Abstract
Introduction
Mitogen Activating Protein (MAPK) kinase phosphatase-1 (MKP-1) has been shown to be a key negative regulator of the MAP kinase pathways of the innate immune system. The impact of MKP-1 in an endodontic model has yet to be studied. Thus, the purpose of this study was to determine the role of MKP-1 in a bacterial-driven model of pathological endodontic bone loss.
Methods
Pulps were exposed in both lower 1st molars of 10-week old mkp-1+/+ and mkp-1−/− mice and left open to the oral environment for either 3 or 8 weeks. At sacrifice, mandibles were harvested and scanned by microcomputed tomography (μCT) to determine periapical bone loss. Histopathological scoring was then performed on the samples to determine the amount of inflammatory infiltrate within the periapical microenvironment.
Results
Significant bone loss and inflammatory infiltrate were found in all experimental groups when compared to control. No statistical difference was found between mkp-1+/+ and mkp-1−/− at either time point with respect to bone loss or inflammatory infiltrate. At 8 weeks, male mkp-1−/− mice were found to have significantly more bone loss and inflammatory infiltrate when compared to female mkp-1−/− mice. There was also a significant correlation between an increase in bone loss and increase in inflammatory infiltrate.
Conclusions
A sexual dimorphism exists in the periapical inflammatory process, where male mkp-1−/− mice have more inflammation than female mkp-1−/− mice. The increase in inflammatory infiltrate correlates to more bone loss in the male mice.
Keywords: MAP kinase phophatase-1, sexual dimorphism, periapical bone loss, inflammation
Introduction
Periapical lesions of endodontic origin occur as a consequence of the host immune inflammatory response to oral pathogens. Lipopolysaccharide (LPS) derived from endodontic pathogens can stimulate the production of IL-1β, TNF-α, IL-6 and RANKL, either directly or indirectly. The production of inflammatory cytokines is a tightly controlled process that involves both transcriptional and posttranscriptional mechanisms. In stimulated cells, maximal induction of cytokine expression requires activation of the mitogen activating protein kinase (MAPK) stress kinase pathway. The production of inflammatory cytokines is the consequence of activation of key intracellular pathways, including MAPK pathways (1-3) following activation of toll-like receptor (TLR) complexes that recognize bacterial constituents. MAPKs consist of three major families, including extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 MAPK. MAPK pathways relay, amplify and integrate signals, which modulate a series of physiological responses including cellular proliferation, differentiation, development, inflammatory responses and apoptosis (4).
The innate immune response is activated in multiple cell types associated with the endodontic lesions including monocytes/macrophages, granulocytes, pulpal fibroblasts, osteoclast precursors and mesenchymal cells (5, 6). Activation of these receptors leads to the stimulation of multiple pro-inflammatory cytokines, including IL-1β, TNFα, and IL-6, and has been associated with enhanced RANKL production, osteoclastogenesis and bone resorption (5, 6). In addition to the innate immune system, the adaptive immune response has been shown to play a role in bone resorption in lesions of endodontic origin (7). The predominant cell types in endodontic lesions in a rat model were shown to be T-cells followed by B-cells and monocytes/macrophages (8).
Although the activation of MAPK pathways is critical to initiate an innate immune response against invading pathogens, sustained production of pro-inflammatory cytokines can result in bone resorption. A number of studies have demonstrated that LPS from gram-negative bacteria was capable of inducing bone resorption in vivo (9-11). Therefore, modulating MAPK immune response to an appropriate level is essential to attenuate bone resorption associated with bacterial infection. In mammalian cells, MAPKs are activated by phosphorylation on critical tyrosine, serine or threonine residues. Inactivation of activated MAPKs occurs via dephosphorylation by MAP kinase phosphatases (MKPs,) a group of dual-specificity protein phosphatases (DUSP) (12). To date, at least 11 MKP family members have been identified in mammalian cells, with MKP-1 being the first discovered. Studies have shown that mkp-1−/− mice are susceptible to endotoxic shock and exhibited a marked increase in production of pro-inflammatory cytokines TNF-α, IL-6, and an anti-inflammatory cytokine IL-10 as compared with wild-type animals (13). MKP-1−/− mice also had a marked increase in both the incidence and severity of experimentally induced autoimmune arthritis (14, 15), and exhibited more bone loss after LPS challenge as compared with wild-type animals (16). These results highlight the significance of MAPK/MKP-1 regulation on innate immune response to LPS-induced inflammation and maintaining bone homeostasis, suggesting that restriction of activated MAPKs is a potential therapeutic strategy for diseases associated with exaggerated MAPK responses. Recent studies confirmed that MKP-1 is a key negative regulator of periodontal disease progression in LPS-driven models of experimental periodontal disease (16, 17), but its role in a bacterial-driven environment has not been determined. Thus, the purpose of this study was to determine the role of MKP-1 in a bacterial-driven model of pathological endodontic bone loss.
Material and Methods
Animals
The Institutional Animal Care and Use Committee (IACUC) at the Medical University of South Carolina approved all experimental protocols. Mice used for this application were initially obtained through a material transfer agreement (MTA) from Bristol Myers Squibb. Generation of homozygous MKP-1 knockout mice (KO) was done through mating MKP-1+/− mice to obtain MKP-1 null mice and maintained on a mixed C57/129 genetic background.
Periapical Bone Loss Model: Pulp exposure was obtained as described in previous endodontic models (18,19.) Briefly, 10-week old male and female mice were anesthetized using intraperitoneal injection of Ketamine (80mg/kg) and xylazine (10mg/kg) in sterile phosphate-buffered saline after induction with inhalational isoflurane. For experimental groups, lower first molars were accessed and pulps exposed under surgical operating microscope (Olympus Highlight 3100/SZ61; Olympus Imaging America, Center Valley, PA) with a dental handpiece (Aseptico; Woodinville, WA) and ¼ round bur. Access allowed penetration of #6 endodontic hand file to the mesial and distal root canals. The pulp was then left exposed to the oral environment for three or eight weeks. Euthanization occurred by CO2 asphyxiation. Mandibles were harvested and sectioned into two halves at the midline for analysis. Control mice were mixed gender and did not receive exposure.
MicroCT Analysis
Anatomic sections were performed to include the mandibular 1st through 3rd molars, as well as surrounding osseous tissues. Samples were placed in 10% formalin for 24 hours and then stored in 70% ethanol. Cone-beam microcomputed tomography (μCT) scans were obtained using high-resolution desktop μCT (μCT40 scanner; Scanco USA, Inc., Wayne, PA). Initial data reconstruction was performed using Scanco Medical Open VMS software (Scanco USA, Inc.) For visualization, samples were digitally reconstructed so that a two-dimensional slice could be obtained showing a patent mesial and distal canal in the first molar.using GE MicroView software (GE Healthcare BioSciences, Chalfont St. Giles, UK). Data was exported into ImageJ for further analysis. Periapical regions of interest were obtained by digital cropping and measured by the software in square pixels. All measurements were performed by the same trained examiners and repeated at separate time intervals. Mean bone loss cross-sectional area was compared in mkp-1−+/+ and mkp-1−/− mice.
Histology and Inflammation Indices
Following μCT analysis, specimens were decalcified in a 0.5 M EDTA solution pH 8.0 for 4 weeks at 4°C. Mandibular sections were paraffin-embedded, and 7 μm sagittal sections prepared. Some sections were stained with hematoxylin and eosin (H&E) for descriptive histology. Scoring of inflammatory infiltrate was performed with a trained pathologist (HY). Scoring of inflammation corresponded with the following index: 0 = No neutrophils near the root; 1 = sparse (<5%) neutrophils adjacent to the root apex; 2 = 5-20% neutrophil infiltration adjacent to the root apex; 3 = 20-50% neutrophils in the area limited adjacent to root apex; 4 = numerous (>50%) neutrophils in area greater than 50% adjacent to root apex.
Statistical analysis
Non-parametric Wilcoxon (Mann-Whitney) rank-sum test were used with an alpha=0.05 two-sided significance level to evaluate potential differences between mkp-1−/− and wild-type mice in bone loss and histological scoring measures per hemi-mandible. To accommodate for the within-cluster dependence of the data, we applied the corrected variance formula for the Wilcoxon rank-sum statistics developed by Rosner and colleagues (19). We report mean and standard error results estimated utilizing mixed effect regression models. We also used multivariable mixed effect regression models to assess associations between bone loss and histological scoring, while adjusting for time, gender, and mkp-1−/− versus mkp-1+/+ status. All possible 3- and 2-way interactions were tested in this multivariable model. All statistical analyses were performed using SAS® Proprietary software, 9.2, © 2002-2008, SAS Institute Inc., Cary, NC, USA.
Results
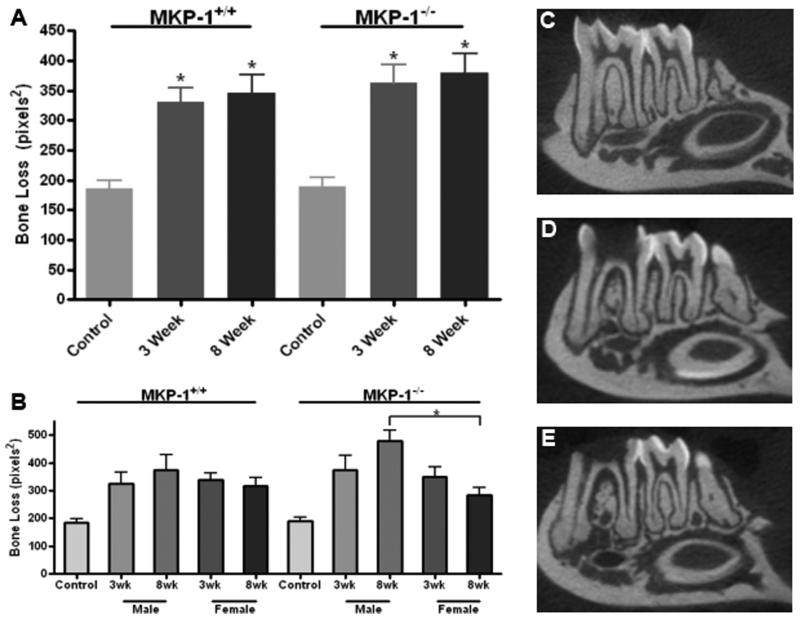
Post-endodontic exposure, μCT analysis of mandibular samples revealed that a significant amount of periapical bone loss was achieved using this experimental protocol compared to untreated controls in all groups (Fig. 1A). Although analysis of mkp-1−/− mice showed numerically greater amounts of bone loss than bone observed in age-match wild-type littermate controls, significant differences in bone loss were not detected at either 3- or 8-week time points. However, among mkp-1−/− mice at 8 weeks, significantly more bone loss occurred in males compared to age-match female mice (P<.05), a result that was not observed among wild-type mice (Fig. 1B).
Figure 1. Microcomputed tomography indicates significant periapical bone loss from mkp-1−/− male mice.
(A) Mean periapical bone loss area as determined by μCT analysis from control and 3-and 8-week exposed molar groups in wild type and mkp-1 deficient male and female mice. (B) Average periapical bone loss separated by gender. Representative μCT splines of control group (C), 3-week treatment group (D) and 8-week treatment group (E). All μCT images are from mkp-1−/− male mice. (N= 10/genotype in 3 and 8 week groups; *P<.05)
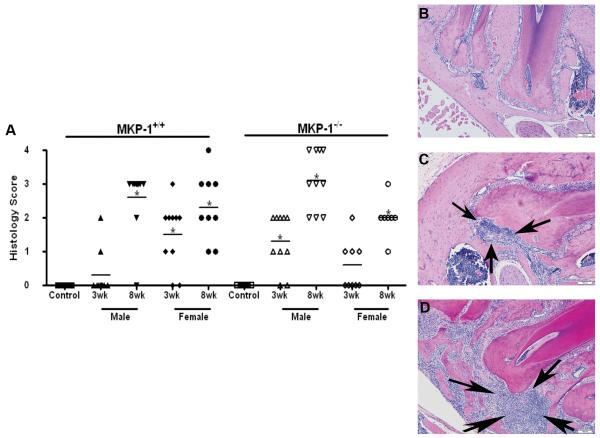
To determine if the degree of inflammation was consistent with μCT data, inflammatory infiltrate was evaluated and quantitated in the periapical area. Similar to data from μCT analysis, there was not a significant difference in the amount of neutrophil infiltrate between mkp-1−/− and mkp-1+/+ mice at either 3- or 8-week time points (Fig 2A), although the mean scoring for mkp-1−/− mice was higher than that of age-match wild-type control mice. As with bone loss, there was a significantly higher histopathology score in mkp-1−/− males compared to mkp-1−/− females at 8 weeks (P<.05) (Fig. 2A). There was a significant correlation found to exist between histopathology score and amount of bone loss among all groups (P<.05; data not depicted in figures).
Figure 2. Histopathology indicates that male mkp-1−/− male mice have increased neutrophil infiltrate.
(A) Scatter plot analysis with mean histopathologic scoring of neutrophil infiltrate indicated by horizontal line from control and 3-and 8-week treated groups in wild type and mkp-1−/− mice. Representative H&E slides corresponding to periapical areas used for histopathologic analysis of control group (B), 3-week treatment group (C) and 8-week treatment group (D), All histological images are from mkp-1−/− male mice. Arrowheads indicate neutrophil infiltrate and scale bar is 100μM. (N= 10/group; *P<.05).
Discussion
Invasion of the dental pulp system by bacterial constituents, such as LPS, leads to the stimulation of the innate immune system through multiple intracellular signaling pathways, including the MAPK cascade. Innate immune cells, i.e., neutrophils and macrophages, within the periapical microenvironment are activated to both produce and respond to pro-inflammatory cytokines leading to enhanced osteoclastogenesis and bone resorption. In previous studies, MKP-1 has been shown to be a key component in the attenuation of bone loss through a role as negative regulator of the MAPK system. Although in this study, a trend existed where mkp-1−/− mice had more bone loss than wild-type counterparts, this did not reach significance. There was, however, a significant difference in bone loss and histological scoring between male and female genders of the 8-week mkp-1−/− group. Males showed an increased periapical inflammatory response as compared to females with respect to bone loss and histological score.
There have been previous studies showing a sexual dimorphism related to inflammation similar to that observed in this study. The protective role of estrogen has been shown in a study of ovariectomized rats with induced periapical lesions. This protective effect has been hypothesized to be due to effect on the synthesis and release of pro-inflammatory cytokines (21). In the periodontal literature, it has been shown that males are more prone to periodontal infection than females (22). Elevation of LPS binding protein, CD14 and TLR-4 in males, was noted as a plausible explanation for the gender difference (23).
The clinical significance of sexual dimorphism in periapical inflammation has not been fully elucidated. In a previous endodontic outcome study, it was found that male patients were more likely to be associated with preoperative radiolucency than were female patients. The same study showed that presence or absence of a preoperative radiolucency was the most significant predictor for success in initial root canal therapy (24). One could potentially make the conclusion that males, especially those with preoperative apical periodontitis, could have a lower success rate in endodontics. In one large population study, it was shown that males had a significantly lower success rate of initial root canal treatment when compared to females. It was also noted that association with apical periodontitis decreased the success rate (25). Based upon findings in this study, further investigation is necessary to determine whether sexual dimorphism associated with periapical inflammation is clinically significant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang K, Jiang Y, Han J, Gu J. The binding of actin to p38 MAPK and inhibiting its kinase activity in vitro. Sci China C Life Sci. 2003;46(1):87–94. doi: 10.1007/BF03182688. [DOI] [PubMed] [Google Scholar]
- 2.Whitmarsh AJ. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta. 2007;1773(8):1285–98. doi: 10.1016/j.bbamcr.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Whitmarsh AJ, Davis RJ. Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene. 2007;26(22):3172–84. doi: 10.1038/sj.onc.1210410. [DOI] [PubMed] [Google Scholar]
- 4.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12(2):186–92. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 5.Hirao K, Yumoto H, Takahashi K, Mukai K, Nakanishi T, Matsuo T. Roles of TLR2, TLR4, NOD2, and NOD1 in pulp fibroblasts. J Dent Res. 2009;88(8):762–7. doi: 10.1177/0022034509341779. [DOI] [PubMed] [Google Scholar]
- 6.Bar-Shavit Z. Taking a toll on the bones: regulation of bone metabolism by innate immune regulators. Autoimmunity. 2008;41(3):195–203. doi: 10.1080/08916930701694469. [DOI] [PubMed] [Google Scholar]
- 7.Graves DT, Oates T, Garlet G. Review of osteoimmunology and the host response in endodontic and periodontal lesions. Journal of Oral Microbiology. 2011;3:1–14. doi: 10.3402/jom.v3i0.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stashenko P, Wang CY. Characterization of bone resorptive mediators in active periapical lesions. Proc Finn Dent Soc. 1992;88(Suppl 1):427–32. [PubMed] [Google Scholar]
- 9.Orcel P, Feuga M, Bielakoff J, De Vernejoul MC. Local bone injections of LPS and M-CSF increase bone resorption by different pathways in vivo in rats. Am J Physiol. 1993;264(3 Pt 1):E391–7. doi: 10.1152/ajpendo.1993.264.3.E391. [DOI] [PubMed] [Google Scholar]
- 10.Nishida E, Hara Y, Kaneko T, Ikeda Y, Ukai T, Kato I. Bone resorption and local interleukin-1alpha and interleukin-1 beta synthesis induced by Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis lipopolysaccharide. J Periodontal Res. 2001;36(1):1–8. doi: 10.1034/j.1600-0765.2001.00637.x. [DOI] [PubMed] [Google Scholar]
- 11.Rogers JE, Li F, Coatney DD, Rossa C, Bronson P, Krieder JM, et al. Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol. 2007;78(3):550–8. doi: 10.1902/jop.2007.060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26(22):3203–13. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, et al. MAP kinase phosphatase-1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203(1):131–40. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase-1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103(7):2274–9. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176(3):1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- 16.Sartori R, Li F, Kirkwood KL. MAP kinase phosphatase-1 protects against inflammatory bone loss. J Dent Res. 2009;88(12):1125–30. doi: 10.1177/0022034509349306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H, Li Q, Herbert B, Zinna R, Martin K, Junior CR, et al. Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss. Gene Ther. 2011;18:344–53. doi: 10.1038/gt.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawashima N, Niederman R, Hynes RO, Ullmann-Cullere M, Stashenko P. Infection-stimulated infraosseous inflammation and bone destruction is increased in P-/E-selectin knockout mice. Immunology. 1999;97:117–23. doi: 10.1046/j.1365-2567.1999.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou L, Sasaki H, Stashenko P. Toll-like receptor 4-deficient mice have reduced bone destruction following mixed anaerobic infection. Infection and Immunity. 2000;68:4681–7. doi: 10.1128/iai.68.8.4681-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosner B, Glynn RJ, Lee ML. Extension of the rank sum test for clustered data: two-group comparisons with group membership defined at the subunit level. Biometrics. 2006;62(4):1251–9. doi: 10.1111/j.1541-0420.2006.00582.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Bain JL, Caskey CP, Sandifer LC, Johnson RB. Effects of gender on serum biomarkers of systemic inflammation coincident to experimentally-induced preiapical lesions. Arch Oral Biology. 2011;56:168–76. doi: 10.1016/j.archoralbio.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Desvarieux M, Schwahn C, Volzke H, Demmer RT, Ludemann J, Kessler C, et al. Gender differences in the relationship between periodontal disease, tooth loss, and atherosclerosis. Stroke. 2004;35:2029–35. doi: 10.1161/01.STR.0000136767.71518.36. [DOI] [PubMed] [Google Scholar]
- 23.Shiau HJ, Reynolds MA. Sex differences in destructive periodontal disease: exploring the biologic basis. J Periodontol. 2010;81:1505–17. doi: 10.1902/jop.2010.100045. [DOI] [PubMed] [Google Scholar]
- 24.Marquis VL, Dao T, Farzaneh M, Abitbol S, Friedman S. Treatment outcome in endodontics: The Toronto study. Phase III: Initial treatment. J Endod. 2006;32:299–306. doi: 10.1016/j.joen.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 25.Swartz DB, Skidmore AE, Griffin JA. Twenty years of endodontic success and failure. J Endod. 1983;9:198–202. doi: 10.1016/S0099-2399(83)80092-2. [DOI] [PubMed] [Google Scholar]