae Leopoldino-Carolinae, Naturae cuiosorum. 1824;12:302–312. [Google Scholar]
- 3.Padget DH. The cranial venous system in man in reference to development, adult configuration and relation to the arteries. Am J Anat. 1956;98:307–355. doi: 10.1002/aja.1000980302. [DOI] [PubMed] [Google Scholar]
- 4.Padget DH. The development of the cranial venous system in man, from the viewpoint of comparative anatomy. Contributions to Embryology. 1957;36:79–140. [Google Scholar]
- 5.Braun JP, Tournade A, Ammerich H. Transverse anastomoses of the veins at the base of the brain. Neuroradiology. 1956;12:165–169. doi: 10.1007/BF00341861. [DOI] [PubMed] [Google Scholar]
- 6.Lasjaunias P, Berenstein A, Ter Brugge KG. 2nd edition. Vol 1. Berlin Heidelberg New York: Springer Verlag; 2001. Surgical Neuroangiography. [Google Scholar]
- 7.Thron AK. Vascular anatomy of the spinal cord. Wien New York: Springer Verlag; 1988. [Google Scholar]
- 8.Torres-Vazquez J, Kamei M, Weinstein BM. Molecular distinction between arteries and veins. Cell Tissue Res. 2003;314:43–59. doi: 10.1007/s00441-003-0771-8. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein BM. Vessels and nerves: marching to the same tune. Cell. 2005;120:299–302. doi: 10.1016/j.cell.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Cullen SP, Ozanne A, et al. The bihemispheric posterior inferior cerebellar artery. Neuroradiology. 2005;47:809–812. doi: 10.1007/s00234-005-1427-z. [DOI] [PubMed] [Google Scholar]