Summary
We describe an unconventional endovascular approach in a young patient with large high-flow traumatic carotid cavernous fistula that could not be treated by detachable balloon procedure. Two coronary stent-grafts were used to close the large tear of internal carotid artery.
After the failure of stenting procedure, the fistula was successfully treated by trapping with two detachable balloons.
Key words: traumatic carotid cavernous fistula, Stent-graft
Introduction
A direct carotid cavernous fistula (CCF) is a high-flow shunt between the internal carotid artery (ICA) and the cavernous sinus (CS) caused by trauma or rupture of a preexisting cavernous aneurysm.
The treatment of choice for CCFs is transarterial or transvenous balloon embolization or coil occlusion of the cavernous sinus with preservation of the parent artery. When the venous compartment of the fistula or the fistulous communication do not allow the use of the balloons or coils, the next treatment option is trapping of the ICA1. A different technical approach has recently been introduced using coronary stent placement into the cavernous segment of the ICA. We report the failure of a post-traumatic CCF treatment by two coronary stentgrafts, finally resolved with trapping of the ICA.
Methods
A 16-year-old male had a craniofacial trauma following a traffic accident in September 2004. A head CT did not show any brain or skull base lesions.
In December 2004, because of progressive proptosis, conjunctival injection, eye pain and a pulsatile bruit exophthalmus over the left eye, a head CT was performed. Evidence of both left cavernous sinus and left superior ophthalmic vein enlargement suggested the presence of a high-flow carotid cavernous fistula.
Standard cerebral angiography was performed by a femoral approach to assess the feeding arteries, the fistulous site and the drainage.
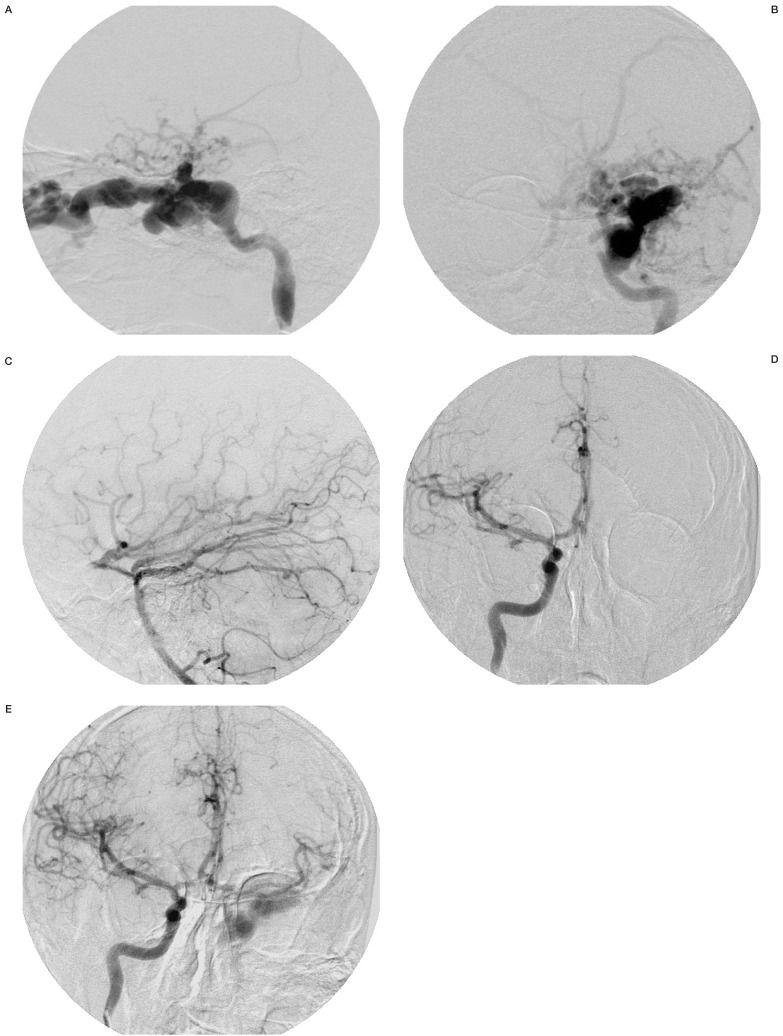
Angiography confirmed a high-flow Barrow type A CCF2 originating from the left cavernous segment of the ICA. The exact site of the fistula could not be identified; venous effluence was through the left superior ophthalmic vein and cortical veins (figure 1A,B). Left hemispheric arterial communication was filled by the vertebrobasilar artery and right ICA (figure 1C-E).
Figure 1.
Left carotid angiography (A: lateral view, B: A-P view) showing a CCF with venous effluence through the left superior ophthalmic vein and cortical veins. Left vertebral angiography (C: lateral view) and right carotid angiography (D: A-P view, E: A-P view after manual compression of left carotid artery) showing left hemispheric arteries filled by the vertebrobasilar artery and right ICA.
After discussion of the clinical and angiographic findings and the therapeutic options, the patient requested an endovascular procedure.
Two weeks later the patient underwent an endovascular treatment. At the first treatment session a balloon test occlusion (BTO) was performed for a neurological and angiographic evaluation using a 2F non detachable balloon microcatheter, 6 F guiding catheter via a right femoral approach and continuous heparinization, more than double the baseline activated clotting time (ACT).
At the second treatment session a detachable latex balloon (Goldbal 1 Balt) mounted at the tip of a 1.8 F microcatheter (Magic Balt) was used to close the fistula under general anaesthesia. The site of the fistula communication was assumed to be in the middle portion of the cavernous segment of the ICA. The balloon placed inside the cavernous sinus was inflated until the maximum pressure without total occlusion of the fistula and with stenosis of the parent vessel. The venous compartment and the tear in the ICA was felt to be too large to guarantee complete closure of fistula with detachable balloons or coils.
At the third treatment session a coronary stent graft (Jostent 16 × 4.5 mm) was easily brought through an 8 F catheter, via the left femoral approach, into the injured cavernous ICA and bridged the supposed tear.
Angiographic control showed persistence of the CCF. A second stent graft (19 × 4.5 mm) was placed next to the first one.
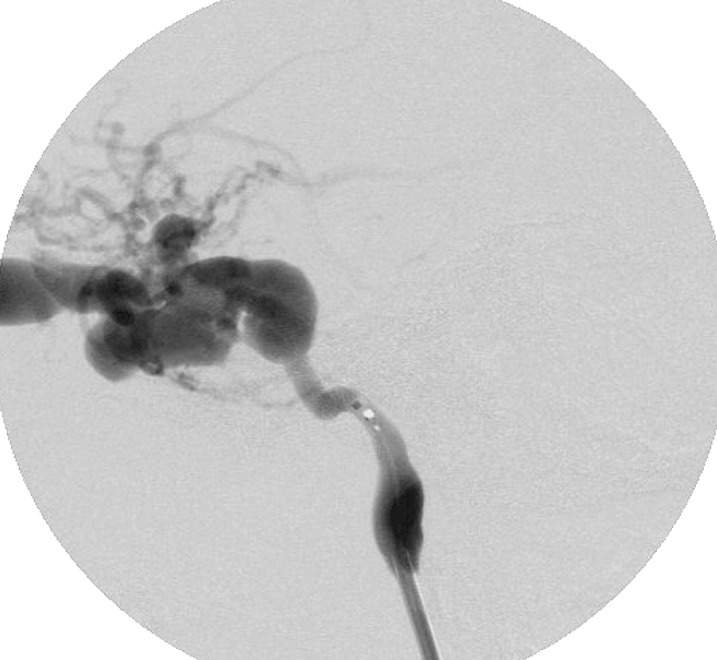
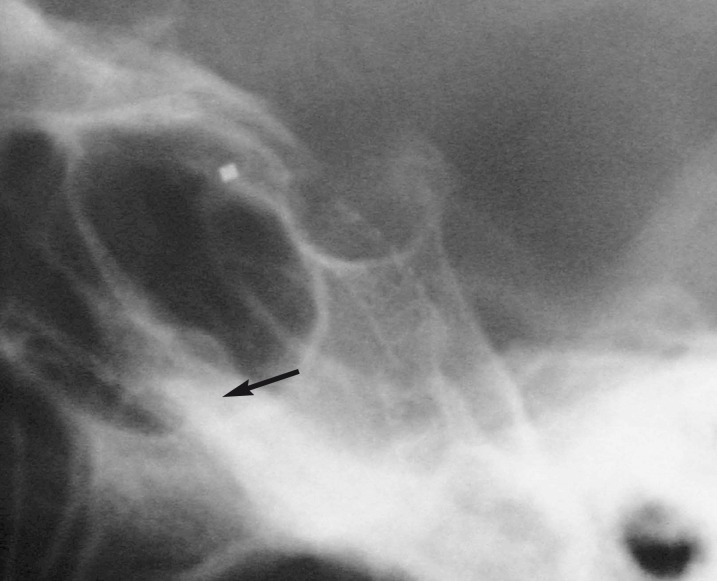
The angiographic control showed only a slowdown of the flow and persistence of the fistula (figure 2). The suspicion of ICA stenosis or occlusion at the level of the stents was confirmed by angiographic control of the right ICA and vertebral artery that showed the retrograde injection of the left carotid siphon and persistence of a low injection of CCF (figure 3A,B). Radiographic control (figure 4) showed a partial offset of the extremities of stentgrafts.
Figure 2.
Left carotid angiography, after releasing of two stent-grafts into cavernous segment of ICA, showing the persistence of the fistula.
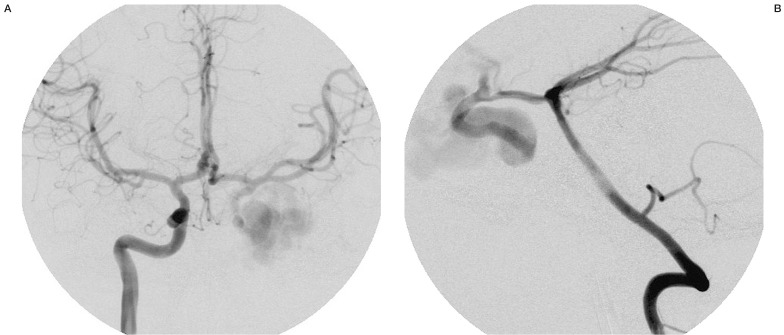
Figure 3.
Right carotid angiography (A) and left vertebral angiography (B) after left ICA stenting, showing retrograde injection of the left carotid siphon and persistence of slow injection of CCF.
Figure 4.
Radiographic film (lateral view); partial offset of the extremities of stent-grafts.
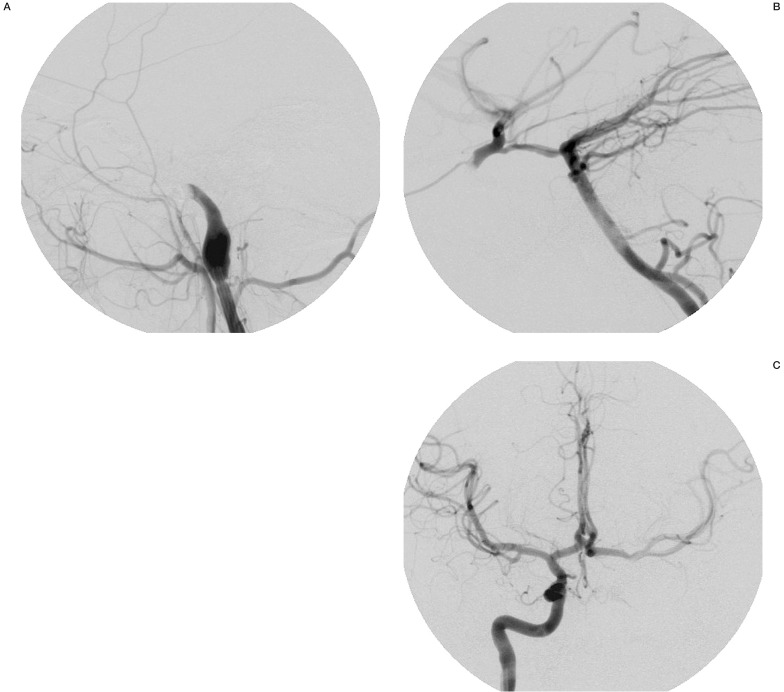
At the fourth and final treatment session, trapping of left carotid siphon was performed with two detachable balloons; the distal one was brought into the siphon via the posterior communicating artery and detached next to the origin of ophthalmic artery. The proximal balloon was released at the end of petrosal carotid canal resulting in complete closure of the fistula with improvement of left hemispheric circulation by collateral vessels (figure 5A-C).
Figure 5.
Left carotid angiography A) Left vertebral angiography B) and right carotid angiography (C: A-P view) and after endovascular trapping showing the closure of the fistula with improvement of left hemispheric circulation by collateral vessels.
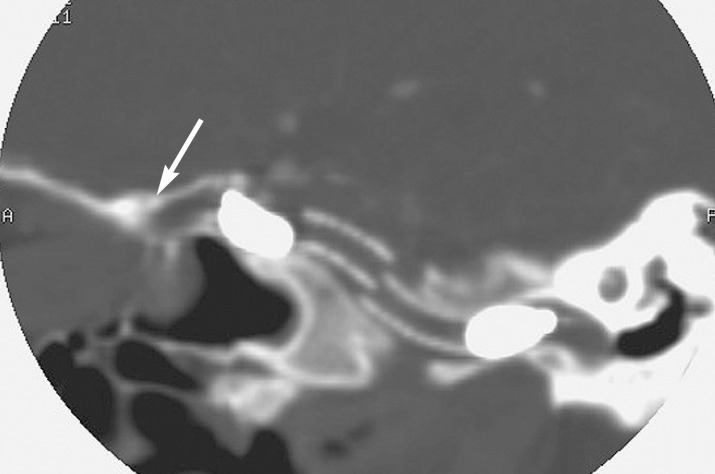
Follow-up CT angiography performed three days after the endovascular treatment confirmed the complete occlusion of the fistula, the correct position of balloons and a partial offset of covered stents (figure 6).
Figure 6.
CT angiography after trapping, showing the detached balloons at the stent-grafts and a partial offset (white arrow) of stent-graft
No neurological complication was noted. Full regression of ophthalmologic symptoms was achieved in two weeks.
Discussion
Most CCFs occur following skull base trauma or aneurysm rupture. Symptoms and sequelae are caused by arterialization of venous outflow from the CS and include proptosis, pulsatile bruit exophthalmus, ophthalmoplegia, impaired visual acuity and intracranial haemorrhage.
Since 19813, CCF have been successfully treated with preservation of the internal carotid artery (ICA), usually achieved by placing detachable balloons in the venous compartment by arterial route through the vessel tear. The CS must be large enough to receive one or more detachable balloons, but sometimes it must be smaller to allow access of balloons. However if the size of the fistula is too large and the embolization balloon may retract to the parent ICA causing a partial or complete occlusion. Precautions must be taken to avoid intracranial balloon detachment or migration. Teng et Al.4 suggested using a double balloon technique when the orifice of the fistula is too small for the passage of the deflated or slightly inflated balloon, or when the width of the cavernous sinus is smaller than the tear of the portant vessel (combination of a large fistula with a small CS). Moreover a delayed spontaneous deflation of the balloon over some weeks is expected. This results in a rate of pseudoaneurysms or pouch formation at the site of the healed CCF as high as 30%5.
Since 1991 detachable coils have been proposed as an alternative procedure via transarterial6 or transevenous routes7. The disadvantages of coil occlusion are an increased cost of the technique and possible coil occlusion of critical parenchymal veins afferent to the cavernous sinus. The mesh of coils within the cavernous sinus should present, in case of large communications, an interface to the parent vessel, like an endovascular occlusion of intracranial aneurysms, with the risk of clot formation and acute thromboembolic complications that can be reduced with heparinization and antiplatelet therapy. Halbach et Al. 6 described some failures of the platinum coil procedure in CCFs. In one case, finally treated with endovascular trapping of ICA, the cast of platinum coils previously released into the CS projected through the fistula into the parent artery. In a second one the closure of anterior drainage was achieved, but complicated by distal migration of platinum coils with aggravation of ocular symptoms.
Endovascular cavernous sinus embolization with balloons or coils may be responsible for cranial nerve palsy. Sencer et Al. 8 reported a case with mechanical compression of the lateral rectus muscle by balloons. These difficulties could be resolved with an intracranial covered stent.
In 2001 Weber et Al. 9 described a case of direct carotid cavernous fistula cured by endovascular porous coronary stent. This kind of device is highly flexible and easily follows the curves of vessels.
In 2002 Kocer et Al. 10 reported a case of an iatrogenic carotid cavernous fistula successfully treated with endovascular stent-graft (Jostent 12 × 4 mm).
Covered stent or stent-graft provides waterproof sealing of the bridged vessel segment11. It would provide ideal features for many neurovascular purposes such as aneurysms, vessel wall dissections and arteriovenous fistulas. Stent-graft has a cover of polytetrafluoroethylene, used for many years as a graft material during vascular surgery12. It has a low incidence of complications, including acute stent thrombosis. Use of this device is the therapy of choice for acute coronary rupture.
Jostent, provided by Jomed, barely follows the curves of intracranial vessels. It can be used to reach only the first segment of intracranial ICA, the intracavernous segment. This poor navigability is closely connected to the length of device. For this reason we started using a small Jostent (16 × 4.5 mm), too short for the size of the tear. The second stent (19 × 4.5 mm) was placed next to the first one but its release was not perfect. That resulted in partial offset of their extremities with consequent ICA stenosis and persistent CCF, leading us to perform an endovascular trapping.
Although the therapeutic goal is the occlusion of the fistula with preservation of the ICA, in case of failure of a preservative procedure or further difficulties during an endovascular treatment, trapping must be considered1. Therefore previous balloon test occlusion must be always performed.
Without any type of temporary test occlusion, the incidence of stroke after permanent carotid artery occlusion ranges from 17% to 30%13.
Conclusions
There are few reports in the literature of treatment of CCF with a stent-graft. When a traditional endovascular procedure cannot be performed, the stent-graft could be used before trapping, always after balloon test occlusion. We expect a positive outcome from the initial experiences. Future technical developments of this device will cancel some of current limitations, responsible for our failure.
References
- 1.Stuart C Coley, Hament Pandya, et al. Endovascular trapping of Traumatic Carotid-Cavernous Fistulae. Am J Neuroradiol. 2003;24:1785–1788. [PMC free article] [PubMed] [Google Scholar]
- 2.Barrow DL, Spector RH, et al. Classification and Treatment of spontaneous carotid cavernous fistulas. Neurosurgery. 1985;62:248–256. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 3.Debrun G, Lacour P, et al. Treatment of 54 traumatic carotid cavernous fistulas. J Neurosurgery. 1981;55:678–692. doi: 10.3171/jns.1981.55.5.0678. [DOI] [PubMed] [Google Scholar]
- 4.Michael Mu Huo Teng, Cheng-Yen Chang, et al. Cavernous Fistulas. Am J Neuroradiol. 2000;21:1753–1756. [PMC free article] [PubMed] [Google Scholar]
- 5.Pearse P Morris. Balloon Reconstructive Technique for the Treatment of a Carotid Cavernous Fistula. Am J Neuroradiol. 1999;20:1107–1109. [PMC free article] [PubMed] [Google Scholar]
- 6.Halbach VV, Higashida RT, et al. Transarterial platinum coil embolization of carotid-cavernous fistula. Am J Neuroradiol. 1991;12:429–433. [PMC free article] [PubMed] [Google Scholar]
- 7.Halbach VV, Higaschida RT, et al. Transvenous embolization of direct carotid-cavernous fistulas. Am J Neuroradiol. 1988;9:741–747. [PMC free article] [PubMed] [Google Scholar]
- 8.Serra Sencer, Ozenc Minareci, Arzu Poyanli. Treatment of Direct Carotid Cavernous Fistula. Am J Neuroradiol. 1999;20:1465–1466. [PMC free article] [PubMed] [Google Scholar]
- 9.Weber W, Henkes H, et al. Cure of a Direct Carotid Cavernous Fistula by Endovascular Stent Deployment. Cerebrovasular Diseases. 2001;12:272–275. doi: 10.1159/000047715. [DOI] [PubMed] [Google Scholar]
- 10.N Kocer, O Kizilkilic, et al. Treatment of Iatrogenic Internal Carotid Artery Laceration and Carotid Cavernous Fistula with Endovascular Stent-Graft Placement. Am J Neuroradiol. 2002;23:442–446. [PMC free article] [PubMed] [Google Scholar]
- 11.Cocer Celil, Dursum Engin, et al. Intractable epistaxis related to cavernous carotid artery pseudoaneurysm: treatment of a case with covered stent. Auris Nasus Larynx. 2004;31:275–278. doi: 10.1016/j.anl.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Elsner M, Auch-Schwelk W, et al. Coronary Stent-Graft Covered by a Polytetrafluoroethylene Membrane. Am J Cardiology. 1999;84:335–338. doi: 10.1016/s0002-9149(99)00289-1. [DOI] [PubMed] [Google Scholar]
- 13.Carotid Artery Balloon Test Occlusion. Am J Neuroradiol. 2001;22(8 Suppl):S8–9. [PMC free article] [PubMed] [Google Scholar]