Abstract
There has been recent controversy about whether activation in the human inferior frontal gyrus (IFG) and Brodmann Area (BA) 6 when observing actions indicates operation of mirror neurons. Recent functional magnetic resonance imaging (fMRI) data have demonstrated repetition suppression (RS) effects in posterior IFG which are consistent with the presence of mirror neurons in humans. Here we investigated whether there were similar RS effects elsewhere in the IFG and BA6, or whether, instead, activation in other locations may signal operation of alternative mechanisms. Replicating previous findings, we found RS effects in posterior IFG consistent with the operation of mirror neurons. However, these effects were not found in other locations in IFG and BA6. Additionally, activation patterns in anterior regions of IFG suggested dissociable operations when observing and executing actions. Therefore, caution should be exercised when claiming that activations in many locations during action observation indicate the operation of mirror neurons. Activation may instead reflect alternative mechanisms, such as encoding of the semantic features of actions.
Keywords: Mirror neuron, Mirror system, Action observation, Repetition suppression, Adaptation, Semantics
Introduction
Mirror neurons are a class of neuron that was first discovered in the premotor area F5 of the macaque monkey (Di Pellegrino et al., 1992; Gallese et al., 1996; Rizzolatti et al., 2001; Umilta et al., 2001), and subsequently demonstrated to be present in a region of the inferior parietal lobule, area PF (Fogassi et al., 2005; Gallese et al., 2002). The defining property of mirror neurons is that they discharge not only when the monkey executes a certain action (e.g. precision grip) but also when the monkey observes a similar action performed by the experimenter.
Neuroimaging studies have provided evidence of activation in similar cortical areas when humans observe and execute actions (Buccino et al., 2001; Decety et al., 1997; Gazzola and Keysers, 2009; Grezes and Decety, 2001; Hamilton and Grafton, 2006; Rizzolatti et al., 1996). Such human neuroimaging studies have reported activations throughout the inferior frontal gyrus (IFG), including Brodmann Area (BA) 45 and BA44, as well as ventral BA6 (see Dinstein et al., 2007; Morin and Grèzes, 2008) and even dorsal BA6 (Calvo-Merino et al., 2005). Despite such large differences in the loci of activity such effects are all interpreted as reflecting the operation of mirror neurons (Calvo-Merino et al., 2005; Dinstein et al., 2007; Morin and Grèzes, 2008). This is at odds with the data from the macaque monkey where mirror neurons are reported to be mainly located in a subregion of area F5; F5c (Belmalih et al., 2009; Nelissen et al., 2005). It has recently been argued that this apparent disparity in the reported spatial extent between humans and macaque monkeys could reflect the fact that an increase in the BOLD signal in the IFG or BA6 during observation and execution of an action does not necessarily reflect mirror neuron activity (Dinstein et al., 2007, 2008; Dinstein, 2008). Although one would predict that mirror neuron activity during action observation would lead to an increase in the BOLD signal, the reverse inference is not necessarily true — that an increase in the BOLD signal during action observation is driven by mirror neuron activity. It has been proposed that the best approach to attribute the fMRI response to a single neuronal population encoding observation and execution of the same action is fMRI repetition suppression (RS) (Dinstein et al., 2007; Malach, in press). This approach assumes that as stimuli that evoke activity in a specific neuronal population are repeated, the magnitude of the BOLD response decreases or adapts. Areas of the cortex that contain mirror neurons should show RS both when a specific action is executed and subsequently observed and when an action is observed and subsequently executed (as well as when an action is observed or executed twice). Using such an fMRI RS paradigm, a recent study showed significant effects in human IFG that are consistent with the presence of mirror neurons (Kilner et al., 2009). These effects were observed in only the posterior part of the IFG.
The hypothesis that the human IFG and BA6 contain functionally dissociable mechanisms that operate when observing and executing actions is tested here. We employed an fMRI RS paradigm in which fourteen right handed subjects repeatedly observed or executed one of two actions performed by the right hand — a precision grip or an index finger ring pull (Kilner et al., 2009 and Fig. 1). We show (1) that only activity in the most posterior parts of the IFG, at the border of BA44 and ventral BA6, is consistent with the presence of mirror neurons and (2) that the pattern of activity in this region is functionally dissociable from activity in both anterior BA44 and BA47.
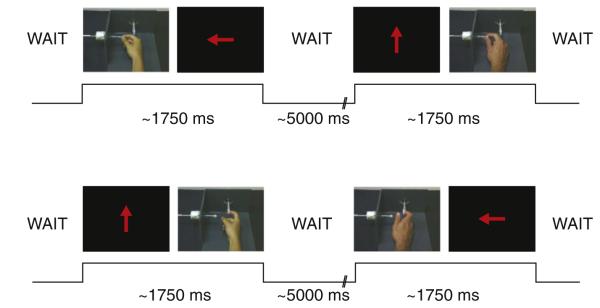
Fig. 1.
Experimental task. Each trial consisted of a pair of stimuli presented one after the other. The pairs either contained two execution conditions, two observation conditions or one execution and one observation condition. The four trials shown in Fig. 1 are cross-modal trials. In the execution conditions, an arrow cue indicated whether a ring pull or precision grip should be executed. The four trials show exemplars of the following trial types — execute–observe same, execute–observe different, execute–observe same and observe–execute different.
Materials and methods
Subjects
Data were recorded from 14 healthy right-handed subjects (7 females, 25–45 yrs). All subjects gave written informed consent prior to testing and the recordings had local ethical committee approval.
Procedure
The task employed in this study was identical to the one we have previously used (Kilner et al., 2009). Subjects were asked to either observe or execute one of two actions performed by the right hand — a precision grip or an index finger pull. The actions were made in a way that required only movements around the wrist and the hand, thus minimising movement artefacts in the fMRI data.
In the scanner on each trial subjects were presented with pairs of stimuli sequentially. These pairs could consist of two executions, two observations, or mixed execution and observation conditions. During the observation conditions subjects observed a video of one of the actions — index finger ring pull or precision grip. In total there were 28 different exemplar videos of each movement made by two different actors; one female and one male. Each video lasted ~750 ms. Subjects were required to execute an action according to the direction in which an arrow on the screen pointed. The arrow either pointed up the screen – indicating the index finger ring pull – or left — indicating the precision grip (Fig. 1). Subjects were trained prior to scanning to execute the correct action as rapidly as possible following the imperative cues. These imperative cues were presented for 500 ms.
Trials were categorised into 8 types: execute–execute same action type (defined by whether it was a ring pull or precision grip; EEs), execute–execute different action type (EEd), observe–observe same (OOs), observe–observe different (OOd), execute–observe same (EOs), execute–observe different (EOd), observe–execute same (OEs) and observe–execute different (OEd). Within each trial there was a 500 ms gap between presentations of the two stimuli and there was a between-trial jittered ‘wait’ with a mean of 5000 ms (sd 1000 ms). Subjects performed 3 sessions, where each session consisted of 96 trials with each of the 8 trial types presented 12 times.
Data acquisition and analysis
We acquired T2*-weighted echo-planar images (EPI) with blood oxygen-level dependent (BOLD) contrast on a 3 T whole-body MRI scanner (Magneton TIM TRIO, Siemens Healthcare, Erlangen, Germany) operated with a 32-channel RF head receive and body transmit coil. A total of 186 volumes were collected for each of the 3 sessions. These included 6 dummy volumes at the start of each session to allow for T1 equilibration. In contrast with the previous study (Kilner et al., 2009), here each volume consisted of 48 slices allowing a whole brain analysis as opposed to restricting the analysis to the posterior regions of BA44 and ventral BA6. Imaging parameters were: in-plane resolution 3 mm×3 mm, slice thickness 2 mm with 1 mm interslice gap, TR 70 ms, 64×74 matrix, extended field of view (FoV). T1-weighted structural scans with 1 mm isotropic resolution (MDEFT; Deichmann et al., 2004) were collected for each subject and were coregistered to the mean EPI image generated by the spatial realignment procedure.
Data pre-processing of the EPI functional scans included spatial realignment, normalisation to a standard EPI template in Montreal Neurological Institute (MNI) stereotactic space, and smoothing with a 4 mm (full-width at half-maximum) Gaussian Kernel, using SPM5 (www.fil.ion.ucl.ac.uk/spm). Inhomogeneities in the sensitivity profile of the 32-channel RF coil were corrected by applying the unified segmentation and bias correction to the EPI time-series as the first step (Ashburner and Friston, 2005).
The event-related fMRI data were then analysed using a linear convolution model in the usual way: Stimulus functions comprised a set of delta functions corresponding to the onset times of the different conditions. The first stimulus of each within-trial pair was modelled by two regressors, one for a pair beginning with an execution condition and one for a pair beginning with the observation condition. For the second within-trial stimulus there were 8 different stimulus functions depending upon the trial category, modelled by a further 8 regressors (see above). In addition, the ‘wait’ period that occurred between every pair of stimuli was explicitly modelled. These functions were convolved with a canonical hemodynamic response function for explanatory variables or regressors. Subject-specific movement parameters and drift terms (high pass filter cut-off period, 128 s) were also modelled as covariates of no interest. Therefore, for each session there were 17 regressors at the first level. Condition-specific estimates of neural activity (betas), corresponding to the amplitude of the modelled response were computed at each voxel for each subject.
For each effect of interest, contrasts were taken at the first level for each subject and taken to the second level (thus conforming to a classic random effects design). The contrasts of interest taken at the first level were the main effect of action type, same or different (EEs−EEd+OOs−OOd+OEs−OEd+EOs−EOd), the interaction between action type and modality switch ((EEs−EEd+OOs−OOd)−(OEs−OEd+EOs−EOd)) and the interaction between action type, modality switch and first event prime ((EEs−EEd+OEs−OEd)−(OOs−OOd+EOs−EOd)). All voxels reported conform to MNI (Montreal Neurological Institute) co-ordinate space. For display, the right side of the image corresponds to the right side of the brain. In this study we employed three different statistical approaches. As the studied was intended to look at effects in the IFG and BA6 an initial analysis employed a single region of interest (ROI) approach, where the ROI consisted of left and right BA6, BA44, BA45 and BA47. This volume was created from published cytoarchitectonic maps (Eickhoff et al., 2005). These regions were selected because they have previously been shown to be active during action observation and execution tasks. Any effects significant at p<0.005 uncorrected within this ROI are reported, along with FWE correction for both peak and cluster levels where appropriate. Additionally, to test if any effects were consistent with previous results (Kilner et al., 2009) we employed a second ROI approach by applying a small volume correction of a sphere of 6 mm radius centred on the average co-ordinates of the previously published repetition suppression effects (Table 2 in Kilner et al., 2009; [−52, 6, 22] and [56, 6, 24] for the left and right hemispheres respectively). Finally, all other effects outside of this ROI are reported controlling for FWE across the whole brain at the cluster level correction of p<0.05, having first thresholded the images at an uncorrected threshold of p<0.005. All further reported post-hoc analyses are based on the values extracted from peak co-ordinates from independent contrasts. These are described in detail in the text where appropriate.
Post-hoc analysis
Based on the results of the analysis described above we performed a post-hoc analysis to test the hypothesis that different regions of the IFG have significantly different patterns of activation. To this end we divided the IFG into six regions of equal volume and calculated the average beta for the EEs–EEd, OOs–OOd, EOs–EOd and OEs–OEd contrasts for each subject. The six regions were defined as: Left BA44/ BA6: x = −40: −60, y = −4: 4, z = 5: 25, Right BA44/BA6: x = 40: 60, y = −4: 4, z = 5: 25, Left anterior BA44: x = −45: −65, y = 6: 14, z = 5: 25, Right anterior BA44: x = 45: 65, y = 6: 14, z = 5: 25, Left BA47: x = −35: −55, y = 16: 24, z = −10: 10, Right BA47: x = 35: 55, y = 16: 24, z = −10: 10.
The x and z coordinates were chosen to cover the anatomical areas in question (notably, the x-coordinates were chosen so that the majority of the volume for each section was in the brain, but as lateral as possible). The y-coordinates were selected likewise, but with an arbitrary split in BA44 to create equally sized ROIs with no shared voxels. To test whether the patterns of activations were significantly different between areas we performed a 3×2×2×2 repeated measures ANOVA where the factors were ROI (BA44/BA6, anterior BA44, BA47), hemisphere (Left or Right), Modality switch (within or cross modality) and first event prime (Execute or Observe). The degrees of freedom of the ANOVA were corrected for non-sphericity using a Greenhouse–Geisser correction. It should be noted that although we were as unbiased as possible when selecting these regions, this is a post-hoc analysis and these regions could not be defined either anatomically or a priori, therefore it is statistically biased.
Results
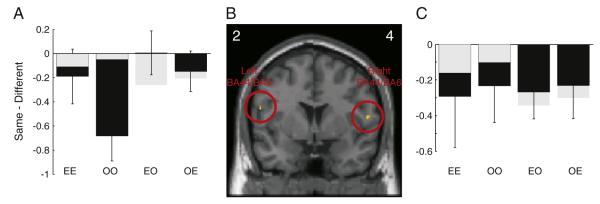
Two brain areas in the IFG showed lower activation when the same action was executed or observed for a second time, compared with a different action, irrespective of whether the first action was observed or executed or a modality switch (RS effect, see Fig. 2). This pattern was observed bilaterally in posterior regions of the IFG, corresponding to the border of BA44 and BA6 (peak voxel MNI co-ordinates [−56, 2, 20] and [56, 4, 12]; p<0.005 uncorrected, t = 3.69 and 3.42 respectively; Figs. 2A–C). However, neither of these effects survived FWE correction when correcting for the whole of the left and right IFG ROI (see Materials and methods). To test whether these effects were consistent with those previously published (Kilner et al., 2009) we employed a correction based on a sphere of 6 mm radius centred on the previously reported co-ordinates (see Materials and methods). Based on this small volume correction the activation in the left hemisphere was significant at p<0.05 corrected for FWE at the peak and cluster level. Both of these BA44/BA6 areas also showed the basic properties of mirror neurons, namely that there was significantly greater BOLD response during both action execution and observation compared to rest (p<0.05, t = 2.34 and 3.60 for left and right hemispheres respectively, t-test at the voxel of interest from the previous orthogonal analysis). This pattern of modulation satisfies the criteria necessary to demonstrate mirror neuron activity (Dinstein et al., 2008; Dinstein, 2008) and replicates the results of a previous study with the same paradigm and actions (Kilner et al., 2009). No other brain areas in the IFG or BA6 showed an RS effect regardless of modality of the action (observed or executed).
Fig. 2.
Main effect of repetition suppression for action type, whereby there was lower activation when the same action was executed or observed for a second time, compared with a different action, irrespective of whether the first action was observed or executed. (B) shows SPMs of the t-values from the group level. The SPM is shown for the coronal slice at maximal activity in the left and right hemispheres separately. The y co-ordinates for the peak voxel are shown in the left and right corners. The SPM image is thresholded for display at p<0.005 uncorrected. The open red circles indicate the significant clusters. (A,C) black bars show the mean difference in beta values between the repetition of the same or different action type at the peak voxel shown in B (E = executed/O = observed action). Negative values are consistent with repetition suppression. Error bars are standard error of the mean. The grey bars in A and C show the effects at the peak voxel for just the cross-modal conditions that was within a small volume of 1 cm radius from the peak previously reported for this contrast (Kilner et al., 2009).
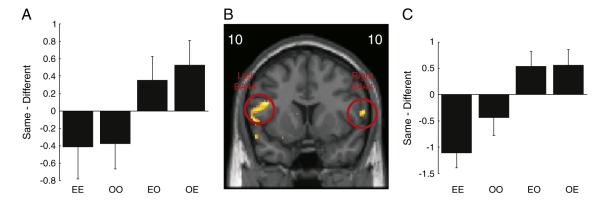
Two areas of IFG, middle-to-anterior BA44 bilaterally, showed a significant interaction between the action type, same or different, and modality switch, within or cross modality (peak voxel MNI co-ordinates [−46, 10, 22] and [62, 10, 12]; p<0.005 uncorrected, p<0.05 cluster level corrected within ROI, t = 5.73 and 4.76 respectively; Figs. 3A–C). Here in both right and left hemispheres there was an RS effect within-modality (t(13) = −3.2, p<0.05 and t(13) = −1.91, p = 0.08 for right and left BA44 peak coordinates respectively) but a repetition enhancement (RE) effect cross-modality (t(13) = 2.9, p<0.05 and t(13) = 2.05, p = 0.06 for right and left BA44 peak coordinates respectively). When the task, execute or observe, remained the same, the BOLD response showed a decrease on repetition of the same action (OO and EE, Fig. 3). In contrast, when there was a task switch (OE and EO) for the same action, there was an increase in the BOLD response.
Fig. 3.
Interaction between action type and modality switch. (B) shows SPMs of the t-values from the group level. The SPM is shown for the coronal slice at maximal activity in the left and right hemispheres separately. The y co-ordinates for the peak voxel are shown in the left and right corners. The SPM image is thresholded for display at p<0.005 uncorrected. The open red circles indicate the significant clusters. (A,C) black bars show the mean difference in beta values between the repetition of the same or different action type at the peak voxel shown in B. Negative values are consistent with repetition suppression, and positive values with repetition enhancement. Error bars are standard error of the mean.
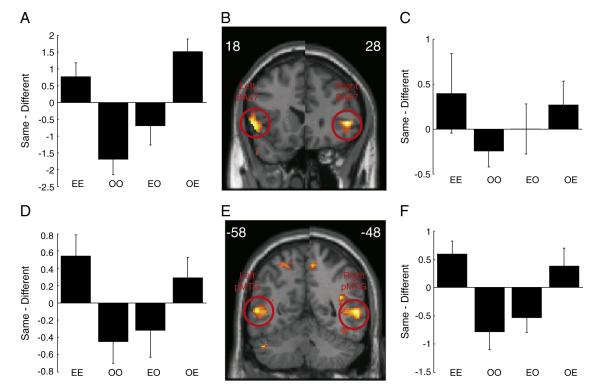
At further anterior regions of IFG, corresponding to BA47, there was a significant interaction between the action type, same or different, modality switch (within or cross modality) and first event prime (execute or observe), (peak voxel MNI co-ordinates [−56, 18, 0] and [48, 28, 2]; p<0.005 uncorrected, p<0.05 cluster level FWE corrected for whole brain, t = 7.62 and 9.25 respectively; Figs. 4A–C). In these regions the interaction was driven by the magnitude of the BOLD response being less when an observed action was the same as the previous event irrespective of whether the first event was observed or executed, consistent with RS (OO and EO conditions in Fig. 4; t(13) = −3.6, p<0.05 and t(13) = −3.5, p<0.05 for right and left BA47 peak coordinates respectively). In contrast, when the second event was action execution, the BOLD response increased when the action was the same as the previous event compared to when the previous action was different, again irrespective of whether the first event was observed or executed (t(13) = 2.3, p<0.05 and t(13) = 3.5, p<0.05 for right and left BA47 peak coordinates respectively).
Fig. 4.
Interaction between action type, modality and first event prime. (B and E) show SPMs of the t-values from the group level. The SPM is shown for the coronal slice at maximal activity in the left and right hemispheres separately. The y co-ordinates for the peak voxel are shown in the left and right corners. The SPM image is thresholded for display at p<0.005 uncorrected. The open red circles indicate the significant clusters. (A,C,D and F) black bars show the mean difference in beta values between the repetition of the same or different action type at the peak voxel shown in B and E. Negative values are consistent with repetition suppression. Error bars are standard error of the mean.
This same pattern was also observed bilaterally in a region of middle temporal gyrus (MTG) corresponding to BA21/37 (peak voxel MNI co-ordinates [−54, −58, 6] and [58, −48, 4]; p<0.005 uncorrected, p<0.05 FWE whole brain cluster level corrected, t = 6.49 and 8.08 respectively; Figs. 4D–F).
Having performed a series of mass univariate analyses and shown evidence that different regions of the IFG show distinct patterns of modulation, we tested whether the pattern of BOLD responses was significantly different between ROIs by performing a post-hoc analysis (see Fig. 5 and Materials and methods). The only significant effects of the 3×2×2×2 repeated measures ANOVA were a 2 way interaction between modality switch and the prime (F(1,13) = 11.6, P<0.05) and the 3 way interaction between ROI, modality switch and the prime (F(1.62,21.03) = 12.52, P<0.05; Fig. 5B). In other words, the pattern of RS or RE effects of the type shown in Figs. 2, 3 and 4 are significantly different throughout the IFG.
Fig. 5.
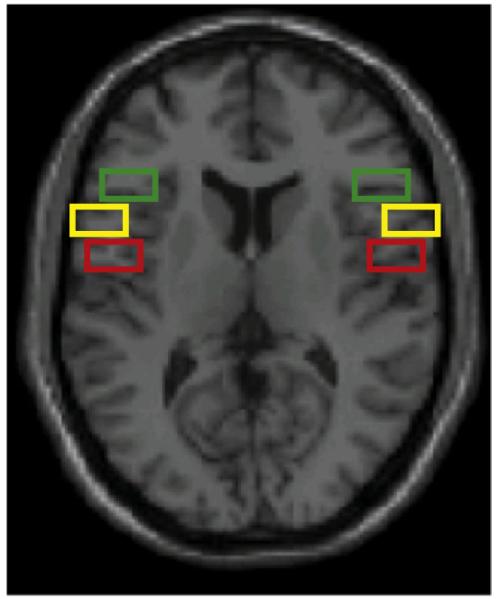
Post-hoc region of interest analysis. The 6 ROIs are displayed on a single slice of the canonical single subject structural image. Red boxes show the extent of the left and right BA44/BA6 ROI, yellow boxes show the extent of the left and right anterior BA44 ROI and the green boxes show the extent of the left and right BA47 ROI (see Materials and methods for more details).
To investigate this further we performed three separate 2×2×2×2 repeated measures ANOVAs. When comparing BA44/BA6 and anterior BA44 ROIs the only significant effect was a significant interaction between the ROI and the modality switch (F(1,13) = 6.56, p<0.05). When comparing BA44/BA6 and BA47 there was a 2 way interaction between the modality switch and the prime (F(1,13) = 11.6, p<0.05) and a 3 way interaction between ROI, modality switch and the prime (F(1,13) = 17.0, p<0.05). When comparing anterior BA44 and BA47 there was a significant effect of modality switch (F(1,13) = 7.11, p<0.05), a 2 way interaction between modality switch and the prime (F(1,13) = 15.83, p<0.05) and a 3 way interaction between ROI, modality switch and the prime (F(1,13) = 11.7, p<0.05). However, it should be noted that although we were as unbiased as possible when selecting these regions, this analysis is post-hoc.
Discussion
It has been assumed for most of the last two decades that activations in many locations throughout the IFG and BA6 when humans observe actions reflect the operation of mirror neurons (e.g. Rizzolatti and Craighero, 2004). However, these activations may serve a variety of functions; some of which will not involve mirror representations of action. The present study employed an fMRI RS paradigm to address where in the IFG and BA6 demonstrated repetition effects consistent with the presence of mirror neurons and also whether other areas of the IFG and BA6 were functionally dissociable.
The study demonstrated only two areas in IFG that displayed the RS characteristics predicted of mirror neurons, namely 1) lower activation to an action when the same action had been presented just before, and regardless of whether the first and second actions were observed or executed or switched between modalities, and 2) activation when observing and executing actions, relative to baseline. These areas were bilateral BA6/BA44 border. This effect replicates the findings of Kilner et al. (2009), and is consistent with the existence of mirror neurons in humans.
There has been recent controversy surrounding the existence of mirror neurons at all in humans (e.g. Dinstein et al., 2007; Lingnau et al., 2009), due to inconsistency in findings. For example, Dinstein et al. (2007) found RS effects within IFG when rock–paper–scissors actions were repeatedly observed or executed, but no effects when switching modality. Lingnau et al. (2009) found an RS effect in left dorsal premotor cortex when mimed actions were observed and then executed, but not if they were executed and then observed. Of course, these studies should not be taken as evidence of absence of mirror neurons in humans; they reflect simply an absence of evidence. Even if considering these studies in isolation (ignoring the findings of Kilner et al., 2009, and the present study), it might be the case that humans have mirror neurons, but that they do not alter their patterns of activation when stimuli that evoke their response are repeated. There are three underlying neural mechanisms that have been proposed to explain fMRI adaptation with repetition (cf. Grill-Spector et al. 2006): the fatigue model – where the neuronal firing rate is decreased with repetition, the facilitation model – where the input is processed more efficiently with repetition, and the sharpening model — where fewer neurons discharge with repetition. To date it is not known which of these mechanisms might best explain the fMRI adaptation effects observed here as there is no published literature on the effect of repetition on mirror neurons populations. Indeed, to date no study has demonstrated significant repetition suppression effects in single mirror neurons and some have argued that there are mechanistic reasons why mirror neurons might not show crossmodal adaptation (e.g. Rizzolatti and Fabbri-Destro, 2010). However, given that the present study and Kilner et al. (2009) found crossmodal RS effects, there is evidence that repetition has an effect on the BOLD signal in areas that contain mirror neurons in the macaque monkey, and this needs to be explained. It should be noted though that the magnitude of these reported RS effects at the group level are statistically small and only significant here with a restricted search volume based on the co-ordinates from a previous study.
The fact that the present study only revealed activations consistent with mirror neuron activity in bilateral BA6/BA44 border suggests that extra caution should be exercised in interpretation when simple observation of action generates activation in the IFG and BA6. We are not claiming that activations elsewhere will not be found to reflect operation of mirror neurons; mirror representations of different actions may reside in alternative locations. For example, it has been suggested that dorsal BA6 contains mirror representations of foot actions, whereas mirror representations of hand actions lie ventral to these (Buccino et al., 2001). It is also worth noting that the precise locus of the RS effects even for these action types is likely to differ with a different sample. However, the majority of previous neuroimaging studies investigating action observation have, up until now, placed all activations in approximately motor locations into a similar functional category—they are taken to indicate the operation of mirror neurons. The present study suggests that this assumption is likely to be incorrect. Rather, we suggest that the results of this study are more consistent with the idea that mirror neurons in IFG and ventral BA6 in humans are confined to a posterior region, as in the macaque monkey, and that modulation in the BOLD signal in other areas of the IFG is driven by functionally distinct processes. This hypothesis is consistent with the fact that the IFG in humans has been shown to contain cytoarchitecturally distinct regions (e.g. Amunts et al., 1999; Amunts et al., 2010).
In more anterior bilateral BA44 areas, unimodal RS effects were observed but not crossmodal effects (RE effects were observed here). This finding is consistent with the presence of many neurons within the IFG that respond when only observing or executing an action (Rizzolatti and Craighero, 2004), and may suggest that effects here when observing action reflect activation of perceptual representations of action, rather than ‘mirror’ representations, i.e., activated when observing and executing the same action. However, it has been reasoned that it is unlikely that there would be dissociable motor and perceptual populations in common locations which do not communicate (Rizzolatti and Fabbri-Destro, 2010).
Alternatively, the activations in these anterior regions may encode the semantic properties of action, as these effects are in a region of IFG that has previously been proposed to be involved in semantic selection (Thompson-Schill et al., 1999). This interpretation would be consistent with the fact that rather than simply observing no RS effects on crossmodal trials, we found RE effects. RE has been proposed to occur when “priming causes a new process to occur on the target that did not occur on the prime” (Henson, 2003). More specifically, it has been argued that RE effects reflect the acquisition of new semantic representations for unfamiliar stimuli (Henson, 2003). In this BA44 region the BOLD response has been shown to be greater when the repeat between the first and second presentations was accompanied with a task switch (Thompson-Schill et al., 1999), and is therefore consistent with the RE effects found here on crossmodal trials.
In bilateral BA47, RS effects were found on observe trials, but RE effects on execute trials. Of interest, the same pattern was also observed bilaterally in a region of middle temporal gyrus (MTG) corresponding to BA21/37. These four areas have previously been demonstrated to be involved in the retrieval of semantic knowledge (Badre and D’Esposito, 2009; Binder et al., 2009; Turken and Dronkers, 2011; Wagner et al., 2001). It has been found that activity in the temporal regions is greater for automatic retrieval whereas anterior ventral IFG is more active in conditions that require goal-directed access to semantic knowledge. It is possible that the RS effects occurred for repeated pairs in the present study when retrieving semantic representations which were strongly associated with the visual cue—i.e. when the subjects observed the action. In contrast, the RE effects may reflect the consolidation of weakly associated representations—i.e. the association between the executed action and the visual cue of the action, the arrow, which subjects would have learned for the first time in this experiment. If this were the case one would predict that activity in the anterior IFG would be greater when subjects simply executed the action compared to when they observed it, as has been observed previously when comparing the retrieval of weakly and strongly associated representations (Wagner et al., 2001). We found some evidence in support of this. The magnitude of the BOLD response was significantly greater in BA47 when subjects executed compared to when they observed the action (peak voxel [−52, 16, −6] and [48, 26, −2], t = 4.75 and 3.74, respectively; p<0.005 uncorrected).
These different patterns of activation throughout the IFG are consistent with the hypothesis that there is a dissociation between more ‘pragmatic’ representations of an action (the parameters that are relevant to the motor commands when executing and observing an action; therefore the ‘mirror’ representations), and the semantic representations of the same action (including the goal and intentions; de Vignemont and Haggard, 2008; Hickok and Hauser, 2010; Jeannerod, 1994; Kilner, 2011). Within this framework mirror neurons in the posterior IFG (area F5c in monkeys and BA44/BA6 in humans) would encode the pragmatic representations of observed actions and not the more abstract goals and intentions of the action. In contrast, more anterior regions in BA44 and BA47 may encode more abstract semantic representations of action. This dissociation of the more concrete pragmatic representations from the more abstract semantic representations of action along the rostro-caudal axis of the IFG is precisely what would be predicted by a recent model of the frontal lobe (Badre and D’Esposito, 2009; Kilner, 2011).
One consequence of this framework is that it would require that mirror neurons do not encode the semantic representations of the action associated with the abstract goals and intentions, but rather they encode the concrete pragmatic representations of the action. This is consistent with hierarchical models of action understanding where action can be understood at different level of abstraction (Kilner et al., 2007a, b; Kilner, 2011). Future work must establish the extent of communication between pragmatic and semantic pathways, to ascertain whether mirror neurons indeed play a role in understanding the goals and intentions underlying others’ actions, as has been proposed widely (see Hickok, 2009; Hickok and Hauser, 2010; Kilner, 2011).
Conclusions
The present study has found RS effects consistent with the existence of mirror neurons in humans in posterior IFG. More anterior areas of IFG display dissociable adaptation patterns, suggesting that caution should be exercised when proposing that activation simply when observing or executing action indicates the operation of mirror neurons. Effects in these more anterior regions of IFG are consistent with processing of semantic, rather than motoric, features of action.
Acknowledgments
JMK and NW were funded by the Wellcome Trust, UK. CP was funded by an Interdisciplinary Postdoctoral Fellowship awarded by the MRC and ESRC.
References
- Amunts K, Schleicher A, Bürgle U, Mohlberg H, Uylings HBM, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J. Comp. Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Lenzen M, Friederici AD, Schleicher A, Morosan P, Palomero-Gallagher N, Zilles K. Broca’s region: novel organizational principles and multiple receptor mapping. PLoS Biol. 2010;8(9) doi: 10.1371/journal.pbio.1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat. Rev. Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmalih A, Borra E, Contini M, Gerbella M, Rozzi S, Luppino G. Cortical connections of the visuomotor parietooccipital area V6Ad of the macaque monkey. J. Comp. Neurol. 2009;512:183–217. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grèzes J, Passingham RE, Haggard P. Action observation and acquired motor skills: An fMRI study with expert dancers. Cereb. Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F. Brain activity during observation of actions. Influence of action content and subject’s strategy. Brain. 1997;120:1763–1777. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. NeuroImage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- De Vignemont F, Haggard P. Action observation and execution: what is shared? Soc. Neurosci. 2008;3:421–433. doi: 10.1080/17470910802045109. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Hasson U, Rubin N, Heeger DJ. Brain areas selective for both observed and executed movements. J. Neurophysiol. 2007;98:1415–1427. doi: 10.1152/jn.00238.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Behrmann M, Heeger DJ. A mirror up to nature. Curr. Biol. 2008;18:R13–R18. doi: 10.1016/j.cub.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I. Human cortex: reflections of mirror neurons. Curr. Biol. 2008;18:R956–R959. doi: 10.1016/j.cub.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp. Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fogassi L, Fadiga L, Rizzolatti G. Action representation and the inferior parietal lobule. In: Prinz W, Hommel B, editors. Attention & Performance XIX. Common Mechanisms in Perception and Action. Oxford; Oxford UP: 2002. pp. 334–355. [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb. Cortex. 2009;19:1239–1255. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum. Brain Mapp. 2001;12:1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Goal representation in human anterior intraparietal sulcus. J. Neurosci. 2006;26:1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Prog. Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. J. Cogn. Neurosci. 2009;21:1229–1243. doi: 10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Hauser M. Mis)understanding mirror neurons. Curr. Biol. 2010;20:R593–R594. doi: 10.1016/j.cub.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M. The representing brain. Neural correlates of motor intention and imagery. Behav. Brain Sci. 1994;17:187–245. [Google Scholar]
- Kilner JM. More than one pathway to action understanding. Trends Cogn. Sci. 2011;15:352–357. doi: 10.1016/j.tics.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD. Predictive coding: an account of the mirror neuron system. Cogn. Process. 2007a;8:159–166. doi: 10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD. The mirror-neuron system: a Bayesian perspective. Neuroreport. 2007b;18:619–623. doi: 10.1097/WNR.0b013e3281139ed0. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Neal A, Weiskopf N, Friston KJ, Frith CD. Evidence of mirror neurons in human inferior frontal gyrus. J. Neurosci. 2009;29:10153–10159. doi: 10.1523/JNEUROSCI.2668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingnau A, Gesierich B, Caramazza A. Asymmetric fMRI adaptation reveals no evidence for mirror neurons in humans. Proc. Natl Acad. Sci. U. S. A. 2009;106:9925–9930. doi: 10.1073/pnas.0902262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R. Targeting the functional properties of cortical neurons using fMR-adaptation. Neuroimage. doi: 10.1016/j.neuroimage.2012.01.002. (in press) 10.1016/j.neuroimage.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Morin O, Grèzes J. What is “mirror” in the premotor cortex? A review. Neurophysiol. Clin. 2008;38:189–195. doi: 10.1016/j.neucli.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Luppino G, Vanduffel W, Rizzolatti G, Orban GA. Observing others: multiple action representation in the frontal lobe. Science. 2005;310:332–336. doi: 10.1126/science.1115593. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, Fazio F. Localisation of grasp representations in humans by PET: 1. Observation versus execution. Exp. Brain Res. 1996;111:246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M. Mirror neurons: from discovery to autism. Exp. Brain Res. 2010;200:223–237. doi: 10.1007/s00221-009-2002-3. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front. Syst. Neurosci. 2011;5 doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G. I know what you are doing. A neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]