Abstract
Recent studies have expanded our understanding of the role of the anti-inflammatory cytokine interleukin (IL)–10, produced by multiple lineages of both human and murine T cells, in regulating the immune response. Here, we demonstrate that the small percentage of circulating CD4+ T cells that secrete IL-10 can be isolated from human peripheral blood and, importantly, we have optimized a protocol to expand these cells in both antigen-specific and polyclonal manners. Expanded CD4+IL-10+ T cells abrogate proliferation and T helper (Th) 1–like cytokine production in an antigen-specific manner, and to a lesser extent exhibit bystander suppressive capacity. CD4+IL-10+ T cells are suppressive in a cell contact–dependent way, though they do not require secretion of IL-10 for their suppressive role in vitro. CD4+IL-10+ T cells have an activated phenotype, with high expression of CD25, CD69, and cytotoxic T-lymphocyte antigen-4, and are largely FoxP3 negative. This novel method for the isolation and expansion of suppressive IL-10–secreting T cells has important implications both for further research and clinical therapeutic development.
Keywords: Interleukin-10, Induced regulatory T cells
1. Introduction
The selection of a broad T-cell repertoire during thymic development introduces the potential of self-reactive T cells. Many of these are dealt with during central tolerance, either by deletion of T cells with a high receptor affinity for self, or the selection of CD25+FoxP3+ (forkhead box protein 3) natural regulatory T cells (nTregs) [1], which afford protection in the peripheral immune system. Because of the incomplete nature of central tolerance, multiple mechanisms of peripheral tolerance exist, including induction of anergy or apoptosis, immune deviation and sequestration, and the expansion of regulatory populations, including transforming growth factor (TGF)-β–secreting T helper (Th) 3 cells [2], TGF-β–induced FoxP3+ cells [3], and interleukin (IL)-10–secreting regulatory cells [4-6]. Although IL-10 was initially described in Th2 cells [7], it has since emerged as a potent anti-inflammatory cytokine produced by multiple cell lineages. Both human and murine IL-10–secreting cells can be elicited in vitro by antigen stimulation in the presence of excess IL-10 [6], IL-10 and interferon-α (IFN-α) [8], vitamin D3 and dexamethasone [9-11], anti-CD3/CD46 [12], or immature dendritic cells (DCs) [13]. In vivo, chronic exposure to antigen [5,14-17] or infection [18,19], repeated administration of superantigen [20,21], and treatment with immunosuppressive agents [22,23] generate anergic IL-10–secreting cells. IL-10–secreting T cells can play a role in suppressing effector responses to autoimmune [24] or allergic [25] diseases, chronic infections [26], and transplantation [27,28]. In animal models of the autoimmune disease multiple sclerosis, IL-10–deficient mice have exacerbated disease [29,30], whereas mice transgenic for human IL-10 are protected [31]. Despite differences in induction protocols and subsequent cytokine profiles, all of these IL-10–secreting cells demonstrate regulatory activity with hyporesponsiveness in vivo.
Recent evidence has shown that IL-10–secreting regulatory cells can be derived from effector Th1 cells and dampen immune pathology in a range of chronic infections in mice and in human beings [32]. Furthermore, our group has shown that chronic administration of peptide antigen leads to the development of IL-10–secreting regulatory cells derived from T-bet–expressing Th1 precursors [33]. These cells can suppress autoimmune disease in an IL-10–dependent manner [5].
Previous studies have identified IL-10+ T cells in human peripheral blood by expression of specific cell surface proteins on CD4+ T cells. Naive CD4+CD45RA+ T cells were found to secrete low levels of IL-10, with a significant increase in the CD4+CD45RA− memory population [34]. Recent work has demonstrated the phenotype of human peripheral blood mononuclear cells (PBMCs) sorted according to naive and memory, and then CD25 and CD127 (IL-7R), populations, showing that the IL-7R− subset cosecreted IFN-γ and IL-10 and suppressed naive cells in coculture to a degree, in an IL-10–dependent fashion [35]. However, these studies were hampered by the high percentage of dead cells found after activation of this subset [36]. We therefore took the approach of specifically isolating IL-10–secreting T cells from PBMCs strictly according to IL-10 secretion as opposed to population markers. A recent study has used similarly isolated cells to examine the IL-10 locus during homeostatic expansion of selected IL-10–secreting T cells [37]. Herein, we describe the phenotype of IL-10–secreting T cells expanded by antigen-specific and polyclonal stimulation. These IL-10–secreting T cells are both anergic and suppressive, in a cell-contact–dependent manner, and represent the human equivalent of IL-10–secreting regulatory T cells derived from effector T-cell populations.
2. Subjects and methods
2.1. Reagents
Human myelin basic protein (MBP) was prepared from brain white matter as previously described [38], with purity > 95% as assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Mycobacterium tuberculosis–purified protein derivative (PPD; UK Central Veterinary Laboratory, Surrey, UK) and MBP were used at previously determined optimal concentrations of 50 μg/mL. Anti-CD3/CD28 Dynabeads (Invitrogen, San Diego, CA) were always used at a concentration of 1.25 μl/106 cells of culture, which equates to 1 bead per 20 cells. CD4 (RPA-T4), CD127 (hIL-7R-M21), CD152 (BNI3), and CD69 (FN 50) antibodies were sourced from BD Biosciences (Franklin Lakes, NJ); CD25 (4E3) from Miltenyi Biotec (Bergisch Gladbach, Germany); and FoxP3 (PCH101) from eBioscience (San Diego, CA). Complete RPMI refers to RPMI 1640 supplemented with 2 mM glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin and 20 μM HEPES buffer (Cambrex BioScience, East Rutherford, NJ), and culture medium was complete RPMI supplemented with 10% autologous, heat-inactivated plasma or AB serum (National Health Service Blood and Transplant, Bristol, UK). MACS buffer comprised phosphate buffered saline (PBS) with 0.5% AB serum (or autologous plasma) and 2 mM ethylenediamine tetraacetic acid (Sigma-Aldrich, St. Louis, MO). FACS buffer was composed of PBS with 2% AB serum (or autologous plasma) and 2 mM ethylenediamine tetraacetic acid; FACS staining buffer was the same, but with 20% AB serum (or autologous plasma). Recombinant human IL (rhIL)-2 and rhIL-15 were from R&D Systems (Minneapolis, MN). Recombinant human IL-10 soluble receptor alpha (rhIL-10sRα) was obtained from R&D Systems.
2.2. Cell isolation
Healthy volunteer blood donors were recruited by advertisement from members of staff within the University of Bristol. Volunteers gave written informed consent, as approved by the Salisbury and South Wiltshire Research Ethics Committee (REC reference number: 05/Q2008/23). About 100 mL of peripheral blood was collected into citrate-phosphate-dextrose anticoagulant solution with adenine (Sigma-Aldrich) at a ratio of 1:9 of citrate-phosphate-dextrose anticoagulant solution with adenine to blood. PBMCs were isolated by density centrifugation on Histopaque-1077 (Sigma). Clear plasma was collected and heat inactivated for subsequent use as autologous plasma. After density centrifugation, the interface was collected and washed several times to remove platelets by resuspending in 20 mL cold MACS buffer, spinning at 130 g for 10 minutes at 10°C and discarding the supernatant. After centrifugation, a proportion of cells were set aside for overnight culture without stimulation and were frozen the following day in 50% plasma, 40% complete RPMI, and 10% dimethylsulphoxide (Sigma-Aldrich) for subsequent use as antigen-presenting cells (APCs) or naive responders.
2.3. IL-10 secretion assay and cell sorting
After density centrifugation, PBMCs were resuspended in culture media and stimulated with CytoStim (Miltenyi Biotec) as per manufacturer’s instructions for 16 hours at 37°C, 5% CO2. For cell counting/dead cell analysis studies, after CytoStim treatment, cells were spun and subjected to a Dead Cell Removal Kit (Miltenyi Biotec) as per manufacturer’s instructions. A Miltenyi Biotec human IL-10 Secretion Assay [Cell Enrichment and Detection (PE) Kit] was then performed according to manufacturer’s instructions. For the altered procedure optimized to select live IL-10+ cells, cells were harvested after CytoStim treatment, and CD4+ T cells were purified by negative selection (Miltenyi Biotec) according to manufacturer’s instructions (CD4+ purity typically 90%-95%). The IL-10 Secretion Assay was performed up to and including the labeling with IL-10–PE detection antibody, and cells were subsequently stained with via-Probe (7-amino-actinomycin D, 7-AAD; BD Biosciences) and sorted on a BD FACSVantage Cell Sorter (BD Biosciences) on the basis of IL-10+7-AAD− cells. IL-10−7-AAD− cells were also collected as controls.
2.4. T-cell expansion and proliferation assays
Isolated CD4+IL-10+ T cells were expanded via stimulation with either PPD (50 μg/mL) and irradiated PBMCs for use as APCs (at a ratio of 1:5 T cells:APCs) or anti-CD3/CD28 Dynabeads in the presence of rhIL-2 (60 U/mL) and rhIL-15 (10 ng/mL) in 48-well culture plates (Corning, Corning, NY). Cells were split and supplemented with culture medium and IL-2/IL-15 as required. At days 10–14 of culture, resting cells were either restimulated (as described earlier) or used for proliferation assays. To assess proliferation, 1 × 106 autologous responder PBMCs were cultured with or without varying ratios of expanded IL-10+CD4+ T cells with antigens PPD (50 μg/mL), MBP (50 μg/mL), anti-CD3/CD28 Dynabeads or no antigen in 48-well plates. For transwell assays, cells were cultured in 24–well plates (Corning) with populations separated by high pore density cell culture transwell inserts (Falcon Transwell, BD Biosciences). To assess the role of IL-10 in suppression, rhIL-10sRα (1 μg/mL) was added to block signaling via this cytokine. Supernatant (50 μl) was taken on day 2 of culture and frozen for subsequent cytokine measurements. Between days 4 and 9 of culture, duplicate aliquots of 100 μl were harvested from wells (top well for transwell assays) and transferred to 96-well round bottomed microtiter plates (Corning) and pulsed with 0.5 μCi [3] [H]-thymidine (Amersham International, Buckinghamshire, UK). After 18 hours, cells were harvested onto glass fibermats (LKB-Wallac, Turku, Finland) using a Mach III Harvester 96 (TomTec, Hamden, CT). Fibermats were dried under a heat lamp, and Meltilex melt-on scintillation sheets (Wallac) were applied. Radioactivity was measured on a liquid scintillation β-counter (1450 Microbeta; Wallac), and data are expressed as corrected counts per min (ccpm). Percent suppression was calculated as ccpm suppression coculture/ccpm positive-control culture × 100 = X; suppression was expressed as 100 - X%.
2.5. Flow cytometry
To determine the percentage of CD4+IL-10+ cells in selected populations, cell fractions were washed in FACS buffer and surface stained for 20 minutes at 4°C in FACS staining buffer with anti-CD4FITC and anti-IL-10PE. For IL-10+ cell line phenotyping, selected IL-10+ T cells (or naive PBMCs as controls) were expanded for one round of activation with anti-CD3/CD28, rhIL-2, and rhIL-15 (as described earlier) and were frozen. Cells were thawed and reactivated with anti-CD3/CD28 beads for 1, 5, and 13 days. For cell surface staining for CD4, CD69, CD127, CD25, and cytotoxic T-lymphocyte antigen (CTLA)-4, cells were harvested, washed in FACS buffer and stained for 20 minutes at 4°C in FACS staining buffer at appropriate dilutions. For intracellular staining for Fox-P3, the anti-human FoxP3 staining kit (eBioscience) was used as per manufacturer’s instructions. Briefly, cells were stained with surface CD4 antibody as described previously, fixed and permeabilized (30 minutes at 4°C, buffer from kit), and washed twice in FACS buffer and twice in permeabilization buffer (from kit). Cells were stained in 100 μl permeabilization buffer and 20 μl anti-human FoxP3 at 4°C for 30 minutes, and then washed in permeabilization buffer. Fluorescence intensity was measured on a BD FACScan, BD FACSCalibur, or BD LSRII Flow (BD Biosciences) and analyzed using FlowJo (Tree Star, Inc., Ashland, OR) FACS analysis software or FCS Express software (DeNovo Software, Ontario, Canada).
2.6. Cytokine protein levels
Supernatant cytokine concentration was quantified by Cytometric Bead Array assay using the Human Th1/Th2 kit (BD Biosciences) per manufacturer’s instructions. Standards were used corresponding to 5 ng/mL-20 pg/mL of each cytokine, and fluorescence intensity was measured on a BD FACS Canto flow cytometer and analyzed using BD FACS Diva software (BD Biosciences).
2.7. Statistics
Student’s t test (unpaired, two-tailed) or one-way analysis of variance for multiple comparisons (followed by Bonferroni’s or Dunnett’s Multiple Comparison test where appropriate) was used to establish significance for IL-10–secreting cell purification experiments and suppression assays.
3. Results
3.1. Purification of IL-10–secreting T cells from human peripheral blood
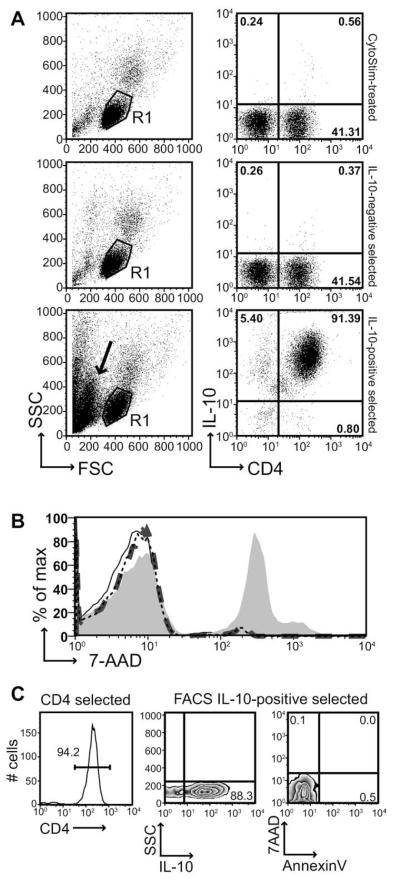
To determine whether IL-10–secreting CD4+ T cells could be isolated from peripheral blood, we first separated PBMCs from whole blood of healthy volunteers by density centrifugation. These cells were stimulated overnight with the Miltenyi reagent CytoStim, a pan-cytokine stimulus which activates T cells by crosslinking T-cell receptor with major histocompatibility complex molecules on APCs. Optimization studies determined that maximal IL-10 secretion was achieved at 16 hours post-activation with CytoStim (1, 2, 4, and 16 hours tested; data not shown). Initially, we used the Miltenyi Human IL-10 Secretion Assay Cell Enrichment and Detection Kit to isolate IL-10–secreting T cells after stimulation. Briefly, cells were harvested after 16 hours and dead cells were removed via the Dead Cell Removal Kit (Miltenyi). Cells were then labeled with IL-10 catch reagent, a double-ended anti-CD45/anti-IL-10 capture antibody, which coats the surface of T cells to capture secreted IL-10 during a subsequent secretion phase. IL-10–secreting cells were then detected with an anti-IL-10–PE antibody followed by anti-PE-MicroBeads for selection; the IL-10–labeled cells were positively selected on a Miltenyi MS magnetic column. The IL-10+–labeled cells were eluted from the column and counted by trypan blue exclusion. We found that a starting PBMC pool of 1.81 ± 0.64 × 106 cells/mL peripheral blood (mean ± standard deviation [SD]; n = 7; all data represented refer to average numbers for noted “n” number of individuals) yielded 4.96 ± 2.02 × 103 IL-10+ T cells/mL blood, meaning that 0.32 ± 0.24% of the initial PBMC pool was isolated as IL-10+ T cells (Table 1). Flow-cytometric analysis of the selection procedure demonstrated that the small percentage (0.56% in a representative FACS plot; Figure 1A) of CD4+IL-10+ T cells seen in whole PBMCs after 16-hour treatment with CytoStim was significantly enriched after column selection with greater than 90% of selected cells found to be CD4+IL-10+ (representative plot in Figure 1A). However, the forward scatter/side scatter (FSC/SSC) plot of the IL-10–positive selected fraction versus the whole PBMC and IL-10–negative fraction plots showed an increase in low FSC/SSC cells, typically regarded as a “dead cell” population (black arrow, Figure 1A). Indeed, we found a viability of only 6.83 ± 18.08% for isolated IL-10+ cells counted by trypan blue exclusion, suggesting that the vast majority of cells in the isolated IL-10+ fraction were dead after column selection. This was confirmed by cell viability staining with 7-AAD (which intercalates in double-stranded DNA but can only enter cells with compromised membranes), which revealed only 1.74 ± 1.00% 7-AAD–positive cells in whole naive PBMCs and 2.31 ± 1.40% of the CytoStim-treated PBMCs, but 35.27 ± 11.31% 7-AAD positively stained IL-10+ column-selected cells, even when gating in FACS plot FSC/SSC only on what typically would denote a “live cell” lymphocyte gate (Figure 1B, Table 1) (mean ± SEM; n = 5; p < 0.05 when comparing IL-10+ cells to whole naive or CytoStim-treated PBMCs). Furthermore, only 2.69 ± 1.41% of the nonselected (IL-10–negative) cells were 7-AAD positive (Figure 1B, Table 1), suggesting that exposure to the selection procedure or column alone did not result in enhanced cell death.
Table 1.
Selection and viability of IL-10+ cells isolated from human peripheral blood
| Cells/mL peripheral blood ± SD |
% Whole PBMCs ± SD |
%CD4+T ± SD |
|
|---|---|---|---|
| Column-selected (n = 7) | |||
| Whole PBMCs | 1.81 × 106 ± 0.64 | ||
| IL-10+ cells | 4.96 × 103 ± 2.02 | 0.32 ± 0.24 | |
| Sort-selected (n = 3) | |||
| Whole PBMCs | 1.70 × 106 ± 0.41 | ||
| CD4+ T cells | 4.40 × 105 ± 2.09 | 25.20 ± 8.06 | |
| IL-10+CD4+7-AAD− T cells |
1.08 × 103 ± 0.42 | 0.062 ± 0.011 | 0.26 ± 0.08 |
|
Column-selected viability (n = 5) |
% 7-AAD+ ± SEM | ||
| Whole PBMCs | 1.74 ± 1.00 | ||
| 16-h CytoStim–treated PBMCs |
2.31 ± 1.40 | ||
| IL-10− cells | 2.69 ± 1.41 | ||
| IL-10+ cells | 35.27 ± 11.31^ |
Comparison of selection numbers (represented as cells/mL peripheral blood ± SD) of IL-10+ cells selected directly by Miltenyi Human IL-10 Secretion Assay Cell Enrichment and Detection kit (called “column-selected”) vs. IL-10+ cells selected by FACS-sorting for live (7-AAD-negative) cells after modified IL-10 Secretion Assay and CD4 column purification (called “sort-selected”). Viability based on 7-AAD staining is represented as percent population ± SEM for each stage of column-based selection (whole PBMCs preselection; PBMCs after CytoStim-treatment but before selection; IL-10–negative cells eluted from MS magnetic columns; selected IL-10+ cells eluted from MS magnetic columns). ^p < 0.05 for IL-10+ cells when compared with whole PBMCs or CytoStim-treated PBMCs. All cell numbers and percentages are averaged from the noted n number of individuals and are represented as cell number ± SD or percent ± SEM.
Fig. 1.
Column selection of IL-10–secreting T cells leads to accumulation of dead cells. CytoStim-treated PBMCs were harvested after 16 hours and subjected to a Dead Cell Removal Kit. IL-10–positive T cells (and IL-10–negative fraction) were separated by MS columns after an IL-10 Secretion Assay. Cells were sampled at each stage for immunophenotyping. (A) Representative FACS plots show FSC/SSC of human PBMCs after CytoStim treatment (top), in the IL-10–negative fraction (middle), and in the IL-10–positive fraction (bottom). R1-gated cell staining for surface CD4FITC/IL-10PE is also depicted. Plots are representative of one of six experiments. Circled gates are R1; arrow in bottom FSC/SSC plot denotes low FSC/SSC, “dead” cell population. (B) 7-AAD staining was performed on each cell fraction to assess viability. The FACS histogram depicts lymphocyte-gated 7-AAD staining for whole naive PMBCs (thin black line), CytoStim-treated PBMCs (thin black dotted line), IL-10–negative fraction (thick dark gray dashed line) and IL-10+ cells (filled light gray histogram). The data are representative of one of five experiments. (C) The FACS histogram depicts lymphocyte-gated CD4 PE staining after negative selection for CD4+ cells. The dot plots show side scatter (SSC) versus IL-10–PE and 7-AAD versus AnnexinV FITC post-IL-10 flow cytometry sorting. The data are representative of one of five experiments.
Given the high percentage of dead cell contamination in the IL-10+ fraction post-column isolation, we modified the manufacturer’s protocol in two key ways, similar to that described by Dong et al. [37]. PBMCs were treated with CytoStim as previously for 16 hours. Cells were then harvested, and CD4+ T cells were first purified by negative selection before the IL-10 Secretion Assay. With a starting population of 1.70 ± 0.41 × 106 cells/mL blood (mean ± SEM; n = 3), we used negative selection to isolate 4.4 ± 2.09 × 105 cells/mL CD4+ T cells, or 25.20 ± 8.06% of initial PBMC count (Table 1). CD4+ purity was typically 90%–95% (Figure 1C; 94.2% CD4+ in representative histogram). IL-10–positive cells were then isolated as per the IL-10 Secretion Assay protocol, up to and including the step of labeling with anti-IL-10–PE. At this stage, cells were also labeled with viability dye 7-AAD and then isolated by cell sorting according to live cells (7-AAD-negative) that expressed IL-10 (IL-10–PE positive). IL-10–negative cells were also sorted as controls. The final yield of CD4+IL-10+7-AAD− T cells was 1.08 ± 0.42 × 103 cells/mL, which is 0.062 ± 0.011% of the initial PBMC count and 0.26 ± 0.08% of the starting CD4+ T-cell population (Table 1). Although this yield was approximately five times less than that isolated by the column selection, the purity was good (Figure 1C; 88.3% IL-10+ cells after sorting; note, IL-10–PE labeling is purposely low to avoid contamination, and hence cells lose brightness by the end of sorting, so it is likely that purity is even higher than that represented by this stain at the end of sorting). Most importantly, the purified cells were not contaminated by dead cells, as demonstrated by the fact that sorted IL-10+ cells were both 7-AAD- and AnnexinV-negative (Figure 1C). Therefore, starting with this highly pure population of live CD4+IL-10+ T cells, we sought to expand their numbers through stimulation and also to assess their functional phenotype.
3.2. Expanded IL-10–secreting T cells are anergic and can suppress naive cells in coculture
To assess the phenotype and function of isolated IL-10+CD4+ T cells, we expanded the cells through in vitro stimulation. It is well established that individuals who have been vaccinated with Bacillus Calmette–Guérin mount a secondary response to stimulation with M. tuberculosis–purified protein derivative (PPD). Therefore, we stimulated the IL-10+CD4+ T cells (or IL-10− T cells as controls) with PPD (50 μg/mL) and irradiated whole PBMCs as APCs, or used polyclonal anti-CD3/CD28 (1.25 μl/106 cells; 1 bead/20 cells used throughout all experiments) stimulation of the CD4+IL-10+ T cells alone, in the presence of cell growth and survival factors rhIL-2 (60 U/mL) and rhIL-15 (10 ng/mL), which have been shown to induce proliferation of anergic, T regulatory type 1 (Tr1) cells [39]. One round (RI) of expansion typically resulted in at least a 50-fold increase in IL-10+CD4+ T cells, with an average 169 ± 73-fold (mean ± SD; n = 3) expansion. Cells were maintained as a T-cell line (TCL) by restimulating resting cells (days 10–14) through a second round of activation, with an average 4.5 ± 2.4-fold expansion. Thus, the IL-10 TCL retained viability through expansion. The IL-10 TCL also retained IL-10 secretion through two rounds of stimulation (Table 2; data given for anti-CD3/28 stimulation, but were similar for PPD stimulation [data not shown]), though at a decreasing level. IL-10 TCL secreted tumor necrosis factor (TNF)-α upon initial activation (Table 2), and high levels of IFN-γ throughout culture, reminiscent of the phenotype seen in many types of induced IL-10–secreting regulatory cells [10]. We routinely detected only low levels of IL-4 and IL-5 in IL-10+ TCL, and TGF-β was not detected above background levels (data not shown).
Table 2.
IL-10+ T-cell lines secrete both IL-10 and IFN-γ
| Day 2 | Day 13 | Day 18 | |
|---|---|---|---|
| IL-10 | >5000 | 1352 | 982 |
| TNF-α | 1911 | 0 | 22 |
| IFN-γ | >5000 | 585 | >10000 |
IL-10+ T cells (selected from human PBMCs) were expanded by stimulation with anti-CD3/CD28 beads (in the presence of IL-2 and IL-15) for the indicated number of days (d2 cells had only one round stimulation; d13 and 18 cells were in their second round stimulation; day counted from initial stimulation of sorted cells). Supernatant was taken on indicated days and used in a CBA assay. Cytokine concentrations represented as pg/mL. Each day shown represents one distinct donor; data representative of three experiments.
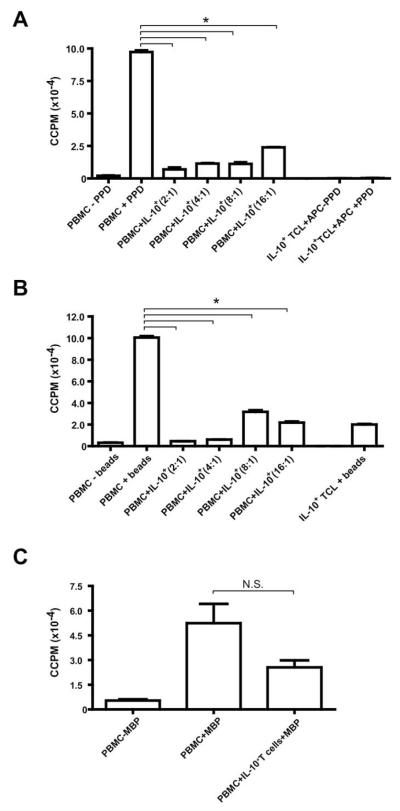
We next sought to determine the functional phenotype of the expanded IL-10–secreting T cells. Naive autologous PBMCs and the resting IL-10+ PPD-expanded TCL (day 10 post-sort) were reactivated in vitro. The peak response to PPD for healthy donors typically occurs between days 5–7 of activation (data not shown). While naive PBMCs proliferated strongly in response to PPD stimulation at day 7 (~100,000 cpm), IL-10+ T cells were profoundly anergic in response to restimulation with PPD (Figure 2A). These anergic IL-10+ T cells also suppressed naive PBMC responders in coculture (93% suppression at a 2:1 ratio of responders to IL-10+ T cells; all % suppressions given are for data from depicted representative experiments), and this suppression titrated through to a ratio of 1 IL-10 cell to 16 responders (76% suppression; p < 0.01 for all suppressive cultures relative to positive PBMC + PPD control culture) (Figure 2A). This suppressive phenotype extended to polyclonal restimulation via anti-CD3/CD28 beads (Figure 2B), where IL-10+ T cells also suppressed naive PBMCs at the peak day 4 response (95% suppression at a 2:1 ratio of responders to IL-10+ T cells; p < 0.01 for all suppressive cultures relative to positive PBMC + beads control culture), though IL-10+ T cells on their own proliferated marginally in response to the strong anti-CD3/CD28 stimulus (~20,000 cpm; Figure 2B). Furthermore, IL-10+ T cells may partially suppress the response of naive PBMCs to a self-antigen, MBP. It has previously been demonstrated that healthy subjects have autoreactive MBP-specific T cells circulating in the blood and can mount a primary response to in vitro stimulation with MBP [40,41]. At the peak day 9 response to MBP, there was a trend suggesting that IL-10+ T cells may partially suppress naive PBMCs (52% suppression at a 2:1 ratio of PBMCs to IL-10+ T cells), though this suppression was not statistically significant (Figure 2C).
Fig. 2.
PPD-specific IL-10+ T cells are anergic and suppressive in a dose-dependent fashion. IL-10+ T cells were selected from human peripheral blood and expanded with PPD, IL-2, and IL-15 for 10 days. Autologous naive PBMCs (1 × 106 cells/condition) were activated in 1-mL cultures in 48-well plates in the presence (+) or absence (–) of (A) 50 μg/mL PPD, (B) anti-CD3/CD28 beads (1.25 μl/106 cells), or (C) 50 μg/mL MBP (after one additional round of restimulation with PPD). 1 × 106 IL-10+ T cells were titrated into wells with naive PBMCs in a dilution series from 5 × 105 cells/well to 6.25 × 104 cells/well in 2-fold dilutions (three PBMCs: one IL-10+ T-cell ratio [1 × 106:3 × 105] used for MBP treatment). IL-10+ T cells (1 × 106 cells/well) were also stimulated with irradiated APCs (3 × 106 cells/well), with or without PPD, or alone with anti-CD3/CD28 beads. At the peak response days (day 7 for PPD; day 4 for anti-CD3/CD28 beads; and day 9 for MBP), 100-μl duplicates were harvested into 96-well plates and pulsed with 0.5 μCi 3[H]-thymidine. Results are expressed as corrected counts per minute (ccpm); error bars represent standard error (SE). TCL, T-cell line. *p < 0.01; NS, not significant. Data for PPD and anti-CD3/CD28 stimulation representative of three experiments; MBP data are from one experiment (one distinct individual donor per experiment).
Taken together, these data demonstrate that IL-10+ T cells are anergic, though they can be expanded in the presence of IL-2 and IL-15. Furthermore, these IL-10+ T cells broadly suppress the response of PBMCs to their own antigen (PPD), as well as to polyclonal (anti-CD3/CD28) stimulation, and may partially suppress the response to a different antigenic specificity (MBP). IL-10+ TCL eventually became nonsuppressive by the third or fourth round of activation (data not shown) as IL-10 secretion decreased and IFN-γ secretion increased.
3.3. Anergic IL-10+ T cells also suppress effector cytokine secretion
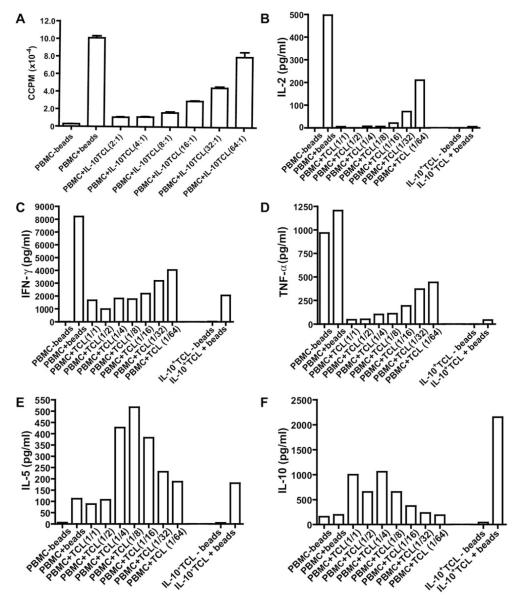
Selected IL-10+ T cells could also be initially expanded by polyclonal activation with anti-CD3/CD28 beads (in the presence of IL-2 and IL-15) for two rounds of stimulation. Similar to that seen for the PPD-specific IL-10+ TCL, the anti-CD3/CD28-expanded IL-10+ TCL was anergic upon restimulation with beads (peak day 4 response) and suppressed naive PBMCs in coculture (89% suppression at a 2:1 ratio of PBMCs to IL-10+ T cells) (Figure 3A).
Fig. 3.
Anti-CD3/CD28 expanded IL-10+ T cells suppress naive PBMC proliferation and effector cytokine production. IL-10+ T cells were expanded with anti-CD3/CD28 beads, rhIL-2 and rhIL-15 for two rounds of stimulation; cells were used at day 9 of RII. Autologous naive PBMCs (1 × 106 cells/condition) were activated in the presence (+) or absence (–) of anti-CD3/CD28 beads. IL-10+ T cells were titrated into wells with naive PBMCs in a dilution series from 5 × 105 (A) or 1 × 106 (B–F) cells/well to 3.125 × 104 cells/well in 2-fold dilutions. IL-10+ T cells (1 × 106 cells/well) were also stimulated with or without anti-CD3/CD28 beads alone. (A) At the peak response (day 4), 100-μl duplicates were harvested into 96-well plates and pulsed with 0.5 μCi 3[H]-thymidine. Results are expressed as ccpm; error bars represent SE. (B-F) supernatant was collected from the cultures at day 2 and used in a cytometric bead array (CBA) to measure cytokine secretion. Results for (B) IL-2, (C) IFN–γ, (D) TNF–α, (E) IL-5, and (F) IL-10 are expressed in pg/mL. Data representative of three experiments (one distinct individual donor per experiment). TCL, T-cell line. In A, ratios represented as # PBMC: # TCL. For B–F, ratios are written as # TCL/# PBMC.
To assess the cytokine secretion phenotype associated with this suppression, supernatant was taken at day 2 of restimulation cultures and assayed by cytometric bead array. Th1 cytokines IL-2 (Figure 3B), IFN-γ (Figure 3C), and TNF-α (Figure 3D) were found to directly correlate with the anergic and suppressive phenotypes, with high levels of these three effector cytokines when naive PBMCs were stimulated on their own, which were suppressed in the presence of IL-10+ T cells (Figure 3B-D). The IL-10+ T cells did not produce any IL-2 themselves in response to restimulation with anti-CD3/CD28, and also suppressed IL-2 in cocultures with naive PBMCs (Figure 3B). While some IFN-γ (~2,000 pg/mL) and TNF-α (~50 pg/mL) was secreted by IL-10+ T cells themselves, as seen in supernatant from the TCL (Table 2), in response to anti-CD3/CD28 stimulation, the IL-10+ T cells did suppress the secretion of these cytokines by naive PBMCs, in response to anti-CD3/CD28, to these “background” levels (Figure 3C, D). The IL-10+ T cells themselves also secreted IL-5 (in response to restimulation) as did the naive PBMCs, and coculture levels were at these background levels or enhanced (Figure 3E). IL-4 levels were very low (<13 pg/mL) for all conditions (data not shown). In addition to the suppression of effector cytokines, the IL-10+ T cells also secreted high levels of IL-10 when reactivated with anti-CD3/CD28 (~2,200 pg/mL) and in coculture with naive PBMCs (Figure 3F). Less IL-10 is seen with decreased ratios of IL-10+ TCL (i.e., PBMC: TCL 1:16, 1:32, and 1:64) given that less IL-10–secreting cells were added to these cultures. Taken together, these data demonstrate that IL-10+ T cells suppress Th1 effector cytokine secretion in naive PBMCs, and have the phenotype often described for Tr1 cells [10], with secretion of IFN-γ, IL-5, and IL-10.
3.4. IL-10+ T cells require cell-cell contact, but not IL-10, to mediate suppression in vitro
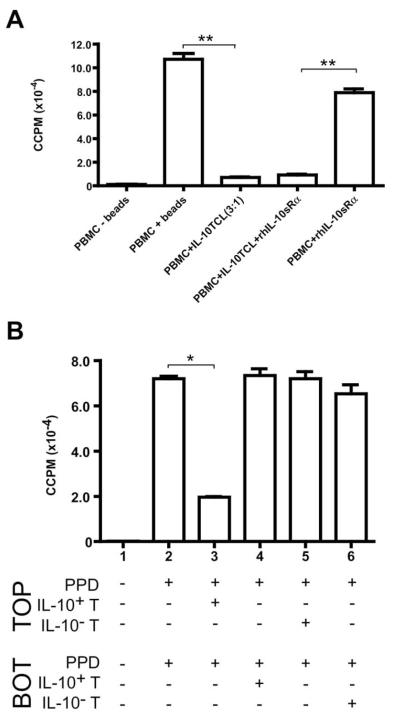
Previous studies have demonstrated that Tr1 cells suppress both primary and memory responses through secretion of both IL-10 and TGF-β [6,8,39,42]. Given that our cells secrete IL-10 and not TGF-β, we sought to determine whether active IL-10 secretion was required to mediate the suppression observed by IL-10+ T cells on naive PBMCs. We blocked IL-10 signaling by the inclusion of a recombinant human soluble IL-10 receptor alpha (rhIL-10sRα). Resting IL-10+ T cells (expanded for two rounds of stimulation with anti-CD3/CD28, IL-2, and IL-15) did suppress naive PBMCs in restimulation as seen previously (p < 0.001 for PBMC+ IL-10TCL [3:1] culture relative to positive PBMC+ beads control culture) (Figure 4A). This suppression was not affected by the addition of rhIL-10sRα (p < 0.001; PBMCs+ rhIL-10sRα versus PBMCs+ IL-10TCL+ rhIL-10sRα) (Figure 4A). We have seen in a distinct activation system using IL-10+ TCL that the addition of rhIL-10sRα to cocultures does partially reduce suppression, demonstrating that the recombinant receptor is sufficient to block IL-10 and can affect other roles of IL-10+ TCL (data not shown).
Fig. 4.
Cell contact, but neither IL-10 nor TGF-β, is required for IL-10+ T–cell–mediated suppression in vitro. (A) IL-10+ T cells were expanded with anti-CD3/CD28 beads, rhIL-2 and rhIL-15 for two rounds of stimulation; cells were used at day 9 of RII. Autologous naive PBMCs (1 × 106 cells/condition) were activated in the presence (+) or absence (−) of anti-CD3/CD28 beads. IL-10+ T cells were titrated into wells with naive PBMCs at a 3:1 ratio (PBMCs: IL-10+ T cells). rhIL-10sRα (1 μg/mL) and/or rhTGF-βsRII (100 ng/mL) were added to cultures where indicated. At the peak response (day 3), 100-μl duplicates were harvested into 96-well plates and pulsed with 0.5 μCi 3[H]-thymidine. Results are expressed as ccpm; error bars represent SE. (B) IL-10+ and IL-10− T cells (selected and sorted from the same donor) were stimulated with PPD, IL-2 and IL-15 for 13 days. Autologous naive PBMCs (1 × 106/ chamber) were added to the top and bottom chambers of transwells (separated by a porous membrane in 24-well plates), with or without PPD (50 μg/mL) in both chambers. Resting day 13 PPD-specific IL-10+ or IL-10− T cells were added to chambers where indicated at 3 × 105 cells/chamber. On day 7, 100-μl duplicates were harvested into 96-well plates and pulsed with 0.5 μCi 3[H]-thymidine. Results are expressed as ccpm; error bars represent standard error. *p < 0.01; **p < 0.001. Data representative of three experiments (one distinct individual donor per experiment). TCL, T-cell line; Top, top well; bot, bottom well.
Given the observation that IL-10 was not required to mediate suppression, and previous work in murine models demonstrating that cell contact was required for in vitro IL-10 T regulatory cell-mediated suppression [16,23], we used transwell porous membranes to separate IL-10+ T cells from naive PBMCs to assess the role of cell contact in the suppressive phenotype. We further compared the proliferation of naive PBMCs in the presence of PPD-expanded IL-10+ or IL-10− T cells (day 13 post-sort). Naive PBMCs proliferated robustly in response to PPD-stimulation when seeded in both the top and bottom chambers of the transwell system (only top well proliferation measured; Condition 2, Figure 4B). This proliferation was suppressed when IL-10+ T cells were included in the top chamber with naive PBMCs (73% suppression, Condition 3, Figure 4B; p < 0.01 relative to Condition 2, positive control culture). However, when IL-10+ T cells were in the bottom chamber, separated from the measured PBMCs by the porous membrane, proliferation of the naive PBMCs was not suppressed (Condition 4, Figure 4B), indicating that secreted proteins alone were not sufficient to suppress proliferation and that cell contact was required. Furthermore, inclusion of IL-10− T cells in either the top (Condition 5) or bottom (Condition 6) chamber of the wells, did not suppress naive PBMC proliferation, demonstrating that PPD-expansion alone was not sufficient to endow a suppressive phenotype and that suppression required coincubation with the IL-10+ T-cell population (Figure 4B).
3.5. IL-10–secreting T cells have an activated phenotype
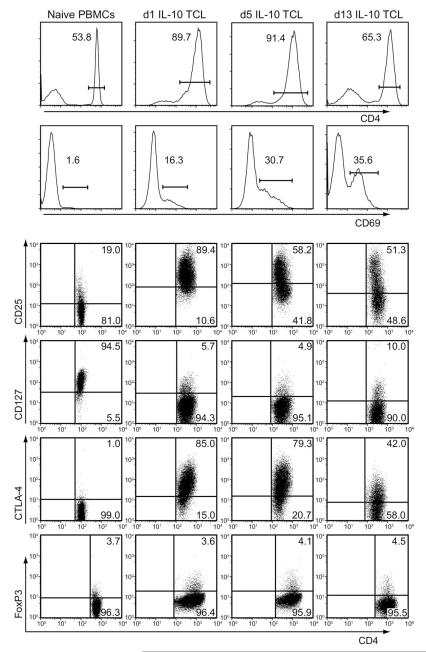
We further phenotyped these isolated IL-10–secreting CD4+ T cells by assessing cell surface activation–associated proteins at several time points after polyclonal stimulation with anti-CD3/CD28 beads. CD4+IL-10+ T cells highly upregulated the early activation-associated protein CD69, the high affinity IL-2 receptor alpha chain CD25, and costimulatory molecule CTLA-4 (CD152) after one day of activation (compared with autologous naive PB-MCs) (Figure 5, Table 3). The CD25 upregulation most likely correlated with activation state as opposed to expansion of CD25+ nTregs, given the fact that FoxP3 staining did not increase after stimulation (Figure 5, Table 3). By day 13 post-stimulation, these activation-associated proteins were largely downregulated, correlating with the resting phenotype (Figure 5, Table 3). CD4 was also slightly downregulated (from 89.7% to 65.3%), which is often seen in long-term T-cell culture lines.
Fig. 5.
Anti-CD3/CD28 stimulated IL-10+ T cells have an activated cell phenotype. IL-10+ T cells (selected from human PBMCs) were expanded by stimulation with anti-CD3/CD28 (in the presence of IL-2 and IL-15) for one round, and then frozen. Thawed cells were subsequently washed and reactivated with anti-CD3/CD28 for 1, 5, or 13 days; stains depicted show autologous naive PBMC controls, d1 IL-10 TCL, day 5 IL-10 TCL, and day 13 IL-10 TCL. These cells were harvested and stained at each time point. Top two row histograms represent cell-surface staining for CD4 and CD69. Dot plots show CD4-gated cell surface stains for CD25 (third row), CD127 (fourth row), and CTLA-4 (fifth row). Bottom row shows dot plots for CD4-gated, intracellularly stained FoxP3 expression. Protein expression was measured by flow cytometry (BD FACS Scan) and data were analyzed with FlowJo software. Numbers represent the percentage expression of the described protein populations on the IL-10+ T-cell line (TCL) at indicated time points. Data are representative of two experiments.
Table 3.
Anti-CD3/CD28 stimulated IL-10+ T cells have an activated cell phenotype
| Nai̇ve PBMCs | 1-Day IL-10+ TCL |
5-Day IL-10+ TCL |
13-Day IL-10+ TCL |
|
|---|---|---|---|---|
| CD4 | 53.8 | 89.7 | 91.4 | 65.3 |
| CD69 | 1.6 | 16.3 | 30.7 | 35.6 |
| CD4+CD25+ | 19.0 | 89.4 | 58.2 | 51.3 |
| CD4+CD127lo | 5.5 | 94.3 | 95.1 | 90.0 |
| CD4+CTLA-4+ | 1.0 | 85.0 | 79.3 | 42.0 |
| CD4+FoxP3+ | 3.7 | 3.6 | 4.1 | 4.5 |
IL-10+ T cells (selected from human PBMCs) were expanded by stimulation with anti-CD3/CD28 (in the presence of IL-2 and IL-15) for one round, and then frozen. Thawed cells were subsequently washed and re-activated with anti-CD3/CD28 for 1, 5, or 13 days. These cells were harvested at each timepoint and shown as cell-surface stained for CD4 or CD69; surface stains for CD127, CD25, and CTLA-4 shown on CD4+ gated cells; and intracellularly stained FoxP3 expression shown on CD4+ gated cells. Autologous naïve PBMCs were stained as done for controls. Protein expression was measured by flow cytometry (BD FACS Scan) and data were analyzed with FlowJo software. Numbers represent the percent expression of the described protein populations on the IL-10+ T-cell line (TCL) at indicated timepoints. Data are representative of two experiments.
Taken together, these data demonstrate that CD4+IL-10+ T cells can be isolated from human peripheral blood, and expanded in both antigen-specific and polyclonal fashions. Expanded CD4+IL-10+ T cells suppress both proliferation and Th1 cytokine secretion from naive PBMCs in a cell contact-dependent manner. Furthermore, these cells have an activated phenotype when expanded and are distinct from FoxP3+ nTregs.
4. Discussion
Herein, we have described a method of isolating and expanding PBMCs based specifically on their secretion of IL-10. One problem we encountered with the commercially available methods for secretion-based selection was the high frequency of dead cells that appear to be “trapped” by selection columns when isolating a low frequency (in this case, <1% cells) population, even after attempting to remove dead cells first by a dead cell exclusion kit. Experiments in our laboratory have also indicated that dead cells themselves appear to have suppressive capacity (data not shown), and previous studies have shown that apoptotic cells can be suppressive, potentially by inhibiting expression of CD69 [43] or sequestering chemokines [44]. Previous studies have also shown that isolation of IL-10 regulatory cells according to cell markers can lead to the accumulation of dead cells after culturing [36]. By adapting the commercial protocol to use CytoStim to induce maximal IL-10 secretion, then purifying CD4+ T cells before IL-10 selection and ultimately selecting according to live IL-10+ cells by flow cytometry sorting [37], we have optimized the procedure to procure pure, CD4+IL-10+ live T cells.
We have also developed a method to expand these CD4+IL-10+ T cells by stimulation with recall antigen PPD or polyclonally via anti-CD3/CD28 in the presence of IL-2 and IL-15 to expand IL-10+ cells from relatively low precursor numbers, allowing for phenotyping and functional assays. These isolated CD4+IL-10+ T cells strongly suppress both the proliferative and effector cytokine phenotype of naive PBMCs when activated with their own (PPD) or polyclonal (anti-CD3/CD28) stimulation, and partially suppress activation of naive PBMCs with a distinct primary antigen (MBP), suggesting that the IL-10+ cells may function in part by mediating bystander suppression [45]. The PPD-specific line likely suppressed anti-CD3/CD28 restimulation strongly due to the T-cell receptor-stimulation initiating activation-dependent function of the IL-10+ cells, and had less effect on MBP-restimulation as activation of the IL-10+ cells by MBP relies on a degree of cross-reactivity for the PPD-specific line. We have also found that the expanded IL-10+ cells continue to secrete IL-10 alone or in coculture with naive PBMCs; however, blocking IL-10 receptor did not prevent suppression, though contact between IL-10+ T cells and naive PMBCs was required. We have previously shown, in our murine Tg4 model, a dichotomy between a requirement for IL-10 secretion in vivo and its dispensability in vitro [46], suggesting a similar possibility for human IL-10+ T cells. Others have demonstrated for IL-10 regulatory cells that a partial block of IL-10 or TGF-β still allows suppression [6], and also that cell contact was required [47].
Dong et al. have also described selection of IL-10–secreting T cells from PBMCs, but in their model they used homeostatic, neutral expansion with IL-7 and IL-15 to examine any potential establishment of IL-10 memory for either IL-10+ single-positive or IL-10+IFN-γ+ double-positive populations [37]. Interestingly, they showed that after one round of stimulation, 90% of the selected cells lost the ability to secrete IL-10, and they suggest that Th1-type cells do not establish IL-10–secretion recall. However, when we activated specifically with antigen or polyclonal anti-CD3/CD28 stimulation, in the presence of growth factors IL-2 and IL-15, we found that IL-10–secreting cells retained the functional capacity to suppress naive cell proliferation and effector Th1 cytokine secretion in coculture (Figures 2 and 3) through at least two rounds of differentiation. Furthermore, while there was some loss of IL-10 secretion, the observed suppression was a specific property of IL-10+ T cells as expansion of IL-10− T cells did not lead to a suppressive phenotype (Figure 4B). This phenotype may in part be established through the continual activation of the cells, which are Th1-like in their ontogeny, in line with recent work suggesting that continual presence of IL-12 may sustain IL-10 expression [48].
We have recently described murine IL-10–secreting regulatory cells derived from the Th1 lineage [33]. Interestingly, our human CD4+IL-10+ T cells also secrete IFN-γ and IL-10 (Table 2), and suppress in coculture. The CD4+IL-10+ T cells described here display an activated phenotype, with strong upregulation of CD69 and CTLA-4, but do not appear to be FoxP3+ nTregs, similar to those described in our murine system [49,50]. We speculate that these CD4+IL-10+ T cells are the human equivalent of IL-10–secreting regulatory cells derived from an effector phenotype, and the isolation and expansion methods described here offer important avenues for potential clinical applications of these suppressive cells.
Acknowledgments
We thank Dr Johan Verhagen for critical reading of this manuscript. This work was supported by the Wellcome Trust and the Multiple Sclerosis Society of Great Britain and Northern Ireland. John D. Campbell is an employee of Miltenyi Biotec Ltd.
Footnotes
G. Mazza and C.A. Sabatos-Peyton contributed equally to this work.
References
- [1].Wraith DC, Nicolson KS, Whitley NT. Regulatory CD4+ T cells and the control of autoimmune disease. Curr Opin Immunol. 2004;16:695–701. doi: 10.1016/j.coi.2004.09.015. [DOI] [PubMed] [Google Scholar]
- [2].Weiner HL. Induction and mechanism of action of transforming growth factor-β-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- [3].Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: From discovery to their clinical application. Semin Immunol. 2006;18:120–7. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- [5].Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: A role for IL-10. Int Immunol. 1999;11:1625–34. doi: 10.1093/intimm/11.10.1625. [DOI] [PubMed] [Google Scholar]
- [6].Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- [7].Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- [8].Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-α and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–9. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- [9].Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, et al. In vitro generation of interleukin 10–producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roncarolo MG, Gergori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10–secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- [11].Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, et al. Reversing the defective induction of IL-10–secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–55. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–92. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- [13].Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Buer J, Lanoue A, Franzke A, Garcia C, von Boehmer H, Sarukhan A. Interleukin 10 secretion and impaired effector function of major histocompatibility complex class II-restricted T cells anergized in vivo. J Exp Med. 1998;187:177–83. doi: 10.1084/jem.187.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10–secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887–98. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sundstedt A, O’Neill EJ, Nicolson KS, Wraith DC. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J Immunol. 2003;170:1240–8. doi: 10.4049/jimmunol.170.3.1240. [DOI] [PubMed] [Google Scholar]
- [17].Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–32. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- [18].Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4+CD25−Foxp3− Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–97. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, et al. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–83. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miller C, Ragheb JA, Schwartz RH. Anergy and cytokine-mediated suppression as distinct superantigen-induced tolerance mechanisms in vivo. J Exp Med. 1999;190:53–64. doi: 10.1084/jem.190.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sundstedt A, Hoiden I, Rosendahl A, Kalland T, van Rooijen N, Dohlsten M. Immunoregulatory role of IL-10 during superantigen-induced hyporesponsiveness in vivo. J Immunol. 1997;158:180–6. [PubMed] [Google Scholar]
- [22].Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–53. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- [23].Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–96. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- [24].Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: Suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- [25].Reefer AJ, Carneiro RM, Custis NJ, Platts-Mills TA, Sung SS, Hammer J, et al. A role for IL-10-mediated HLA-DR7-restricted T cell-dependent events in development of the modified Th2 response to cat allergen. J Immunol. 2004;172:2763–72. doi: 10.4049/jimmunol.172.5.2763. [DOI] [PubMed] [Google Scholar]
- [26].Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–9. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- [27].Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–7. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- [28].Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bettelli E, Nicholson LB, Kuchroo VK. IL-10, a key effector regulatory cytokine in experimental autoimmune encephalomyelitis. J Autoimmun. 2003;20:265–7. doi: 10.1016/s0896-8411(03)00048-9. [DOI] [PubMed] [Google Scholar]
- [30].Samoilova EB, Horton JL, Chen Y. Acceleration of experimental autoimmune encephalomyelitis in interleukin-10-deficient mice: Roles of interleukin-10 in disease progression and recovery. Cell Immunol. 1998;188:118–24. doi: 10.1006/cimm.1998.1365. [DOI] [PubMed] [Google Scholar]
- [31].Cua DJ, Groux H, Hinton DR, Stohlman SA, Coffman RL. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J Exp Med. 1999;189:1005–10. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–43. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gabryšová L, Nicolson KS, Streeter HB, Verhagen J, Sabatos-Peyton CA, Morgan DJ, et al. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10–secreting Th1 cells. J Exp Med. 2009;206:1755–67. doi: 10.1084/jem.20082118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yssel H, De Waal Malefyt R, Roncarolo MG, Abrams JS, Lahesmaa R, Spits H, et al. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992;149:2378–84. [PubMed] [Google Scholar]
- [35].Haringer B, Lozza L, Steckel B, Geginat J. Identification and characterization of IL-10/IFN-γ-producing effector-like T cells with regulatory function in human blood. J Exp Med. 2009;206:1009–17. doi: 10.1084/jem.20082238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Metzler B, Anderton SM, Manickasingham SP, Wraith DC. Kinetics of peptide uptake and tissue distribution following a single intranasal dose of peptide. Immunol Investig. 2000;29:61–70. doi: 10.3109/08820130009105145. [DOI] [PubMed] [Google Scholar]
- [37].Dong J, Ivascu C, Chang HD, Wu P, Angeli R, Maggi L, et al. IL-10 is excluded from the functional cytokine memory of human CD4+ memory T lymphocytes. J Immunol. 2007;179:2389–96. doi: 10.4049/jimmunol.179.4.2389. [DOI] [PubMed] [Google Scholar]
- [38].Deibler GE, Martenson RE, Kies MW. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2:139–65. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- [39].Bacchetta R, Sartirana C, Levings MK, Bordignon C, Narula S, Roncarolo MG. Growth and expansion of human T regulatory type 1 cells are independent from TCR activation but require exogenous cytokines. Eur J Immunol. 2002;32:2237–45. doi: 10.1002/1521-4141(200208)32:8<2237::AID-IMMU2237>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [40].Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:973–84. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ponsford M, Mazza G, Coad J, Campbell MJ, Zajicek J, Wraith DC. Differential responses of CD45+ve T-cell subsets to MBP in multiple sclerosis. Clin Exp Immunol. 2001;124:315–22. doi: 10.1046/j.1365-2249.2001.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sakaguchi S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- [43].Sun E, Zhang L, Zeng Y, Ge Q, Zhao M, Gao W. Apoptotic cells actively inhibit the expression of CD69 on Con A activated T lymphocytes. Scand J Immunol. 2000;51:231–6. doi: 10.1046/j.1365-3083.2000.00666.x. [DOI] [PubMed] [Google Scholar]
- [44].Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–16. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Anderton SM, Wraith DC. Hierarchy in the ability of T cell epitopes to induce peripheral tolerance to antigens from myelin. Eur J Immunol. 1998;28:1251–61. doi: 10.1002/(SICI)1521-4141(199804)28:04<1251::AID-IMMU1251>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- [46].O’Neill EJ, Day MJ, Wraith DC. IL-10 is essential for disease protection following intranasal peptide administration in the C57BL/6 model of EAE. J Neuroimmunol. 2006;178:1–8. doi: 10.1016/j.jneuroim.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- [48].Chang HD, Helbig C, Tykocinski L, Kreher S, Koeck J, Niesner U, et al. Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. Eur J Immunol. 2007;37:807–17. doi: 10.1002/eji.200636385. [DOI] [PubMed] [Google Scholar]
- [49].Metzler B, Burkhart C, Wraith DC. Phenotypic analysis of CTLA-4 and CD28 expression during transient peptide-induced T cell activation in vivo. Int Immunol. 1999;11:667–75. doi: 10.1093/intimm/11.5.667. [DOI] [PubMed] [Google Scholar]
- [50].Nicolson KS, O’Neill EJ, Sundstedt A, Streeter HB, Minaee S, Wraith DC. Antigen-induced IL-10+ regulatory T cells are independent of CD25+ regulatory cells for their growth, differentiation, and function. J Immunol. 2006;176:5329–37. doi: 10.4049/jimmunol.176.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]