Summary
There are many reasons for patients infected with human immunodeficiency virus (HIV) to develop cerebrovascular disease. The HIV virus itself however may be a cause of vessel wall pathology. We present a clinical and pathological study of a patient who was HIV positive and presented with a subarachnoid haemorrhage. Cerebral angiography and later histology confirm that there was extensive vessel wall injury with dissection and a false aneurysm of the right middle cerebral artery.
Key words: HIV, arterial dissection, cerebro-vascular disease
Introduction
Patients infected with HIV appear to be prone to developing vasculitis with a reported incidence of 23%1. A large range of pathologies have been described affecting different vessel sizes in most organs. It would appear however that the skin and central nervous systems are the systems most often involved 2. As in any immunocompromised state an opportunistic infection may be the direct cause of a vasculitis and in HIV patients cytomegalovirus and tuberculosis are often responsible 3. The vasculitis may be due to other causes however, such as hypersensitivity reactions, poly-arteritis nodosa-like vasculitis or primary HIV vasculitis, particularly of the central nervous system. Along with these pathological descriptions there are also clinical and radiological descriptions of vasculopathy, particularly the dilated arteriopathy of cerebral vessels seen in advanced HIV infection4,5.
We previously reported this dilated arteriopathy in association with subarachnoid haemorrhage (SAH). Based on angiography findings we felt that the most likely cause of SAH in these patients was arterial dissection related to pathological changes in cerebral vessels5. The following case report, we believe strengthens the argument the HIV infection results in weakening of cerebral vessels and may cause dissection and SAH.
Case Report
A 28-year-old HIV positive patient presented following two episodes of severe headache. The headaches were three days apart and the last was described as a typical thunder-clap headache with associated neck stiffness. On examination the patient had meningism but was fully conscious and had no focal signs. A lumbar puncture confirmed the diagnosis of SAH. A CT scan demonstrated acute blood in the cisternal spaces but particularly in the right sylvian fissure.
On day three after her second headache onset she had a cerebral angiogram which demonstrated irregularity of multiple vessels and a right sided middle cerebral artery aneurysm. No neck could be defined and because of subtle vessel stenosis and dilatation related to the aneurysm we felt it represented a false aneurysm following transmural vessel dissection. As parent vessel occlusion was the only surgical option we made the decision to place undersized coils into the false lumen endovascularly. Our hope was that this would initiate some thrombosis and allow for spontaneous healing as we had seen in other cases. The procedure was performed under local anaesthetic as no anaesthetist was available at that time. A 14 microcatheter was easily placed into the false lumen and a 10 soft 3 mm by 8 cm helix coil was deployed but not detached. At this point the patient suffered a grand mal seizure and the procedure was delayed while she was intubated and anaesthetized. Control runs showed the coil was still in place and it was then detached. Two further ten soft coils of 3 mm by 6 cm were placed and detached. At this point thrombosis of the false lumen was occurring and distal flow in the right middle cerebral artery was slowing. The procedure was stopped and the patient was taken for a CT scan.
Figure 1.
Lateral and oblique angiogram images of the right carotid showing the false aneurysm as well as an irregular M2 branches with areas of dilatation and narrowing.
Figure 2.
The final angiogram after placing three undersized soft coils into the false lumen. At this point there was already delayed flow in the distal middle cerebral vessels.
The scan revealed new SAH and a 2 cm haematoma in the right sylvian fissure. This had presumably occurred during the seizure as no extravasation was observed during any angiography runs. The patient was kept ventilated overnight and was extubated the following day. At this time she had a left hemiparesis and was localizing to painful stimulus. Twelve hours after extubation she had a sudden deterioration in her level of consciousness and a CT scan was repeated. The scan demonstrated a right parietal area infarct in keeping with middle cerebral artery occlusion. Unfortunately despite intensive care the patient continued to deteriorate and was declared brain dead the following day.
A full post mortem was performed including brain dissection following fixation in formol saline. Relevant findings included surface SAH and what appeared to be coils extruding from the region of the right middle cerebral bifurcation artery into the subarachnoid space. No saccular aneurysm could be identified. Cerebral vessels were further dissected and prepared for histological examination.
Sections through both the right middle cerebral artery and basilar artery demonstrated the following features; very marked intimal proliferation, disruption of the internal elastic lamina and peri-adventitial new vessel formation, with ecstatic thin walled vessels. Furthermore there was evidence of dissection of the middle cerebral artery, close to the aneurysm, with blood clot seen within the media layer. Also observed were giant cells present within the adventitia although no evidence of tuberculosis infection could be found. Ziehl-Neelsen stains for acid fast bacilli did not demonstrate M tuberculosis. There was extensive anti-mortem thrombus within the right middle cerebral artery but not other vessels.
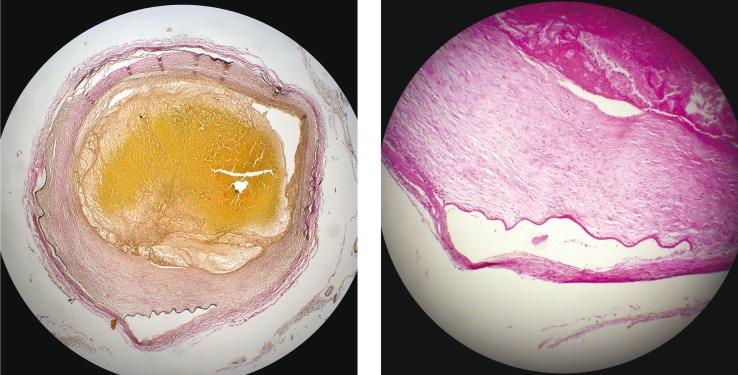
Figure 3.
Haematoxylin and eosin stain (H & E). Section through the basilar artery showing areas of intimal thickening, disruption of the internal elastic lamina and new vessels in the adventitia.
Figure 4.
Elastic von Giesson stain (EVG). A high power view of a dissected area of the middle cerebral artery with blood tracking into the media layer of the vessel. Numerous new vessels can also be seen within the adventitia layer.
Figure 5.
EVG stain and H & E stain. A low power and high power view of the distal middle cerebral artery showing organizing thrombus within the lumen, an irregularly thickened intima and disruption of the internal elastic lamina. Poor cohesion between internal elastic lamina and adventitia creates artefactual splits.
Discussion
The incidence of intracerebral haemorrhage and ischaemic stroke in AIDS have both been reported as 0.2% per year in a recent population based study by Cole. Even when known risks for stroke or haemorrhage were excluded such as infective endocarditis or opportunistic intracranial infection the relative risk was still increased for ischaemic stroke at 9·1 (95% CI, 3·4-24·6) and 12·7 (95% CI, 4·0-40·0) for intracerebral haemorrhage. The patients included in this study were not on highly active antiretroviral therapy (HAART) as the study was conducted at a time when patients were only offered AZT6. This is important as the antiproteases in HAART have been associated with dyslipidaemia and insulin resistance which accelerate atherothrombosis 7.
The implication of Coles study is that AIDS may predispose patients to cerebrovascular events without there being any other associated risk factors. This theory is supported by work done by Blum showing that high HIV viral load is associated with endothelial dysfunction8. There have been various pathological descriptions of vasculitis of cerebral vessels associated with HIV infection as well as angiographic descriptions of a dilated cerebral angiopathy that has been associated with both stroke and haemorrhage2,4,9. We previously reported three patients with AIDS who presented with SAH and on angiography had features of dilated cerebral angiopathy and what we felt was transmural vessel dissection. If dissection was a risk in patients with AIDS it may offer an explanation for both occlusive stroke and intracerebral haemorrhage. Of course there may be other factors which could be causative or contributory. Both hyercoagulable and hypocoagulable states have been described in HIV infection. The presence of antiphospholipid-anticardiolipin antibody and deficiency in Protein S have been described as causes of thrombosis and autoimmune thrombocytopaenia has been reported as causing haemorrhage in these patients10,11.
The histological findings in this case are similar to other reports where intimal proliferation and damage to the internal elastic lamina have been reported4,12. As well as this however there was histological evidence of vessel wall dissection with intramural blood. Two possibilities exist for this finding. Firstly the endothelial proliferation may not allow for normal tight junctions between cells making their union weaker and susceptible to a luminal entry of blood into the media. Alternatively the new vessel proliferation observed in the adventitia may offer a point of weakness for haemorrhage into the vessel wall, similar to that described with vasavasorum rupture in large arteries. Vasa-vasorum have been reported to occur in intracranial arteries, and are more frequently noted in atherosclerotic cases and in elderly patients. They occur more often in the adventitia but have also been noted within the media layer13,14,15. The dilated new vessels seen in the adventitial layer of this patient's arteries may represent proliferating vasa-vasorum in response to some unknown trigger.
The outcome in this case was unfortunate. We have previously had success in partial coiling of false aneurysms in this setting, resulting in thrombosis of the false lumen and repair of the native vessel as seen on later angiography. Because the placement of coils into a false lumen carries a high risk we have intentionally undersized the coils to limit any force that may be placed on fragile clot. In this instance however two complications occurred. Firstly we had aneurysm rupture and secondly propagation of thrombus and middle cerebral artery occlusion. Because there appears to be widespread histological damage to vessels in AIDS we would caution against any reconstructive procedures such as stenting. If there is a place for any intervention it should be the simplest and least traumatic.
There are many reasons for patients with HIV and AIDS to present with neurovascular disease. In the absence of other stroke or haemorrhage risks however a primary vasculopathy with dissection should be considered.
References
- 1.Gherardi R, Belee L, et al. The spectrum of vasculitis in human immunodeficiency virus infected patients. Arthritis Rheum. 1993;36:1164–1174. doi: 10.1002/art.1780360818. [DOI] [PubMed] [Google Scholar]
- 2.Chetty R. Vasculitides associated with HIV infection. J Clin Pathol. 2001;54:275–278. doi: 10.1136/jcp.54.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lie JT. Vasculitis associated with infectious agents. Curr Opin Rheumatol. 1996;8:26–29. doi: 10.1097/00002281-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kure K, Park Yd, et al. Immunohistochemical localization of an HIV epitope in cerebral aneurysmal arteriopathy in pediatric acquired immunodeficiency syndrome (AIDS) Pediatr Pathol. 1989;9:655–667. doi: 10.3109/15513818909022373. [DOI] [PubMed] [Google Scholar]
- 5.Taylor A, LeFeuvre D, et al. Arterial dissection and subarachnoid haemorrhage in HIV infected patients. A report of 3 cases. Interventional Neuroradiology. 2004;10:137–141. doi: 10.1177/159101990401000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole JW, Pinto AN, et al. Acquired immunodeficiency syndrome and the risk of stroke. Stroke. 2004;35:51–56. doi: 10.1161/01.STR.0000105393.57853.11. [DOI] [PubMed] [Google Scholar]
- 7.Mheta N, Reilly M. Atherosclerotic cardiovascular disease risk in the HAART treated HIV1 population. HIV Clin Trials. 2005;6:5–24. doi: 10.1310/HT0W-NX2N-U2BM-7LUU. [DOI] [PubMed] [Google Scholar]
- 8.Blum A, Hadas V, et al. Viral load of the human immunodeficiency virus could be an independent risk factor for endothelial dysfunction. Clin Cardiol. 2005;28:149–153. doi: 10.1002/clc.4960280311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patsalides AD, Wood LV, et al. Cerebrovascular Disease in HIV-Infected Pediatric Patients: Neuroimaging Findings. American Journal of Roentgenology. 2002;179:999–1003. doi: 10.2214/ajr.179.4.1790999. [DOI] [PubMed] [Google Scholar]
- 10.Shen YN, Frenkel EP. Thrombosis and a hypercoagulable state in HIV-infected patients. Clin Appl Thromb Hemost, 2004;10:277–280. doi: 10.1177/107602960401000311. [DOI] [PubMed] [Google Scholar]
- 11.Silvestrini M, Floris R, et al. Spontaneous subarachnoid haemorrhage in an HIV patient. Ital J Neurol Sci. 1990;11:493–495. doi: 10.1007/BF02336570. [DOI] [PubMed] [Google Scholar]
- 12.Joshi VV, Pawel B, et al. Arteriopathy in children with acquired immune deficiency syndrome. Pediatr Pathol. 1987;7:261–275. doi: 10.1080/15513818709177129. [DOI] [PubMed] [Google Scholar]
- 13.Takaba M, Endo S, et al. Vasa Vasorum of the intracranial arteries. Acta Neurochir. 1998;140:411–416. doi: 10.1007/s007010050118. [DOI] [PubMed] [Google Scholar]
- 14.Connolly ES, Huang J, Goldman JE. Immunohistochemical detection of intracranial vasa vasorum: a human autopsy study. Neurosurgery. 1996;38:789–793. [PubMed] [Google Scholar]
- 15.Aydin F. Do human intracranial arteries lack vasa vasorum? A comparative immunohistochemical study of intracranial and systemic arteries. Acta Neuropathol. 1998;96:22–28. doi: 10.1007/s004010050856. [DOI] [PubMed] [Google Scholar]