Abstract
Pre-eclamptic toxaemia (PET), characterised by pregnancy related hypertension and proteinuria, due to widespread endothelial dysfunction, is a primary cause of maternal morbidity. Altered circulating factors, particularly the VEGF family of proteins and their receptors, are thought to be key contributors to this disease. Plasma from patients with PET induces numerous cellular and physiological changes in endothelial cells indicating the presence of a circulating imbalance of the normal plasma constituents. These have been narrowed down to macromolecules of the VEGF family of proteins and receptors. It has been shown that responses of endothelial cells in intact vessels to plasma from patients with pre-eclampsia is VEGF dependent. It has recently been shown that this may be specific to the VEGF165b isoform, and blocked by addition of recombinant human PlGF. Taken together with results that show that sVEGFR1 levels are insufficient to bind VEGF-A in human plasma from patients with pre-eclampsia, and that other circulating macromolecules bind but do not inactivate VEGF-A, suggest that novel hypotheses involving altered bioavailability of VEGF isoforms resulting from either reduced, or bound PlGF, or increased sVEGFR1 increasing biological activity of circulating plasma could be tested. This suggests that knowing how to alter the balance of VEGF family members could prevent endothelial activation, and potentially some symptoms, of pre-eclampsia.
Introduction
Pre-eclampsia (pre-eclamptic toxaemia, PET) occurs in 3-5% of first pregnancies and is characterised by widespread endothelial dysfunction[1], resulting in clinical vascular manifestations including hypertension, proteinuria, cerebral oedema and infarction, eclampsia (seizures), pulmonary oedema, liver haemorrhage, renal failure and coagulopathy. The clinical picture is resolved with removal of the placenta suggesting a placental source for the systemic effects of the disease. The condition remains a leading cause of maternal morbidity and mortality in the UK[2], but the fetus may also be severely affected – either by growth restriction due to placental insufficiency or by premature delivery[3].
Pre-eclampsia (Pre-eclamptic toxaemia, PET)
In pregnancy inadequate trophoblast invasion results in high resistance vessels and placental underperfusion leading to multiple metabolic changes including hypoxia and oxidative stress, and disturbances in the maternal circulation that result in the systemic abnormalities described above[4]. Plasma from women with pre-eclampsia has biological activity that is not present in plasma from women with normal pregnancy[5-7]. A number of factors that may link abnormal placental development to systemic endothelial dysfunction in PET have been proposed[4], but the primary candidates have been linked to the VEGF family of proteins and their receptors, in particular sVEGFR1, PlGF and VEGF-A[8-10]
The VEGF family of proteins and receptors in pre-eclampsia
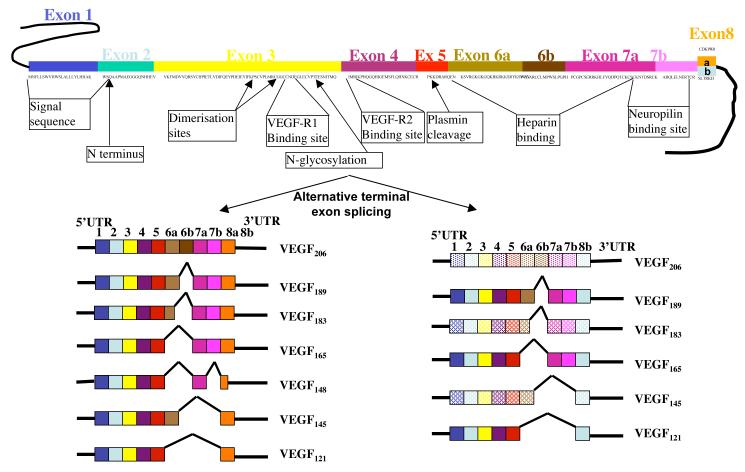
VEGF was first termed Vascular Permeability Factor when it was partially isolated in ascitic fluid in 1983 due its ability to increase vascular permeability[11]. This family now numbers five members in humans, VEGF-A, -B, -C, and -D and Placental Growth Factor (PlGF). The most widely studied form, VEGF-A (or simply VEGF), is expressed as numerous isoforms caused by alternative exon splicing resulting in mature proteins varying from 121 to 206 amino acids. VEGF165 is the dominant angiogenic molecule in physiological and pathological angiogenesis[12]. It is produced by a variety of cells and tissues including the placental syncytiotrophoblasts and placental endothelial cells[9, 10] and its production is generally increased by hypoxia[13, 14]. Alternative splicing of VEGF-A can also result in an alternative family of anti-angiogenic isoforms, such as VEGF165b[15] (figure 1). These isoforms act as weak agonists of VEGFR2 preventing VEGF165 from inducing angiogenesis. While there is little evidence for a role of VEGF-C and -D in pre-eclampsia, PlGF is also integrally linked, as it is predominantly produced by the placenta, is significantly downregulated in pre-eclampsia[16], this downregulation occurs under hypoxia[17], and at or even before the onset of pre-eclamptic symptoms[8], implicating that it could be a contributory factor to the symptomology of the disease.
Figure 1.
Alternative splicing of VEGF-A pre-RNA results in multiple isoforms of two families with alternative terminal exon structures resulting in two different families. Boxes are coding sequence. Lines are untranslated regions. Functional domains shown on RNA. Light coloured boxes indicate predicted mRNA species (not yet described)
VEGF Receptors
VEGFs bind VEGFR1, VEGFR2 and VEGFR3, tyrosine kinase receptors through which their signal transduction can be initiated. VEGF-A binds VEGFR1 and VEGFR2, but although it has a higher affinity for VEGFR1 the VEGF165 isoform acts mainly through VEGFR2 to initiate increased permeability, angiogenesis and vasodilatation[18]. In contrast, VEGF165b is a weak agonist for VEGFR2 and prevents VEGF165 mediated signalling that results in angiogenesis[19-21], but use of receptor specific antagonists has shown VEGF165b increases hydraulic conductivity (Lp, the permeability of vessels to convective water flux) through VEGFR1[22]. PlGF only binds VEGFR1, but does not increase hydraulic conductivity in the same system[23]. VEGFRs are also produced as alternative splice variants. VEGFR1[24] and VEGFR2[25] have secreted splice variants that lack the transmembrane and cytoplasmic domains. These soluble VEGFRs bind to VEGFs and inactivate them[24].
VEGF and VEGFR expression in PET
VEGF
The properties of VEGF led to its investigation as a potential patho-physiological molecule in PET. However, there is a substantial, critical and serious discrepancy in the literature concerning the level of circulating VEGF in pre-eclamptic plasma (see table 1). Abnormally high levels of VEGF in PET plasma and serum[26, 27], amniotic fluid[28], umbilical cord serum[29] and urine[30] have been described using radioimmunoassay or competitive enzyme immunoassay, while commercial VEGF ELISAs show a decrease in VEGF levels[31-33]. This discrepancy has been proposed to be due to interference by VEGF binding molecules in the ELISA[34], but whether this results in reduced biologically active VEGF has only ever been an assumption[35],[36], and is contradicted by studies demonstrating biological activity of VEGF in pre-eclamptic plasma[6, 7, 37]. For example, a polyclonal VEGF RIA showed that total circulating VEGF concentrations in women who develop PET is 34ng/ml compared to normotensive controls (13ng/ml)[34, 38]. Post-delivery VEGF concentrations fall in both PET and control women suggesting that the placenta is the main source of VEGF production[38]. VEGF mRNA studies have been inconclusive and contradictory[9, 39],[10]. We recently showed that circulating VEGF165b in patients with pre-eclampsia was raised during pregnancy, but that it was not significantly higher in PET plasma than in normal pregnancy at term, although there was a lack of upregulation earlier on in pregnancies that subsequently developed pre-eclampsia[40]. When both VEGF165b and total VEGF were estimated, normal pregnancies had VEGF levels that were calculated (from total VEGF and VEGF165b measurements) at ~50% VEGF165b and 50% VEGF165, whereas in the small number of PET patients in which both could be reliably estimated, this was estimated at 69% VEGF165b, 31% VEGF165.[40]. However, there is still a need to determine the relative levels of the two sts of isoforms, using assays that are not interfered with by circulating macromolecules.
Table 1.
Measured concentrations of VEGF, sVEGFR1 and PlGF in plasma or serum from women with pre-eclampsia compared with controls (references up to 2005). No new information since 2005 has described VEGF-A, PlGF or sVEGFR1 levels in any more detail except for reference 40, which describes the VEGF165b levels.
| VEGF-A (ng/ml) | sVEGFR1 (ng/ml) |
PlGF (ng/ml) | Sample | Ref | VEGF method |
|||
|---|---|---|---|---|---|---|---|---|
| Normal | PET | Normal | PET | Normal | PET | |||
| 11.7 | 32.7 | Plasma | [78] | cEIA | ||||
| 0.166 | 0.0129 | Serum | [32] | ELISA | ||||
| 13.6 | 47 | Plasma | [79] | cEIA | ||||
| 0.018 | 0.0003 | 0.498 | 0.054 | Serum | [16] | ELISA | ||
| 13.9 | 51.7 | Plasma | [80] | RIA | ||||
| 0.00626 | 0.0185 | 0.531 | 0.119 | Plasma | [31] | ELISA | ||
| 5.1 | 11.8 | Plasma | [81] | RIA | ||||
| BDL | BDL | 0.225* | 0.08* | Serum | [82] | ELISA | ||
| 0.014* | 0.003* | 1.5 | 7.5* | 0.46* | 0.03* | Plasma | [35] | ELISA |
| 1.13 | 7.79 | [43] | ELISA | |||||
| 0.0648 | 0.0285 | 0.231 | 0.055 | [50] | ELISA | |||
| 6.83 | 25.2 | 0.12 | 2.69 | 0.585 | 0.067 | Plasma | [42] | EIA&ELIS A |
| 0.006 | 0.0139 | 1.6 | 4.3 | 0.669 | 0.137 | Serum | [8] | ELISA |
| 14* | 37.7* | 1.9* | 3.2* | Serum | [44] | RIA | ||
| 0.0136 | 0.016 | 0.22 | 0.636 | 0.012 | 0.012 | Plasma | [83] | ELISA |
| 3.4 | 9.9 | 0.169 | 0.082 | Serum | [84] | ELISA | ||
Estimated from graph. BDL – reported as below detection limit
sVEGFR
Both VEGFR have soluble splice variants. While soluble VEGFR2 levels do not change in pre-eclamptic plasma (5-6ng/ml)[41], higher circulating levels of sVEGF-R1 occur in PET plasma and serum than in normal pregnancy[8, 42, 43]. In animal models, adenovirus mediated over-expression of sVEGFR1 results in pre-eclampic like symptoms (proteinuria and hypertension) in pregnant animals. This led to the widespread, but untested, concept in the field that sVEGFR1 may be causal for pre-eclampsia in humans. However, there are a number of significant, critical and serious inconsistencies in the interpretation and extension to human disease from the experimental design of these animal studies. First, levels of sVEGFR1 (388ng/ml)[35] in the pregnant animal models were two orders of magnitude greater than that seen in humans in pregnancy (3.1-4.3 ng/ml, or 25pM)[8, 44]. Secondly, in humans the VEGF-A levels are ~800pM during PET, as measured by RIA[44]. As VEGF:sVEGFR1 binding is equimolar, the sVEGFR1 levels should not be high enough to affect VEGF levels in humans, whereas in the animal model the sVEGFR1 levels would exceed the VEGF-A levels by 3 fold. In fact in one study where “free” (measured by ELISA) and “total” (measured by cEIA) VEGF levels and sVEGFR1 levels were measured the molar ratio of total VEGF-A to sVEGFR1 was levels was 25 fold (i.e. VEGF-A levels were 547pM, and the sVEGFR1 levels were 21.9pM)[42]. Thus with these numbers it is not possible for sVEGFR1 to bind all the VEGF. Thus while it is of no doubt that sVEGFR1 is raised in pre-eclampsia, it cannot account for the reduced VEGF levels seen by ELISA, even when combined with sVEGFR2, and the ELISA must be being interfered with by other molecules that bind VEGF, which may not affect VEGF activity on its receptor. In fact it has been clearly demonstrated that VEGF-A can bind both covalently[45], and, predominantly, non-covalently to α2-macroglobulin, and that this latter interaction does not affect its ability to activate the receptor[46]. As α2-macroglobulin is one of the most common circulating proteins in plasma, and is found at concentrations far exceeding that of VEGF (2-4mg/ml, compared with 5-25ng/ml), it is much more likely that “bound” VEGF is bound, not to sVEGFR1, which might inactivate it, but α2-macroglobulin, which would not. In contrast, the raised levels of sVEGFR1 could affect the levels of free PlGF.
PlGF
PlGF is alternatively spliced to form 4 mRNA species (PlGF1-4), of which only PlGF2 has been found in mouse[47-49], an interesting caveat to rodent models of pre-eclampsia. PlGF-2 and PlGF-4 contain an additional 21 amino acid insert that encodes a heparin binding domain, resulting in cell association. Circulating PlGF in humans is predominantly PlGF-1 and its levels are tightly linked to human pre-eclampsia in that they are have been shown to be significantly reduced[16, 50-52],[40]. However, the role of PlGF in the pathogenesis of PET is not known partly due to a lack of understanding of its physiological actions in general.
Biological activity of PET plasma
It has been postulated for many years that circulating factors altered in pre-eclampsia affect endothelial function[53-55]. Endothelial cells in culture can be stimulated by plasma from women with PET[7, 54], and some effects are blocked by VEGF neutralising antibodies[7], implicating VEGF as a bioactive molecule in pre-eclampsia. Experiments using myometrial resistance vessels obtained at caesarean section using wire myography[56] showed that plasma from women with PET, but not normotensive pregnancies reduced endothelium-dependent relaxation. This response did not occur when the plasma was incubated with anti-VEGF antibodies[56]. Many downstream pathways have now been shown to be activated by PET plasma, and many pathways proposed to be responsible for the symptoms of pre-eclampsia. These include the production or upregulation of superoxide[57], MCP1 and IL8[58] IL6[59], P and E selectin and V-Cam[60], PGI2[61] and cadherin rearrangement[62]. Interestingly, all of these have been shown to be upregulated or induced by VEGF[63-69].
Thus, VEGF may be important in mediating the endothelial response that occurs in PET. In 2004, using an amphibian model to identify permeabilising agents in human plasma, we confirmed that a large molecular weight molecule (>12kDa) circulating in human plasma from severe pre-eclamptic patients results in a transient rapid increase in the hydraulic conductivity of the vessel wall[5] that was qualitatively similar to that seen by VEGF-A in the same system[70]. While there is no suggestion that the permeability increase that we see in this animal model relates to the symptoms of pre-eclampsia, understanding the mechanisms through which it works may give us a potential mechanism for the endothelial dysfunction induced by pre-eclamptic plasma on human endothelium, and thus a hypothesis to test which may reveal a key mechanisms underlying the pathogenesis of pre-eclampsia.
Of interest was a subsequent study that confirmed the biological activity of VEGF in pre-eclamptic plasma. The transient increase in permeability was blocked by neutralising antibodies to VEGF, and was inhibited by a concentration of a VEGFR TKI (SU5416) previously shown to block the VEGF165b effect but not the effect of VEGF165 on these vessels[22]. Moreover, a concentration of an inhibitor previously shown to block VEGF165 mediated permeability through inhibiting VEGFR2 phosphorylation (ZM323881) in this system[71] did not block the pre-eclamptic plasma mediated permeability response. Of particular interest was the effect of a specific antibody to VEGF165b[19], which blocks VEGF165b mediated inhibition of VEGF165-induced migration of endothelial cells, and VEGF165b induced cytoprotection of endothelial and epithelial cells[72]. The permeability increase was blocked by this neutralising antibody to VEGF165b. This was an extremely surprising finding and difficult to reconcile with the lack of any increase in VEGF165b in PET plasma compared with normotensive plasma at term when these samples were taken. The increase in permeability in this model induced by term pre-eclamptic plasma is clearly an effect of VEGF, but not simply due to excess VEGF165b. There are a number of possible mechanisms, but to outline these it is necessary to examine the mechanisms of actions of the three major contributors involved, VEGF, sVEGFR1 and PlGF.
Mechanisms of actions of VEGF, PlGF and sVEGFR1
VEGF-A
The mechanisms of action of the pro-angiogenic isoforms of VEGF have been widely studied. VEGF165 acts through VEGFR2, resulting in a transient calcium influx, and rapid transient increase in permeability followed by a sustained increase due to one or more of a combination of fenestrations, vesiculovacuolar organelles, endothelial gaps, tight junction and adherent junction disassembly[73]. The mechanism of the action of VEGF165b is less well described, but VEGF165b results in a rapid transient increase in Lp that is smaller in magnitude but greater in potency than VEGF165, probably acting through VEGFR1 not VEGFR2[22].
PlGF
PlGF acts through VEGFR1, but its downstream signalling is still not well understood and there are conflicting reports of its biological activity. PlGF-1 does not cause a transient increase in hydraulic conductivity in the same model used to investigate VEGF165b, VEGF165 and VEGF-C signalling[23], although studies using PlGF-2 knockout mice point to a rather more complex role, as these mice have reduced “leak” in response to VEGF-A[74]. However, PlGF-1 has been shown to be a potent vasodilator[75], particularly in uterine arteries. Blockade of PlGF-1 in pregnancy therefore would result in increased vascular tone, and hence hypertension as seen in pre-eclampsia.
sVEGFR1
The role of VEGFR1 has generally been characterised as a decoy receptor. It has been hypothesised that high levels of sVEGF-R1 antagonise the effects of VEGF and PlGF on placental development, vascularisation and maternal endothelial cell function[42], and thus the increase in sVEGFR1 in maternal plasma has been postulated to inhibit VEGF-A. However, VEGF-A induces increased vascular permeability (an hence oedema), vasodilatation and angiogenesis[70, 76]. Increased sVEGFR1 should therefore prevent the permeability responses of pre-eclamptic plasma, not induce them. Thus increased sVEGFR1 acting on VEGF-A by itself does not explain the symptoms of pre-eclampsia or experimental findings of the effect of PET plasma, but sVEGFR1 acing on PlGF, and removing the inhibition of VEGF165b would explain the symptoms. However, this scenario is not only unproven but theoretically difficult to reconcile with measurements made. PlGF binds to sVEGFR1 with the same affinity as VEGF165 (PlGF competes off binding of 10ng/ml radiolabelled VEGF165 with an IC50 of ~10ng/ml)[77]. However, circulating levels of PlGF are an order of magnitude lower than VEGF, and therefore most of the sVEGFR1 should be bound to VEGF-A not PlGF. This discrepancy has yet to be resolved, but it is possible that circulating levels of PlGF are lower in pre-eclamptic plasma because PlGF may be secreted at a lower level in pre-eclamptic pregnancies, and sVEGFR1 binding is irrelevant.
Thus, plasma from normal pregnancy shows no biological activity on endothelium of intact vessels. In contrast, PET plasma increases permeability and blocks vasodilation in the same models, and in the permeability model, this is blocked by a neutralising antibody to VEGF165b, and by VEGFR1 kinase inhibitors. There is no increased VEGF165b, but reduced PlGF and increased sVEGFR1 and VEGF165b/VEGF165 ratio, so an interplay between PlGF, VEGF165, VEGF165b and sVEGFR1 is hypothesised. This was tested by incubating pre-eclamptic plasma with PlGF, which blocked the biological response.
In summary the current models of pre-eclampsia based on the role of sVEGFR1 impacting on vascular permeability, hypertension and proteinuria do not appear to take into account the findings in the literature of the biologically active VEGF levels in women with pre-eclampsia. Alternate models involving sVEGFR1 competition of PlGF mediated repression of VEGF activity, or sVEGFR1 independent mechanisms need to be tested, particularly as it is the low availability of PlGF that may be the key pathological and treatable disorder of pre-eclampsia. Understanding this interplay between PlGF, sFlt-1 and VEGF may therefore reveal mechanisms through which PET pathophysiology occurs in humans.
ACKNOWLEDGEMENT
DOB would like to thank the British Heart Foundation for their support through a BHF Basic Science Lectureship from 2001-2011 (BS/06/005).
References
- 1.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 2.Health D. o. Why Mother’s Die. Report on confidential enquiries into Maternal Deaths in the United Kingdom 2000-2002. TSO; London: 1998. [Google Scholar]
- 3.Health D. o. CESDI Confidential Enquiries into Stillbirths and Deaths in Infancy. TSO; London: 2000. [Google Scholar]
- 4.Foidart JM, Schaaps JP, Chantraine F, Munaut C, Lorquet S. Dysregulation of anti-angiogenic agents (sFlt-1, PLGF, and sEndoglin) in preeclampsia--a step forward but not the definitive answer. J Reprod Immunol. 2009;82:106–111. doi: 10.1016/j.jri.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Neal CR, Hunter AJ, Harper SJ, Soothill PW, Bates DO. Plasma from women with severe pre-eclampsia increases microvascular permeability in an animal model in vivo. Clin Sci (Lond) 2004;107:399–405. doi: 10.1042/CS20040018. [DOI] [PubMed] [Google Scholar]
- 6.Brockelsby J, Hayman R, Ahmed A, Warren A, Johnson I, Baker P. VEGF via VEGF receptor-1 (Flt-1) mimics preeclamptic plasma in inhibiting uterine blood vessel relaxation in pregnancy: implications in the pathogenesis of preeclampsia. Lab Invest. 1999;79:1101–1111. [PubMed] [Google Scholar]
- 7.Brockelsby JC, Anthony FW, Johnson IR, Baker PN. The effects of vascular endothelial growth factor on endothelial cells: a potential role in preeclampsia. Am J Obstet Gynecol. 2000;182:176–183. doi: 10.1016/s0002-9378(00)70510-2. [DOI] [PubMed] [Google Scholar]
- 8.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 9.Chung JY, Song Y, Wang Y, Magness RR, Zheng J. Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2004;89:2484–2490. doi: 10.1210/jc.2003-031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sgambati E, Marini M, Zappoli Thyrion GD, Parretti E, Mello G, Orlando C, Simi L, Tricarico C, Gheri G, Brizzi E. VEGF expression in the placenta from pregnancies complicated by hypertensive disorders. BJOG. 2004;111:564–570. doi: 10.1111/j.1471-0528.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- 11.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Keyt B. Vascular endothelial growth factor: basic biology and clinical implications. Exs. 1997;79:209–232. doi: 10.1007/978-3-0348-9006-9_9. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler T, Elcock CL, Anthony FW. Angiogenesis and the placental environment. Placenta. 1995;16:289–296. doi: 10.1016/0143-4004(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 15.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- 16.Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1999;106:1019–1022. doi: 10.1111/j.1471-0528.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 17.Munaut C, Lorquet S, Pequeux C, Blacher S, Berndt S, Frankenne F, Foidart JM. Hypoxia is responsible for soluble vascular endothelial growth factor receptor-1 (VEGFR-1) but not for soluble endoglin induction in villous trophoblast. Hum Reprod. 2008;23:1407–1415. doi: 10.1093/humrep/den114. [DOI] [PubMed] [Google Scholar]
- 18.Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vascul Pharmacol. 2002;39:225–237. doi: 10.1016/s1537-1891(03)00011-9. [DOI] [PubMed] [Google Scholar]
- 19.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, Foster R, Digby-Bell J, Shields JD, Whittles CE, Mushens RE, Gillatt DA, Ziche M, Harper SJ, Bates DO. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 20.Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008;68:4683–4692. doi: 10.1158/0008-5472.CAN-07-6577. [DOI] [PubMed] [Google Scholar]
- 21.Cebe Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, Manlius C, Wood J, Ballmer-Hofer K. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006;63:2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass CA, Harper SJ, Bates DO. The anti-angiogenic VEGF isoform VEGF165b transiently increases hydraulic conductivity, probably through VEGF receptor 1 in vivo. J Physiol (Lond) 2006;572:243–257. doi: 10.1113/jphysiol.2005.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillman NJ, Whittles CE, Pocock TM, Williams B, Bates DO. Differential effects on Microvascular Hydraulic Conductivity (Lp) of Vascular Endothelial Growth Factor C (VEGF-C) and Placental Growth Factor-1 (PlGF-1) Journal of Vascular Research. 2001;38:176–185. doi: 10.1159/000051044. [DOI] [PubMed] [Google Scholar]
- 24.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albuquerque RJ, Hayashi T, Cho WG, Kleinman ME, Dridi S, Takeda A, Baffi JZ, Yamada K, Kaneko H, Green MG, Chappell J, Wilting J, Weich HA, Yamagami S, Amano S, Mizuki N, Alexander JS, Peterson ML, Brekken RA, Hirashima M, Capoor S, Usui T, Ambati BK, Ambati J. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker PN, Krasnow J, Roberts JM, Yeo KT. Elevated serum levels of vascular endothelial growth factor in patients with preeclampsia. Obstet Gynecol. 1995;86:815–821. doi: 10.1016/0029-7844(95)00259-T. [DOI] [PubMed] [Google Scholar]
- 27.Sharkey AM, Cooper JC, Balmforth JR, McLaren J, Clark DE, Charnock-Jones DS, Morris NH, Smith SK. Maternal plasma levels of vascular endothelial growth factor in normotensive pregnancies and in pregnancies complicated by pre-eclampsia. Eur J Clin Invest. 1996;26:1182–1185. doi: 10.1046/j.1365-2362.1996.830605.x. [DOI] [PubMed] [Google Scholar]
- 28.Tranquilli AL, Bezzeccheri V, Giannubilo SR, Scagnoli C, Mazzanti L, Garzetti GG. Amniotic vascular endothelial growth factor (VEGF) and nitric oxide (NO) in women with subsequent preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2004;113:17–20. doi: 10.1016/S0301-2115(03)00369-5. [DOI] [PubMed] [Google Scholar]
- 29.Galazios G, Papazoglou D, Giagloglou K, Vassaras G, Koutlaki N, Maltezos E. Umbilical cord serum vascular endothelial growth factor (VEGF) levels in normal pregnancies and in pregnancies complicated by preterm delivery or pre-eclampsia. Int J Gynaecol Obstet. 2004;85:6–11. doi: 10.1016/j.ijgo.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Roes EM, Steegers EA, Thomas CM, Geurts-Moespot A, Raijmakers MT, Peters WH, Sweep CG. High levels of urinary vascular endothelial growth factor in women with severe preeclampsia. Int J Biol Markers. 2004;19:72–75. doi: 10.1177/172460080401900110. [DOI] [PubMed] [Google Scholar]
- 31.Livingston JC, Chin R, Haddad B, McKinney ET, Ahokas R, Sibai BM. Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. Am J Obstet Gynecol. 2000;183:1554–1557. doi: 10.1067/mob.2000.108022. [DOI] [PubMed] [Google Scholar]
- 32.Lyall F, Greer IA, Boswell F, Fleming R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in pre-eclampsia. Br J Obstet Gynaecol. 1997;104:223–228. doi: 10.1111/j.1471-0528.1997.tb11050.x. [DOI] [PubMed] [Google Scholar]
- 33.Lyall F, Young A, Boswell F, Kingdom J, Greer IA. Placental expression of vascular endothelial growth factor in placentae from pregnancies complicated by pre-eclampsia and intrauterine growth restriction does not support placental hypoxia at delivery. Placenta. 1997;18:269–276. doi: 10.1016/s0143-4004(97)80061-6. [DOI] [PubMed] [Google Scholar]
- 34.Anthony FW, Evans PW, Wheeler T, Wood PJ. Variation in detection of VEGF in maternal serum by immunoassay and the possible influence of binding proteins. Ann Clin Biochem. 1997;34(Pt 3):276–280. doi: 10.1177/000456329703400309. [DOI] [PubMed] [Google Scholar]
- 35.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jelkmann W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem. 2001;47:617–623. [PubMed] [Google Scholar]
- 37.Bills VL, Salmon AH, Harper SJ, Overton TG, Neal CR, Jeffery B, Soothill PW, Bates DO. Impaired vascular permeability regulation due to the VEGF165b splice variant in pre-eclampsia Bjog. 2011 doi: 10.1111/j.1471-0528.2011.02925.x. in press. [DOI] [PubMed] [Google Scholar]
- 38.Hunter A, Aitkenhead M, Caldwell C, McCracken G, Wilson D, McClure N. Serum levels of vascular endothelial growth factor in preeclamptic and normotensive pregnancy. Hypertension. 2000;36:965–969. doi: 10.1161/01.hyp.36.6.965. [DOI] [PubMed] [Google Scholar]
- 39.Cooper JC, Sharkey AM, Charnock-Jones DS, Palmer CR, Smith SK. VEGF mRNA levels in placentae from pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1996;103:1191–1196. doi: 10.1111/j.1471-0528.1996.tb09627.x. [DOI] [PubMed] [Google Scholar]
- 40.Bills VL, Varet J, Millar A, Harper SJ, Soothill PW, Bates DO. Failure to up-regulate VEGF165b in maternal plasma is a first trimester predictive marker for pre-eclampsia. Clin Sci (Lond) 2009;116:265–272. doi: 10.1042/CS20080270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol. 2006;194:551–556. doi: 10.1016/j.ajog.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 42.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 43.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 44.McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191:1240–1246. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Soker S, Svahn CM, Neufeld G. Vascular endothelial growth factor is inactivated by binding to alpha 2-macroglobulin and the binding is inhibited by heparin. J Biol Chem. 1993;268:7685–7691. [PubMed] [Google Scholar]
- 46.Bhattacharjee G, Asplin IR, Wu SM, Gawdi G, Pizzo SV. The conformation-dependent interaction of alpha 2-macroglobulin with vascular endothelial growth factor. A novel mechanism of alpha 2-macroglobulin/growth factor binding. J Biol Chem. 2000;275:26806–26811. doi: 10.1074/jbc.M000156200. [DOI] [PubMed] [Google Scholar]
- 47.Maglione D, Guerriero V, Viglietto G, Ferraro MG, Aprelikova O, Alitalo K, Del Vecchio S, Lei KJ, Chou JY, Persico MG. Two alternative mRNAs coding for the angiogenic factor, placenta growth factor (PlGF), are transcribed from a single gene of chromosome 14. Oncogene. 1993;8:925–931. [PubMed] [Google Scholar]
- 48.Cao Y, Ji WR, Qi P, Rosin A. Placenta growth factor: identification and characterization of a novel isoform generated by RNA alternative splicing. Biochem Biophys Res Commun. 1997;235:493–498. doi: 10.1006/bbrc.1997.6813. [DOI] [PubMed] [Google Scholar]
- 49.Yang W, Ahn H, Hinrichs M, Torry RJ, Torry DS. Evidence of a novel isoform of placenta growth factor (PlGF-4) expressed in human trophoblast and endothelial cells. J Reprod Immunol. 2003;60:53–60. doi: 10.1016/s0165-0378(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 50.Madazli R, Aydin S, Uludag S, Vildan O, Tolun N. Maternal plasma levels of cytokines in normal and preeclamptic pregnancies and their relationship with diastolic blood pressure and fibronectin levels. Acta Obstet Gynecol Scand. 2003;82:797–802. doi: 10.1034/j.1600-0412.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- 51.Bersinger NA, Odegard RA. Serum levels of macrophage colony stimulating, vascular endothelial, and placenta growth factor in relation to later clinical onset of pre-eclampsia and a small-for-gestational age birth. Am J Reprod Immunol. 2005;54:77–83. doi: 10.1111/j.1600-0897.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 52.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 53.Roberts JM, Taylor RN, Goldfien A. Endothelial cell activation as a pathogenetic factor in preeclampsia. Semin Perinatol. 1991;15:86–93. [PubMed] [Google Scholar]
- 54.Taylor RN, Casal DC, Jones LA, Varma M, Martin JN, Jr., Roberts JM. Selective effects of preeclamptic sera on human endothelial cell procoagulant protein expression. Am J Obstet Gynecol. 1991;165:1705–1710. doi: 10.1016/0002-9378(91)90019-n. [DOI] [PubMed] [Google Scholar]
- 55.Taylor RN, Musci TJ, Rodgers GM, Roberts JM. Preeclamptic sera stimulate increased platelet-derived growth factor mRNA and protein expression by cultured human endothelial cells. Am J Reprod Immunol. 1991;25:105–108. doi: 10.1111/j.1600-0897.1991.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 56.Brockelsby J, Hayman R, Ahmed A, Warren A, Johnson I, Baker P. VEGF via VEGF receptor-1 (Flt-1) mimics preeclamptic plasma in inhibiting uterine blood vessel relaxation in pregnancy: implications in the pathogenesis of preeclampsia. Lab Invest. 1999;79:1101–1111. [PubMed] [Google Scholar]
- 57.Sankaralingam S, Xu H, Davidge ST. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc Res. 2010;85:194–203. doi: 10.1093/cvr/cvp277. [DOI] [PubMed] [Google Scholar]
- 58.Kauma S, Takacs P, Scordalakes C, Walsh S, Green K, Peng T. Increased endothelial monocyte chemoattractant protein-1 and interleukin-8 in preeclampsia. Obstet Gynecol. 2002;100:706–714. doi: 10.1016/s0029-7844(02)02169-5. [DOI] [PubMed] [Google Scholar]
- 59.Takacs P, Green KL, Nikaeo A, Kauma SW. Increased vascular endothelial cell production of interleukin-6 in severe preeclampsia. Am J Obstet Gynecol. 2003;188:740–744. doi: 10.1067/mob.2003.134. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Gu Y, Lewis DF, Wang Y. Reduced cellular glutathione reductase activity and increased adhesion molecule expression in endothelial cells cultured with maternal plasma from women with preeclampsia. J Soc Gynecol Investig. 2006;13:412–417. doi: 10.1016/j.jsgi.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Rowe J, Campbell S, Gallery ED. Plasma from preeclamptic women stimulates decidual endothelial cell growth and prostacyclin but not nitric oxide production: close correlation of prostacyclin and thromboxane production. J Soc Gynecol Investig. 2001;8:32–38. [PubMed] [Google Scholar]
- 62.Groten T, Kreienberg R, Fialka I, Huber L, Wedlich D. Altered subcellular distribution of cadherin-5 in endothelial cells caused by the serum of pre-eclamptic patients. Mol Hum Reprod. 2000;6:1027–1032. doi: 10.1093/molehr/6.11.1027. [DOI] [PubMed] [Google Scholar]
- 63.Ido Y, Chang KC, Lejeune WS, Bjercke RJ, Reiser KM, Williamson JR, Tilton RG. Vascular dysfunction induced by AGE is mediated by VEGF via mechanisms involving reactive oxygen species, guanylate cyclase, and protein kinase C. Microcirculation. 2001;8:251–263. doi: 10.1038/sj/mn/7800079. [DOI] [PubMed] [Google Scholar]
- 64.Hippenstiel S, Krull M, Ikemann A, Risau W, Clauss M, Suttorp N. VEGF induces hyperpermeability by a direct action on endothelial cells. Am J Physiol. 1998;274:L678–684. doi: 10.1152/ajplung.1998.274.5.L678. [DOI] [PubMed] [Google Scholar]
- 65.Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem. 1998;273:15099–15103. doi: 10.1074/jbc.273.24.15099. [DOI] [PubMed] [Google Scholar]
- 66.Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48:1131–1137. doi: 10.2337/diabetes.48.5.1131. [DOI] [PubMed] [Google Scholar]
- 67.Tezono K, Sarker KP, Kikuchi H, Nasu M, Kitajima I, Maruyama I. Bioactivity of the vascular endothelial growth factor trapped in fibrin clots: production of IL-6 and IL-8 in monocytes by fibrin clots. Haemostasis. 2001;31:71–79. doi: 10.1159/000048047. [DOI] [PubMed] [Google Scholar]
- 68.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 69.Wheeler-Jones C, Abu-Ghazaleh R, Cospedal R, Houliston RA, Martin J, Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 in endothelial cells via p42/p44 mitogen-activated protein kinase. Febs Lett. 1997;420:28–32. doi: 10.1016/s0014-5793(97)01481-6. [DOI] [PubMed] [Google Scholar]
- 70.Bates DO, Curry FE. Vascular endothelial growth factor increases hydraulic conductivity of isolated perfused microvessels. American Journal of Physiology: Heart and Circulatory Physiology. 1996;271:H2520–H2528. doi: 10.1152/ajpheart.1996.271.6.H2520. [DOI] [PubMed] [Google Scholar]
- 71.Whittles CE, Pocock TM, Wedge SR, Kendrew J, Hennequin LF, Harper SJ, Bates DO. ZM323881, a novel inhibitor of vascular endothelial growth factor-receptor-2 tyrosine kinase activity. Microcirculation. 2002;9:513–522. doi: 10.1038/sj.mn.7800164. [DOI] [PubMed] [Google Scholar]
- 72.Magnussen AL, Rennel ES, Hua J, Bevan HS, Beazley Long N, Lehrling C, Gammons M, Floege J, Harper SJ, Agostini HT, Bates DO, Churchill AJ. VEGF-A165b is cytoprotective and antiangiogenic in the retina. Invest Ophthalmol Vis Sci. 2010;51:4273–4281. doi: 10.1167/iovs.09-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200:581–597. doi: 10.1046/j.1469-7580.2002.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luttun A, Brusselmans K, Fukao H, Tjwa M, Ueshima S, Herbert JM, Matsuo O, Collen D, Carmeliet P, Moons L. Loss of placental growth factor protects mice against vascular permeability in pathological conditions. Biochem Biophys Res Commun. 2002;295:428–434. doi: 10.1016/s0006-291x(02)00677-0. [DOI] [PubMed] [Google Scholar]
- 75.Osol G, Celia G, Gokina N, Barron C, Chien E, Mandala M, Luksha L, Kublickiene K. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am J Physiol Heart & Circ. 2008;294:H1381–1387. doi: 10.1152/ajpheart.00922.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 77.Barleon B, Totzke F, Herzog C, Blanke S, Kremmer E, Siemeister G, Marme D, MartinyBaron G. Mapping of the sites for ligand binding and receptor dimerization at the extracellular domain of the vascular endothelial growth factor receptor FLT-1. J Biol Chem. 1997;272:10382–10388. doi: 10.1074/jbc.272.16.10382. [DOI] [PubMed] [Google Scholar]
- 78.Sharkey AM, Cooper JC, Balmforth JR, McLaren J, Clark DE, Charnock-Jones DS, Morris NH, Smith SK. Maternal plasma levels of vascular endothelial growth factor in normotensive pregnancies and in pregnancies complicated by pre-eclampsia. Eur J Clin Invest. 1996;26:1182–1185. doi: 10.1046/j.1365-2362.1996.830605.x. [DOI] [PubMed] [Google Scholar]
- 79.Kupferminc MJ, Daniel Y, Englender T, Baram A, Many A, Jaffa AJ, Gull I, Lessing JB. Vascular endothelial growth factor is increased in patients with preeclampsia. Am J Reprod Immunol. 1997;38:302–306. doi: 10.1111/j.1600-0897.1997.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 80.Hunter A, Aitkenhead M, Caldwell C, McCracken G, Wilson D, McClure N. Serum levels of vascular endothelial growth factor in preeclamptic and normotensive pregnancy. Hypertension. 2000;36:965–969. doi: 10.1161/01.hyp.36.6.965. [DOI] [PubMed] [Google Scholar]
- 81.Bosio PM, Wheeler T, Anthony F, Conroy R, O’Herlihy C, McKenna P. Maternal plasma vascular endothelial growth factor concentrations in normal and hypertensive pregnancies and their relationship to peripheral vascular resistance. Am J Obstet Gynecol. 2001;184:146–152. doi: 10.1067/mob.2001.108342. [DOI] [PubMed] [Google Scholar]
- 82.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 83.Muy-Rivera M, Vadachkoria S, Woelk GB, Qiu C, Mahomed K, Williams MA. Maternal plasma VEGF, sVEGF-R1, and PlGF concentrations in preeclamptic and normotensive pregnant Zimbabwean women. Physiol Res. 2005;54:611–622. [PubMed] [Google Scholar]
- 84.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;122:33–39. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]