Abstract
Previous research suggests that the nonpeptide oxytocin receptor (OTR) agonist WAY 267,464 may only partly mimic the effects of oxytocin in rodents. The present study further explored these differences and related them to OTR and vasopressin 1a receptor (V1aR) pharmacology and regional patterns of c-Fos expression. Binding data for WAY 267,464 and oxytocin were obtained by displacement binding assays on cellular membranes, while functional receptor data were generated by luciferase reporter assays. For behavioural testing, adolescent rats were tested in a social preference paradigm, the elevated plus-maze (EPM) and for locomotor activity changes following WAY 267,464 (10 and 100 mg/kg, i.p.) or oxytocin (0.1 and 1 mg/kg, i.p.). The higher doses were also examined for their effects on regional c-Fos expression. Results showed that WAY 267,464 had higher affinity (Ki) at the V1aR than the OTR (113 versus 978 nm). However, it had no functional response at the V1aR and only a weak functional effect (EC50) at the OTR (881 nm). This suggests WAY 267,464 is an OTR agonist with weak affinity and a possible V1aR antagonist. Oxytocin showed high binding at the OTR (1.0 nm) and V1aR (503 nm), with a functional EC50 of 9.0 and 59.7 nm, respectively, indicating it is a potent OTR agonist and full V1aR agonist. WAY 267,464 (100 mg/kg), but not oxytocin, significantly increased the proportion of time spent with a live rat, over a dummy rat, in the social preference test. Neither compound affected EPM behaviour, whereas the higher doses of WAY 267,464 and oxytocin suppressed locomotor activity. WAY 267,464 and oxytocin produced similar c-Fos expression in the paraventricular hypothalamic nucleus, central amygdala, lateral parabrachial nucleus and nucleus of the solitary tract, suggesting a commonality of action at the OTR with the differential doses employed. However, WAY 267,464 caused greater c-Fos expression in the medial amygdala and the supraoptic nucleus than oxytocin, and lesser effects in the locus coeruleus. Overall, our results confirm the differential effects of WAY 267,464 and oxytocin and suggest that this may reflect contrasting actions of WAY 267,464 and oxytocin at the V1aR. Antagonism of the V1aR by WAY 267,464 could underlie some of the prosocial effects of this drug either through a direct action or through disinhibition of oxytocin circuitry that is subject to vasopressin inhibitory influences.
Keywords: oxytocin, vasopressin, adolescent, social behaviour, anxiety
There has been intensified recent interest in actions of the neuropeptide oxytocin on the central nervous system (CNS), particularly with respect to regulation of affiliative behaviours in mammalian species (1). Mice engineered to be deficient in oxytocin or the oxytocin receptor (OTR) display impaired social and maternal behaviour (2,3), whereas low levels of plasma oxytocin have been observed in humans with autism and depression (4,5). Exogenous delivery of synthetic oxytocin can have anxiolytic-like effects in rodents (6–10) and increase social interaction (11,12) and social preference (13). A range of mostly subtle effects of oxytocin on social cognition and social behaviour are evident in humans (14–16).
Clinical trials are now examining the use of oxytocin as an agent for reducing anxiety and increasing social behaviour in disorders such as autism, schizophrenia and social phobia (17). Although these trials provide some preliminary evidence of short-term improvements in social function after intranasal or intravenous oxytocin (18–20), the viability of oxytocin itself as a clinical treatment has been questioned given its lack of oral bioavailability, extremely short half-life and poor blood–brain barrier (BBB) penetration (21). Additionally, oxytocin has relatively low selectivity for the OTR over vasopressin receptor subtypes (22), which is problematic given that vasopressin receptor-mediated effects can be opposite to those of oxytocin (23).
In response to this, drug discovery programmes have developed novel nonpeptide OTR agonists that exhibit high affinity for the OTR and efficient BBB permeability (24). To date, there has been limited in vivo characterisation of these nonpeptide OTR agonists. A notable recent study (25) reported on the effects of WAY 267,464, a first generation nonpeptide OTR agonist. WAY 267,464 had anxiolytic-like effects in mice, similar to those seen with oxytocin, although it failed to exhibit the antidepressant-like properties of oxytocin in the forced swim test. Few effects of WAY 267,464 were evident in rats, apart from a weak antipsychotic-like action when administered at 30 mg/kg in the prepulse inhibition of the acoustic startle paradigm. Given the affinity of oxytocin, and its metabolites (26), for vasopressin receptors, it is possible that differential effects in behavioural models might arise from a distinct profile of oxytocin and synthetic OTR agonists at vasopressin receptors (25). For example, recent research shows that modulation of the central vasopressin system through administration of the selective vasopressin 1a receptor (V1aR) antagonists, SRX251 and JNJ-17308616, reduces aggression (27) and anxiety-related behaviour (28), respectively, in rodents.
This has important implications for further testing of novel OTR ligands, which would typically be compared with oxytocin itself. Therefore, in the present study, we further compared oxytocin and WAY 267,464 with respect to receptor pharmacology, as well as behavioural and neural effects. The ability of oxytocin and WAY 267,464 to displace tritiated oxytocin from OTR and tritiated vasopressin from V1aR cell membrane preparations was determined and the functional response of both compounds at the OTR and V1aR was also examined using nuclear factor of activated T-cell (NFAT)-coupled luciferase reporter assays. This allowed us to compare and contrast these basic pharmacological properties of WAY 267,464 and oxytocin.
With in vivo testing, we examined the ability of WAY 267,464 and oxytocin to modulate social behaviour in rodents using a social preference test. This was deemed important given that prosocial effects are a primary endpoint of interest in human clinical trials involving oxytocin (29–31). We also characterised the effects of the two compounds on anxiety-like behaviour on the elevated plus-maze (EPM) given previous reports of such effects with oxytocin (7,32,33). We also examined the capacity of the two compounds to suppress locomotor activity, given previous reports of sedation following high peripheral and central oxytocin doses (34–36). Because the previous characterisation of WAY 267,464 found few behavioural effects in rats at a maximal dose of 30 mg/kg (25), in the present study, we examined a high dose of WAY 267,464 (100 mg/kg) in rats, as well as a lower dose (10 mg/kg).
The technique of c-Fos immunohistochemistry provides a powerful tool for assessing the similarities and differences in neuronal effects produced by CNS-acting drugs (37). We therefore compared the pattern of c-Fos expression produced by oxytocin and WAY 267,464 using this technique. It was reasoned that, if WAY 267,464 and oxytocin have differential effects on oxytocin and vasopressin circuitry, then this should be readily apparent in the comparative distribution of c-Fos expression produced by each compound. This might in turn be linked back to differential receptor binding and behavioural profiles. We recently reported a strong c-Fos response in oxytocin-synthesising neurones in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) of the hypothalamus following peripheral administration of oxytocin, as well as c-Fos expression in a distinctive set of other limbic and midbrain structures (34).
Materials and methods
Drugs and drug preparation
WAY 267,464 (4-(3,5-dihydroxybenzyl)-N-(2-methyl-4-(1-methyl-1,4,5,10-tetrahydrobenzo [β]pyrrolo[2,3-e][1,4]diazepine-5-carbonyl)benzyl)piperazine-1-carboxamide) was synthesised in accordance with the methods of Hudson et al. (38). Based on proton, carbon nuclear magnetic resonance spectroscopy and mass spectrometry, WAY 267,464 was considered to be of > 95% purity (39). For in vivo tests, WAY 267,464 was dissolved in 15% dimethyl sulphoxide (DMSO), 2% Tween-80 and 83% physiological saline at concentrations of 2.5 and 25 mg/ml and injected into rats (i.p.) at a volume of 4 ml/kg. Oxytocin peptide was purchased from AusPep Ltd (Parkville, Victoria, Australia) and dissolved to concentrations of 0.025 and 0.25 mg/ml using the same vehicle as WAY 267,464. For binding studies, WAY 267,464 was dissolved in 15% DMSO, whereas oxytocin/vasopressin were dissolved in double distilled water (ddH2O). The oxytocin and vasopressin peptides were provided by Muttenthaler et al. (40).
For behavioural testing, a relatively high maximal dose of oxytocin (1 mg/kg) was selected as a result of previous reports showing that it suppresses locomotor activity (35,36) and produces prosocial effects following chronic but not acute administration (12). This high dose of peripheral oxytocin was also chosen given our previous report of significant behavioural effects of this dose in inhibiting methamphetamine self-administration in rats (41). A maximal dose of WAY 267,464 (100 mg/kg) was chosen based on evidence that 30 mg/kg has only a weak behavioural action in rats (25). For both compounds, one additional dose (i.e. one tenth of the maximal dose) was also tested.
Cell culture and binding experiments
Cloning, cell culture, transfection and membrane preparation
OTR and V1aR cDNA sequences were inserted into the pEGFP-N1 plasmids (Clontech, Saint-Germain-en-Laye, France) using SacI and HindIII restriction sites to yield a C-terminal green fluorescent protein (GFP) fusion protein. The conditions for the propagation of HEK293 cells, creation of stably transfected OTR and V1aR cell lines were similar to those described previously (42). Briefly, for CaPO4 transfection, 1.8 × 106 of HEK293 cells per 10 cm dish were prepared. Twenty microlitres DNA, with a concentration of 1 μg/μl, was mixed with 480 μl H2O + CaCl2 (430 μl H2O + 50 μl CaCl2) and added to 500 μl of HEPES-buffered saline buffer. After 6 min, the solution was added to the Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, with L-glutamine and gentamicin (PAA, Pasching, Austria), and the cells were incubated for 4 h at 37 °C. Following glycerol-shock treatment, DMEM was added again and the cells were incubated at 37 °C. Selection of receptor positive clones was achieved by treatment with the antibiotic geneticin (G418 - BC liquid, 50 mg/ml; Biochrom, Berlin, Germany). Cells were harvested and membranes were prepared as described previously (42).
Binding displacement assays
Membranes (30–50 μg/assay) from HEK293 cells stably expressing the OTR or V1aR were incubated in a final volume of 200 μl containing 50 mM Tris–HCl, 5 mM MgCl2, 0.1% bovine serum albumin, pH 7.8 and [3H]-oxytocin (2 nm) or [3H]-vasopressin (0.75 nm) ([tyrosyl-2,6-3H] oxytocin; 47.4 Ci/mmol and [phenylalanyl-3,4,5-3H]-8-arginine vasopressin; 61.2 Ci/mmol were from Perkin-Elmer Life Sciences, Boston, MA, USA). Competitive displacement of radioactive ligand by WAY 267,464 or oxytocin was performed in the presence of logarithmically-spaced concentrations of competing compound. After 60 min at 25 °C, the reaction was terminated by rapid filtration over glass fibre filters (Whatman GF/A 1.6 μm); Whatman International Ltd, Maidstone, UK). Nonspecific binding was determined in the presence of 1 μm oxytocin or vasopressin, respectively. Specific binding represents the difference between total and nonspecific binding and is presented as normalised data. IC50 values were obtained by nonlinear regression fit (sigmoidal, variable slope) using GRAPHPAD PRISM, version 5.0 (GraphPad Software Inc., San Diego, CA, USA) of three independent experiments (each performed in duplicate). Ki values were determined according to Cheng and Prusoff (43) using the Kd values: oxytocin on OTR = 1.5 nm and vasopressin on V1aR = 0.6 nm.
Functional luciferase reporter assays
HEK293 cells stably expressing OTR-GFP and V1aR-GFP were co-transfected with firefly luciferase containing plasmid pGL4.29 luc2P (Promega GmbH, Mannheim, Germany) expressing the downstream DNA binding elements of G protein-coupled receptor activation (i.e. NFAT for Gq-coupled signalling). Using CaPO4 transfection as described above, 6 × 106 of OTR and V1aR cells per 15 cm dish were prepared. After 6 h of transfection at 37 °C, cells were seeded into 96-well plates (5 × 104 cells/well) and incubated with logarithmically-spaced concentrations (range 0–100 μm) of oxytocin and WAY 267,464 in white DMEM supplemented with 10% fetal calf serum (Gibco, Gaithersburg, MD, USA) and antibiotics. Following 18 h of incubation at 37 °C, medium was removed and cells were stored for 12–18 h at −80 °C and then lysed using the luciferase cell culture lysis reagent (Promega GmbH). The fluorescence intensity as well as the luciferase activity was measured using luciferase assay reagent (Promega GmbH) in accordance with the manufacturer’s recommendation on a Victor3 V multilabel plate reader (Perkin-Elmer Life Sciences). The measured luciferase counts were corrected using the fluorescent intensity for the number of cells per well and normalised to percentage of luciferase activity above baseline. Half-maximal effective concentrations (EC50) were calculated using GRAPHPAD PRISM, version 5.0, using sigmoidal nonlinear regression analysis with variable slope of three to four independent experiments (each performed in triplicate). Native vasopressin was used as the control peptide for the V1aR.
Animals
A total of 70 experimentally naive male Albino Wistar rats were used (Animal Resources Centre, Perth, Australia): 22 rats [Postnatal day (PND) 25; 53–85 g] and 24 rats (PND 30; 102–142 g) were used in Experiment 1A (WAY 267,464 behavioural effects) and 1B (oxytocin behavioural effects), respectively, whereas 24 rats (PND 28; 85–114 g) were used in Experiment 2 (c-Fos immunohistochemistry). Young adolescent rats were used as a result of their small body size which maximised in vivo use of our limited supplies of the test compound.
The rats were housed in groups of eight in large plastic tubs (640 × 400 × 220 mm) in a temperature-controlled colony room (21 ± °C) maintained under a reverse 12 : 12 h light/dark cycle (lights on 21.00 h) and were given ad libitum access to food and water, except during testing. All experiments were performed during the dark phase of the rats.
Experimental procedures were conducted under the approval of the University of Sydney Animal Ethics Committee in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (2004).
Behavioural testing
Social preference test
The paradigm used was adapted from that described by Berton et al. (44). The apparatus consisted of a black walled arena (1200 × 1200 × 910 mm) with one short wall 610 mm high on the north side, and a metal mesh floor mounted on a wooden frame (1170 × 1170 × 70 mm). Two aluminium cages (298 × 194 × 406 mm) were placed at either side of the arena on the east and west walls. A novel rat (adolescent male Albino Wistar rat) that the test rat had not previously met was placed in one of the cages, whereas the opposite cage contained a fluffy dummy rat of similar size and appearance. The side of the live and dummy rat was counterbalanced across conditions. The test was conducted in the dark with a 40-W red light used for illumination. Images sent to a computer outside the room enabled video tracking software (TRACKMATE, version 1.0; MotMen Pty Ltd Cooks Hill, NSW, Australia) to automatically measure the total amount of time spent with the live and dummy rat (s) and travelled distance (m). The proportion of time spent interacting with the live rat versus the dummy rat was manually calculated ([total time spent with the live rat/(total time spent with the live rat + total time spent with the dummy rat)] × 100%). The rat was placed into the arena on the north side, and social interaction was scored when the rat was within 150 mm of the cage. The arena and cages were cleaned with a 30% ethanol solution between each 30-min trial.
EPM test
The apparatus comprised two open and closed black plywood arms (450 × 100 mm) arranged in a cross and connected by a central square (100 × 100 mm). The closed arms had 400 mm high red Perspex walls and the entire apparatus was raised to a height of 700 mm. The test was performed in a dark room with a 40-W red light used for illumination. The rat was placed in the centre square with its head facing one of the closed arms at the start of each 5-min trial. Live images were relayed to a computer in the opposite room where automated video tracking software (TRACKMATE, version 1.0; MotMen Pty Ltd, Cooks Hill, NSW, Australia) recorded open and closed arm times (s), the number of open and closed arm entries and risk assessment. Risk assessment was defined as all four paws of the rat on the closed arm with only its head protruding around the walls or down the open arms. The entire maze was cleaned with a 30% ethanol solution between trials. The experimental procedure followed in this study (45) has been used previously by our group.
Locomotor activity test
Locomotor activity was measured in individual rats in large black plastic tubs (770 × 510 × 465 mm) with a floor of grey absorbent paper pellets. A dark room illuminated with a 40-W red light was used for testing. Above each tub was an infrared sensitive camera that sent a live video feed to a computer where video tracking software (TRACKMATE, version 1.0) automatically measured the distance travelled (m) by the rat over the 60-min trial.
c-Fos immunohistochemistry
Immunohistochemistry
Rats were anaesthetised with sodium pentobarbital (120 mg/kg, i.p.) and perfused transcardially with 200 ml of 0.1 m phosphate-buffered saline (PBS; pH 7.3), followed by 200 ml of cold 4% paraformaldehyde in PBS. The perfusion was performed at a flow rate of 48 ml/min using a Gilson perfusion pump (Gilson Inc., Middleton, WI, USA).
The brains were then removed, blocked in the coronal plane immediately caudal to the olfactory bulbs (approximately Bregma +5.0) and midway through the cerebellum (approximately Bregma −12.0) and post-fixed in 4% paraformaldehyde buffered with PBS overnight at 4 °C. They were then transferred into 15% phosphate-buffered (PB) sucrose for 24 h at 4 °C, followed by 30% sucrose in PB for 48 h. The tissue blocks were placed on microtome stages, frozen to −17 °C, and coronally sectioned at 40 μm. Consecutive sections were placed sequentially into four vials containing 0.1 m PB. One vial was used for c-Fos staining, whereas the remaining vials were transferred to freezing solution (45% 0.1 m PB, 30% ethylene glycol, 25% glycerol) and stored at −17 °C for later use if required.
Free-floating sections were incubated for 30 min in 1% hydrogen peroxide in PB, and then for 30 min in 3% normal horse serum in PB at room temperature. They were then incubated in glass vials with 3 ml of primary c-Fos antibody for 72 h at 4 °C (rabbit polyclonal antisera sc-52 reacts with c-Fos p62 of mouse, rat and human; noncross reactive with Fos B, Fra-1 or Fra-2; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The primary antibody was diluted 1 : 2000 in PB horse serum (PBH: 0.1% bovine serum albumin, 0.2% Triton X-100, 2% normal horse serum in PB). Sections were then washed in PB for 30 min, followed by a 1-h incubation period at room temperature in secondary antibody (biotinylated anti-rabbit IgG made in goat; diluted 1 : 500 in PBH; Vector Laboratories, Burlingame, CA, USA). Sections were washed again for 30 min in PB, and incubated for 1.5 h in ExtrAvidin-horseradish peroxidase (diluted 1 : 1000 in PBH; Sigma, St Louis, MO, USA). Subsequent to three consecutive washes (30 min each) in PB, peroxidase activity was visualised with nickel diaminobenzidine and glucose oxidase reaction as described previously (46). This reaction was terminated after 10 min by washing in PB. Sections were then mounted on subbed slides, dehydrated in ascending concentrations of ethanol, xylene cleared and cover slipped.
One of the vials placed in freezing solution was used to visualise c-Fos expression in oxytocin-expressing cells. The tissue was washed three times in PB (30 min each) before staining for c-Fos using the identical procedure described above. This was followed by a second sequence in which oxytocin (Chemicon, Boronia, Victoria, Australia: anti-oxytocin polyclonal antibody diluted 1 : 3000; catalogue number AB911) was added instead of c-Fos as the primary antibody, and nickel ammonium chloride was not included in the visualisation process (47).
Quantification of c-Fos and oxytocin-labelled immunoreactive cells
The method described produces a black oval-shaped immunoprecipitate confined to the cell nucleus of c-Fos immunoreactive (IR) cells. This was manually quantified microscopically by an observer blind to group assignment with a brain atlas used for guidance (48). Sections were viewed under either a × 20 or × 40 objective. Only darkly-labelled round or oval nuclei that fell within an optical graticule were classified as c-Fos IR. The number of c-Fos IR nuclei that fell within a 0.5 × 0.5 mm (20× objective) or 0.25 × 0.25 mm area (× 40 objective) in each region of interest was counted from one section per rat as previously described (49). A schematic of the 37 regions quantified is shown in Fig. 1.
Fig. 1.
Schematic showing the 37 brain regions in which c-Fos immunoreactive (IR) cells were quantified, as adapted from the atlas of Paxinos and Watson (48). Open squares indicate the approximate positions of the grid within which c-Fos counts were made.
In a number of cases, the designated area to be counted was considerably larger than the boundaries of the graticule. Here, the graticule was placed in a fixed position within the region of interest relative to known anatomical landmarks and only c-Fos IR cells within the area of the graticule were counted. In cases where the designated area was smaller than the boundaries of the graticule, only the region of interest was counted.
A similar method was used to quantify: (i) c-Fos-expressing cells; (ii) oxytocin-expressing cells; and (iii) cells expressing both c-Fos and oxytocin (double-labelled) within the SON and PVN. For each rat, bilateral PVN counts were made at two levels of Bregma (−1.40 and −1.80 mm) using a × 20 objective, whereas bilateral counts for the SON were made at −1.30 and −1.40 mm relative to Bregma using a × 40 objective (47). The higher magnification was used for counting the SON because this region was too small to fill the graticule at lower magnifications. Counts were then summed over the four regions and averaged to give a final count for single and double-labelled cells within the SON and PVN. The data were also expressed as a percentage of double-labelled cells based on the number of oxytocin-expressing cells in each nucleus.
Preparation of images
To illustrate the distribution of c-Fos and oxytocin-labelled IR cells in key areas, digital images were made of representative pieces of tissue. The digitised images were produced with an Olympus DP70 12.5 mega pixel camera system (Olympus, Tokyo, Japan) attached to an Olympus Optical BX51 light microscope. Images were acquired with a desktop computer using custom software supplied with the camera system. All images were directly imported into ADOBE PHOTOSHOP CS (Adobe Systems, San Jose, CA, USA) and reduced in size. For images of c-Fos IR, the only post-production enhancements were uniform conversion to black and white, increasing contrast (+20%) and whitening of the non-tissue surfaces using the eraser tool.
Experimental procedures
Experiments 1A and 1B
Rats were randomly allocated to three treatment groups in Experiment 1A: vehicle (n = 9), 10 mg/kg WAY 267,464 (n = 6) and 100 mg/kg WAY 267,464 (n = 7). The three treatment conditions in Experiment 1B included vehicle (n = 8), 0.1 mg/kg oxytocin (n = 8) and 1 mg/kg oxytocin (n = 8). Rats were injected with their allocated treatment and then individually placed in cardboard boxes with straw bedding for 20 min. From 20–50 min post-injection, they were tested in the social preference test and then immediately placed on the EPM for 5 min (50–55 min post-injection). They were then placed in the locomotor activity test chambers for 60 min (55– 115 min post-injection).
Experiment 2
Rats were given three consecutive days of 5 min handling. Over the following two consecutive days, rats were habituated twice to the test procedure. Each rat was given a dummy injection (i.p.) of the vehicle and placed in groups of four into the locomotor activity chambers for 60 min to familiarise them with the apparatus. Cardboard boxes with straw bedding were used to house the rats immediately after injection to allow the effects of WAY 267,464 and oxytocin to emerge. These boxes also enabled easy transportation of rats to the adjacent test room and ensured rats remained isolated before being tested. The extensive habituation rats received to the test procedure and activity chambers aimed to minimise c-Fos expression as a result of novelty or the stress of handling and injections, and reduce any unwarranted stress that might obscure treatment effects.
On the test day, rats were randomly allocated to one of three treatment conditions: vehicle (n = 8), 1 mg/kg oxytocin (n = 8) and 100 mg/kg WAY 267,464 (n = 8). Rats were injected with their allocated treatment and then individually placed in the cardboard boxes for 20 min. They were then placed in the locomotor activity chambers for 60 min (20–80 min post-injection) and activity recorded. At the end of this test, rats were individually perfused in a room far removed from where the other rats were housed or tested.
Statistical analysis
Behavioural data in Experiments 1A and 1B were analysed using one-way ANOVA followed by multiple a priori contrasts with the Bonferroni correction used to control the Type I error rate. In Experiment 2, treatment by time (12 × 5 min bins) effects on locomotor activity were examined using a mixed-design ANOVA, whereas group differences on c-Fos and oxytocin-expressing cell counts were determined with one-way ANOVA. Significant ANOVAs were followed by post-hoc tests (Tukey’s) to enable specific group comparisons. Homogeneity of variance was determined with Levene’s Test. When this requirement was not satisfied, a log10(x + 1) and square-root transformation was applied to the data and the most effective correction for heterogeneity of variance was used for analysis. All analyses were performed using SPSS, version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Pharmacology of oxytocin and WAY 267,464 at the OTR and V1aR
WAY 267,464 and oxytocin were tested for their ability to displace tritiated oxytocin/vasopressin from the OTR and V1aR in isolated cell membrane preparations (stably expressed in HEK293 cells). The measured IC50 and resulting Ki values of oxytocin were 1.0 nm for the OTR and 503 nm for the V1aR. WAY 267,464 displayed Ki values of 978 nm (OTR) and 113 nm (V1aR). In addition to binding experiments, we analysed the ability of WAY 267,464 and oxytocin to activate the G-protein response in recombinant HEK293 cells stably expressing the OTR and V1aR. Functional response of the receptors were measured with luciferase reporter assays, based on the downstream activation, following agonist treatment, of the NFAT response element that has been additionally expressed in those cells as a fusion to the luciferase gene (for details, see Materials and methods). For the OTR, oxytocin had a functional EC50 of 9.0 nm and WAY 267,464 showed weaker activity, with an observed EC50 of 881 nm. Oxytocin generated a functional response at the V1aR with an EC50 of 59.7 nm, whereas WAY 267,464 did not activate the receptor at concentrations as high as 100 μm. The EC50 of a vasopressin control at the V1aR was measured to be 13.5 nm (data not shown). All binding and functional data are presented in Table 1 and corresponding graphs (nonlinear regression sigmoidal fit, variable slope) are shown in Fig. 2.
Table 1.
Pharmacology of Oxytocin and WAY 267,464 at the Oxytocin (OTR) and Vasopressin 1a Receptor (V1aR).
| OTR |
V1aR |
|||
|---|---|---|---|---|
| Compound | Binding Ki (nm)a | Functional EC50 (nm) | Binding Ki (nm)a | Functional EC50 (nm) |
| Oxytocin | 1.0 ± 0.1 | 9.0 ± 0.7 | 503 ± 133b | 59.7 ± 17 |
| WAY 267,464 | 978 ± 71 | 881 ± 383 | 113 ± 32 | > 100 000 |
≥ 3 (except where otherwise noted); data are presented as the mean ± SEM;
Ki values were determined using IC50 values according to Cheng and Prusoff (43) using the following Kd values: oxytocin on OTR = 1.5 nm and vasopressin on V1aR = 0.6 nm;
n = 2.
Fig. 2.
Binding and functional experiments of WAY 267,464 and oxytocin on the oxytocin (OTR) and vasopressin 1a receptor (V1aR). Binding data were obtained by competitive radioligand binding experiments, measuring the displacement of [3H]-oxytocin or [3H]-vasopressin, respectively, by the compounds WAY 267,464 (open squares and dashed lines) and oxytocin (solid squares and lines) from the human OTR (a) and V1aR (b) on isolated cell membranes. The ability of the compounds to signal through Gq and activate the downstream nuclear factor of activated T-cells (NFAT) response element in HEK293 cells stably transfected with the human OTR (c) and V1aR (d) was measured with luciferase reporter gene assays. The NFAT element was fused to the firefly luciferase gene and receptor activation was directly proportional to the emitted light response. Data were fitted with nonlinear regression (sigmoidal, variable slope) and are shown as the mean ± SEM of three to four independent experiments. Binding data are normalised to percentage (%) of specific binding and functional data are normalised to the number of cells and percentage (%) of activation above baseline. The corresponding Ki and EC50 values of each compound are listed in Table 1.
Social preference test
WAY 267,464 had a significant effect on the total amount of time spent with the live rat in Experiment 1A (F2,19 = 7.80, P < 0.01; Fig. 3a). Planned contrasts showed that rats treated with 100 mg/kg WAY 267,464 spent significantly longer interacting with the live rat than those who received vehicle (F1,19 = 14.26, P < 0.001) and 10 mg/kg WAY 267,464 (F1,19 = 8.53, P < 0.01). WAY 267,464 also produced a significant effect on the proportion of time spent interacting with the live rat (F2,19 = 7.92, P < 0.01; Fig. 3c), with a priori contrasts indicating that rats given 100 mg/kg WAY 267,464 spent a significantly greater proportion of time with the live rat compared to vehicle- (F1,19 = 8.32, P < 0.01) and 10 mg/kg WAY 267,464-treated rats (F1,19 = 14.66, P < 0.001). WAY 267,464 had no significant overall effect on distance travelled during the social preference test in Experiment 1A (P > 0.05; Fig. 3e).
Fig. 3.
The behaviours measured during the social preference test including the total amount of time (s) (a, b) and the proportion of time (%) (c, d) spent with the live rat, and distance travelled (m) (e, f). WAY 267,464 given at 100 mg/kg (n = 7) increased the total amount of time and the proportion of time spent interacting with the live rat relative to a dose of 10 mg/kg (n = 6) and the vehicle (n = 9) in Experiment 1A. Oxytocin at 1 mg/kg (n = 8) reduced distance travelled relative to the vehicle (n = 8), but not 0.1 mg/kg oxytocin (n = 8), in Experiment 1B. **P < 0.01, ***P < 0.001. Data are represented as the mean + SEM. VEH, vehicle.
There was no significant overall effect of oxytocin on the total amount of time (P > 0.05; Fig. 3b) and the proportion of time (P > 0.05; Fig. 3d) spent with the live rat in Experiment 1B. Oxytocin had a significant effect on locomotor activity in the social preference test (F2,21 = 5.12, P < 0.05; Fig. 3f), with contrasts indicating that rats administered 1 mg/kg oxytocin travelled less distance than vehicle-treated rats (F1,21 = 9.37, P < 0.01).
EPM test
There was no significant effect of WAY 267,464 in Experiment 1A on any scored behaviour on the EPM (P > 0.05; Table 2). Similarly, oxytocin did not significantly modify the majority of EPM behaviours in Experiment 1B (P > 0.05; Table 3). However, rats treated with 1 mg/kg oxytocin tended to spend less time engaged in risk assessment (F1,21 = 6.069, P = 0.022), as well as engage in fewer entries onto the arms of the maze (F1,21 = 5.091, P = 0.035), compared to rats given the lower oxytocin dose of 0.1 mg/kg.
Table 2.
Results of the Elevated Plus-Maze Test and Locomotor Activity Test in Experiment 1A.
| Experiment 1A | Vehicle (n = 9) |
10 mg / kg WAY 267,464 (n = 6) |
100 mg / kg WAY 267,464 (n = 7) |
|---|---|---|---|
| Elevated plus-maze | |||
| Open arm time (%) | 16.6 ± 2.2 | 14.6 ± 2.4 | 13.7 ± 1.5 |
| Open arm entries (n) | 11.4 ± 1.1 | 7.8 ± 1.5 | 8.4 ± 1.2 |
| Total arm entries (n) | 17.2 ± 1.7 | 13.0 ± 2.4 | 13.1 ± 1.5 |
| Risk assessment (s) | 21.2 ± 2.2 | 35.2 ± 10.8 | 21.0 ± 1.9 |
| Locomotor activity test | |||
| Distance travelled (m) | 35.6 ± 5.2 | 29.2 ± 4.3 | 15.2 ± 1.7a,b |
Significantly different from vehicle;
Significantly different from 10 mg / kg WAY 267,464. Data are represented as the mean ± SEM.
Table 3.
Results of the Elevated Plus-Maze Test and Locomotor Activity Test in Experiment 1B.
| Experiment 1B | Vehicle (n = 8) |
0.1 mg / kg Oxytocin (n = 8) |
1 mg/ kg Oxytocin (n = 8) |
|---|---|---|---|
| Elevated plus-maze | |||
| Open arm time (%) | 18.1 ± 5.7 | 21.3 ± 4.9 | 21.4 ± 11.1 |
| Open arm entries (n) | 9.1 ± 2.5 | 10.6 ± 1.6 | 4.5 ± 0.6 |
| Total arm entries (n) | 14.5 ± 3.4 | 16.5 ± 2.9 | 8.0 ± 1.1 |
| Risk assessment (s) | 22.8 ± 8.3 | 24.3 ± 4.6 | 5.1 ± 1.6 |
| Locomotor activity test | |||
| Distance travelled (m) | 24.2 ± 4.8 | 27.0 ± 3.5 | 20.1 ± 3.3 |
Data are represented as the mean ± SEM.
Locomotor activity test
Experiments 1A and 1B
Measured at 55–115 min post-injection, WAY 267,464 produced a significant effect on locomotor activity in Experiment 1A (F2,19 = 8.56, P < 0.01; Table 2). Planned comparisons revealed that rats given 100 mg/kg WAY 267,464 travelled significantly less distance than those treated with vehicle (F1,19 = 16.22, P < 0.001) and 10 mg/kg WAY 267,464 (F1,19 = 8.25, P < 0.01). There was no significant overall effect of oxytocin in the locomotor activity test in Experiment 1B (P > 0.05; Table 3).
Experiment 2
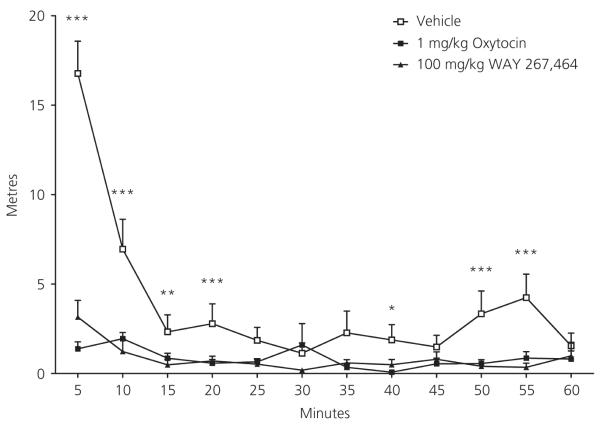
Before perfusion in the c-Fos experiment, locomotor activity in the rats (measured 20–80 min post-injection) was significantly affected by drug treatment (F2,21 = 20.74, P < 0.001), time (F11,231 = 20.96, P < 0.001) and their interaction (F22,231 = 11.23, P < 0.001; Fig. 4). Further comparisons indicated that rats given 1 mg/kg oxytocin (F1,21 = 30.86, P < 0.001), or 100 mg/kg WAY 267,464 (F1,21 = 31.35, P < 0.001), travelled significantly less distance than vehicle-treated rats. These treatment effects were qualified by a significant linear (F2,21 = 13.57, P < 0.001) and quadratic (F2,21 = 57.33, P < 0.001) interaction trend. Vehicle-treated rats travelled a greater distance than those given WAY 267,464 or oxytocin particularly during the first 20 min of the test.
Fig. 4.
Distance travelled (m) over the duration of the 60-min locomotor activity test for vehicle (n = 8), 1 mg/kg oxytocin (n = 8) and 100 mg/kg WAY 267,464 (n = 8) groups. *Vehicle significantly different from 1 mg/kg oxytocin; **Vehicle significantly different from 100 mg/kg WAY 267,464; ***Vehicle significantly different from 1 mg/kg oxytocin and 100 mg/kg WAY 267,464. Data are shown as the mean + SEM.
c-Fos immunoreactivity
Significant overall differences in c-Fos expression were seen in 12 brain regions (Table 4). WAY 267,464 and oxytocin both increased the number of c-Fos IR cells in the PVN (both P < 0.001), central amygdala (CEA) (Fig. 5a–c) (both P < 0.001), lateral parabrachial nucleus (LBN) (both P < 0.001) and nucleus of the solitary tract (NTS) (Fig. 6a–c) (both P < 0.001) relative to the vehicle control.
Table 4.
Mean ± SEM number of c-Fos Immunoreactive Cells in the Regions with Significant Differences between the Vehicle (n = 8), 1 mg / kg Oxytocin (n = 8) and 100 mg / kg WAY 267,464 (n = 8) Conditions.
| Region (Fig. 1) | Bregma | Vehicle | 1 mg/ kg Oxytocin |
100 mg / kg WAY 267,464 |
|---|---|---|---|---|
| Areas with significant WAY 267,464- and oxytocin-induced c-Fos expression above vehicle |
||||
| 18. Paraventricular hypothalamic nucleus | −1.80 | 8.7 ± 2.7 | 72.4 ± 13.5a | 102.4 ± 17.2a |
| 20. Central amygdala | −2.80 | 2.9 ± 0.7 | 25.5 ± 3.2a | 20.6 ± 3.3a |
| 35. Lateral parabrachial nucleus | −9.30 | 2.6 ± 1.3 | 18.0 ± 0.6a | 13.1 ± 2.0a |
| 37. Nucleus of the solitary tract | −13.68 | 1.5 ± 0.7 | 39.3 ± 5.3a | 32.6 ± 6.2a |
| Areas with significant WAY 267,464-induced c-Fos expression above oxytocin and / or vehicle |
||||
| 1. Medial prefrontal cortex / Prelimbic cortex | +2.70 | 9.5 ± 2.0 | 3.9 ± 1.4 | 12.0 ± 2.8b |
| 12. Median preoptic nucleus | −0.26 | 4.9 ± 1.0 | 8.4 ± 1.2 | 11.9 ± 2.2a |
| 16. Supraoptic nucleus (× 40) | −1.30 | 4.6 ± 2.6 | 11.0 ± 4.0 | 41.5 ± 9.3a,b |
| 27. Medial amygdala (posterodorsal) | −3.14 | 6.0 ± 1.5 | 6.0 ± 1.7 | 15.8 ± 1.7b |
| 34. Periaqueductal grey (ventrolateral) | −8.72 | 3.4 ± 1.0 | 6.9 ± 1.4 | 9.4 ± 1.8a |
| Areas with significant oxytocin-induced c-Fos expression above WAY 267,464 and / or vehicle |
||||
| 36. Locus coeruleus | −9.68 | 0.1 ± 0.1 | 5.9 ± 1.3a,c | 1.6 ± 0.8 |
| Areas with significant oxytocin-induced c-Fos expression below WAY 267,464 and / or vehicle |
||||
| 26. Lateral habenula | −3.14 | 6.5 ± 1.8 | 1.1 ± 0.6a,c | 6.3 ± 1.3 |
| Areas with significant oxytocin-induced c-Fos expression above vehicle | ||||
| 11. Bed nucleus of the stria terminalis (dorsolateral nucleus) (× 40) | −0.26 | 3.1 ± 1.0 | 10.8 ± 2.4a | 6.1 ± 1.1 |
Significantly different from vehicle;
Significantly different from oxytocin;
Significantly different from WAY 267,464. All counts were conducted at × 20 magnification, unless stated otherwise (× 40).
Fig. 5.
The distribution of c-Fos immunoreactive cells (black dots) in the central amygdala (CEA; a–c) and the posterodorsal region of the medial amygdala (MePD; d–f) from representative rats treated with vehicle (n = 8) (a, d), 1 mg/kg oxytocin (n = 8) (b, e) and 100 mg/kg WAY 267,464 (n = 8) (c, f). Scale bar = 200 μm (a–c) and 500 μm (d–f). opt, optic tract.
Fig. 6.
The distribution of c-Fos immunoreactive cells (black dots) in the nucleus of the solitary tract (NTS; a–c) and the locus coeruleus (LC; d–f) from representative rats treated with vehicle (n = 8) (a, d), 1 mg/kg oxytocin (n = 8) (b, e) and 100 mg/kg WAY 267,464 (n = 8) (c, f). Scale bar = 500 μm (a–c) and 200 μm (d–f). AP, area postrema; cc, central canal; Me5, mesencephalic trigeminal nucleus; 4V, fourth ventricle.
In comparison to vehicle-treated rats, those administered WAY 267,464, but not oxytocin, exhibited a greater c-Fos response in the median preoptic nucleus (MnPO) (P < 0.05), ventrolateral periaqu-eductal grey (VLPAG) (P < 0.05) and the SON (P < 0.001). Relative to the oxytocin group, WAY 267,464 also increased c-Fos expression in the posterodorsal medial amygdala (MePD) (Fig. 5d–f) (P < 0.05) and prelimbic cortex (PrL) (P < 0.05).
In comparison to vehicle-treated rats, those given oxytocin, but not WAY 267,464, had increased c-Fos expression in the laterodorsal part of the bed nucleus of the stria terminalis (BSTld) (P < 0.01). Oxytocin also uniquely increased c-Fos in the locus coeruleus (LC) (Fig. 6d–f) (versus vehicle: P < 0.001) and decreased the number of c-Fos positive neurones in the lateral habenula (LHb) (versus vehicle: P < 0.05).
c-Fos and oxytocin-labelled immunoreactive cells
The number of c-Fos and oxytocin-labelled IR cells in the SON and PVN are presented in Table 5. WAY 267,464 uniquely increased the number (P < 0.01 versus oxytocin and vehicle) and proportion (P < 0.01 versus oxytocin and vehicle) of double-labelled neurones in the SON (Fig. 7a–c). In the PVN (Fig. 7d–f), WAY 267,464 and oxytocin both increased the number of c-Fos-expressing neurones (WAY 267,464 versus vehicle: P < 0.001; oxytocin versus vehicle: P < 0.01) and double-labelled cells (WAY 267,464 versus vehicle: P < 0.001; oxytocin versus vehicle: P < 0.01). Moreover, rats treated with WAY 267,464 (versus oxytocin: P < 0.05; versus vehicle: P < 0.001) or oxytocin (versus vehicle: P < 0.01) showed a significant increase in the proportion of double-labelled neurones in the PVN.
Table 5.
Mean ± SEM number and proportion of c-Fos and oxytocin-labelled cells in the supraoptic nucleus (SON) and paraventricular hypothalamic nucleus (PVN).
| Region | Vehicle (n = 8) |
1 mg/ kg Oxytocin (n = 8) |
100 mg / kg WAY 267,464 (n = 8) |
|---|---|---|---|
| Supraoptic nucleus (× 40) | |||
| c-Fos only (n) | 1.3 ± 0.6 | 1.6 ± 1.0 | 4.1 ± 1.2 |
| c-Fos + oxytocin (n) | 8.8 ± 3.6 | 10.8 ± 4.7 | 36.7 ± 7.1a,b |
| Oxytocin-labelled cells (n) | 49.4 ± 4.2 | 45.4 ± 4.7 | 60.2 ± 4.4 |
| Percentage double-labelled (%) |
16.5 ± 6.4 | 20.8 ± 7.1 | 59.8 ± 10.8a,b |
| Paraventricular hypothalamic nucleus | |||
| c-Fos only (n) | 11.2 ± 3.4 | 50.7 ± 9.0a | 67.7 ± 9.9a |
| c-Fos + oxytocin (n) | 2.9 ± 1.0 | 21.2 ± 4.6a | 50.2 ± 8.4a |
| Oxytocin-labelled cells (n) | 55.6 ± 2.7 | 65.5 ± 6.6 | 70.4 ± 2.7 |
| Percentage double-labelled (%) |
5.4 ± 2.1 | 31.2 ± 6.4a | 70.0 ± 10.9a,b |
Significantly different from vehicle;
Significantly different from oxytocin.
All counts were conducted at × 20 magnification, unless stated otherwise (× 40).
Fig. 7.
Representative photomicrographs of the distribution of c-Fos (black dots) and oxytocin-labelled (amber immunoprecipitate) immunoreactive cells in the supraoptic nucleus (SON) (a–c) and paraventricular nucleus (PVN) (d–f) for vehicle (n = 8) (a,d), 1 mg/kg oxytocin (n = 8) (b, e) and 100 mg/kg WAY 267,464 (n = 8) (c, f). The single arrow in (c) shows an example of a c-Fos-expressing cell, whereas the double arrow shows an example of a double-labelled cell (expressing both c-Fos and oxytocin). These cells are shown at higher magnification in the insert (c). Scale bar = 100 μm (a–c) and 200 μm (d–f). opt, optic tract; 3V, third ventricle.
Discussion
The present study compared the nonpeptide OTR agonist WAY 267,464 with oxytocin itself on various measures of receptor binding, efficacy, behavioural effects, and regional c-Fos expression. This was an attempt to further characterise the apparent differences between the two compounds first suggested by the behavioural and pharmacological work of Ring et al. (25).
Based on measuring displacement of tritiated oxytocin from the OTR, and tritiated vasopressin from the V1aR, in cell membrane preparations, we found that both ligands can bind to the OTR and V1aR. Functional effects of WAY 267,464 were evident at the OTR, although not at the V1aR at concentrations up to 100 μm. Oxytocin generated a clear functional response at both receptors, which was considerably stronger at the oxytocin receptor.
Behavioural results showed that WAY 267,464, but not oxytocin, had acute prosocial effects in a social preference paradigm, although both compounds failed to affect anxiety-like behaviour on the EPM at the doses tested. Both compounds suppressed locomotor activity at higher doses when tested shortly after injection in an environment to which rats were well habituated. WAY 267,464 and oxytocin produced a similar pattern of c-Fos expression, although WAY 267,464 increased the number of c-Fos positive cells to a greater extent in a number of brain regions including the PrL, MnPO, SON, MePD and VLPAG, whereas oxytocin had greater effects in the BST and LC.
OTR and V1aR binding and functional response
The pharmacological data measured for oxytocin on both receptors were in the expected range according to earlier reports (22,40; M. Muttenthaler and C. W. Gruber, unpublished data) , although the Ki of oxytocin on the V1aR-GFP was approximately five-fold higher than for an unmodified receptor. Displacement binding and functional luciferase reporter assays of WAY 267,464 on the OTR resulted in a Ki value of 978 nm and an EC50 of 881 nm, which is approximately 15-fold higher than the values obtained by Ring et al. (25). The observed difference may be explained by the use of different binding assays [i.e. membrane receptor binding in the present study versus whole cell binding by Ring et al. (25)] and the C-terminal GFP modification of the receptors used for the present functional assays. Despite these differences, we obtained a Ki of WAY 267,464 at the V1aR of 113 nm, which indicated that WAY 267,464 has at least a five-fold higher affinity at the V1aR compared to the OTR, although the compound does not evoke a functional response on the V1aR at concentrations up to 100 μm. The vasopressin control had an EC50 of 13.5 nm, and is therefore in the expected range for the GFP-modified receptor. Hence, the pharmacological experiments indicate that WAY 267,464 is a full agonist (with weak affinity) of the OTR and a possible antagonist at the V1aR. This is in contrast to oxytocin, which is a full agonist with strong affinity at the OTR and a reasonably potent V1aR agonist.
Effects of WAY 267,464 and oxytocin on social behaviour
Peripheral administration of WAY 267,464, but not oxytocin, acutely increased the total amount of time and the proportion of time spent with a live rat (versus a dummy rat). This result appeared to be independent of any nonspecific effects including motor impairment. The effects of WAY 267,464 and oxytocin on social behaviour were assessed in separate experiments and on rats that differed slightly in age and body weight. However, both cohorts of rats were still in the early adolescent period and significant changes in the density of oxytocin and vasopressin receptors in the brain do not occur until late adolescence and again in adulthood (50). Moreover, both groups of rats used in Experiments 1A and 1B displayed a high degree of innate social preference, as shown by the high proportion of time vehicle-treated rats spent with the live rat (Fig. 3c,d). For these reasons, we consider that the direct comparison between the prosocial effects of WAY 267,464 and oxytocin is valid.
It might be argued that the discrepancy in the social effects of WAY 267,464 and oxytocin reflects a mismatch in effective doses in the brain. Although the pharmacokinetic profile of WAY 267,464 has not been reported, oxytocin exhibits poor bioavailability and BBB permeability that impedes effective CNS penetration (21). A single large peripheral dose of oxytocin was used in the present study, whereas the few prosocial effects of oxytocin reported in the literature are with chronic peripheral administration (12) or through i.c.v. infusion (11,13). In the event that oxytocin effectively crossed the BBB, rapid metabolic degradation may have occurred shortly after its release into the extracellular space (25). This may have resulted in oxytocin-derived metabolites exerting agonist activity at receptors other than the OTR to inhibit possible OTR-mediated prosocial effects.
The discrepant prosocial effects of WAY 267,464 and oxytocin may also be a result of the already elevated endogenous levels of oxytocin given that social encounters in male rodents stimulate endogenous oxytocin release (51). This may have prevented peripherally administered oxytocin from further increasing social preference above the high baseline level. More recently, prosocial effects of exogenous oxytocin were only evident in the form of the reversal of stress-induced social deficits (52) or those caused by deletion of the OTR (2), rather than the facilitation of baseline social behaviour (47). For example, Lukas et al. (13) found that i.c.v. infusion of oxytocin did not affect social preference under basal conditions but restored normal social behaviour in rats subjected to social defeat. Chronic stress modulates the expression of the central OTR (53,54), and so this may reflect the interplay between stressor effects on central oxytocin systems and the acute effects of exogenous oxytocin on sociality.
The acute increase in social preference with WAY 267,464 may reflect its possible V1aR antagonist properties in conjunction with its weak OTR agonist effects. Blockade of the V1aR reduces aggression during the resident–intruder test in golden hamsters (55) and adult male rats (27,56), and facilitates social approach in male goldfish that show reduced sociability (57). On the other hand, there is some evidence of prosocial effects of vasopressin including facilitation of pair-bond formation in male prairie voles (58) and social recognition memory in adult male rats (59). In addition, central vasopressin release is positively correlated with maternal care (60) and aggression (61) in female rats. In some studies, vasopressin appears to act in opposition to oxytocin and increase depressive-like and anxiety-like symptomatology, as well as heighten aggression (62,63). Indeed, administration of the selective V1aR antagonists, SRX251 and JNJ-17308616, reduces intermale aggression (27) and produces anxiolytic-like effects (28), respectively, in rodents.
There is not yet conclusive evidence as to whether vasopressin regulates social preference through the V1aR. Lukas et al. (13) found i.c.v. administration of a V1aR antagonist did not affect the innate social preference displayed by adult male Wistar rats. However, Sala et al. (2) showed i.c.v. administration of vasopressin restored social preference and social recognition memory in OTR knockout mice, and these effects were also obtained with central infusion of oxytocin. The prosocial effects of oxytocin were prevented through pre-treatment with the V1aR antagonist SR49059 (2), which indicates V1aR agonism may increase sociability. In non-mammalian species, vasotocin, the vertebrate homologue of vasopressin, increased courtship behaviour (64,65). These effects were blocked (65) or reversed (64) by a V1aR antagonist that further suggests the V1aR may be prosocial. More research is clearly needed to better define the relative prosocial and aggressive effects of vasopressin agonists and antagonists and the role of the V1aR in social preference.
One important caveat in the present study was that the pharmacology of WAY 267,464 and oxytocin at the V1bR was not explored. Previous literature indicates that oxytocin has relatively low affinity at the V1bR (22), whereas the binding profile of WAY 267,464 at this receptor has not been published. The V1bR has dense expression in the hypothalamus and limbic regions, and has also been implicated in social and emotional behaviour through the well characterised prosocial, anxiolytic- and antidepressant-like effects of the V1bR antagonist, SSR149415 (54,67). Mice deficient in the V1bR also display reduced aggression (68) and dampened hypothalamic-pituitary-adrenal axis activity in response to several stressors (69,70).
Effects of WAY 267,464 and oxytocin on anxiety-like behaviour and locomotor activity
By contrast to the report of Ring et al. (25), WAY 267,464 did not have an acute anxiolytic effect on the EPM in the present study. The discrepancy may be a result of differences in the species used (mouse versus rat), the timing of tests, and the limited dose response curve that we employed. Given the possibility that WAY 267,464 may be a V1aR antagonist, one would predict it to reduce anxiety as anxiolytic effects are generally reported after V1a receptor antagonism (28) and downregulation (66,71), and vasopressin itself is generally anxiogenic (72). The failure of WAY 267,464 to affect anxiety-related behaviour on the EPM suggests that its acute prosocial effect in rats was not the result of a general reduction in anxiety-like behaviour, and points to growing evidence that oxytocin-mediated effects on social and nonsocial anxiety may occur somewhat independently (13,73).
Oxytocin also had no effects on the EPM in the present study, also in contrast to previous reports (6,7,32). This may be a result of the effects of oxytocin being assessed in this model 50 min following administration when its effects would be diminished given its short half-life in blood (74) and rapid metabolic degradation (26). The waning effects of oxytocin are evident in the present study from the locomotor activity data, with a significant reduction on distance travelled with oxytocin (1 mg/kg) at 20–80 min post-injection in Experiment 2, but not 55–115 min following injection in Experiments 1A and 1B. It is therefore possible that anxiolytic-like effects of oxytocin would have been evident at an earlier time point.
Another possible explanation for the lack of an acute anxiolytic-like effect with oxytocin in the present study is the relatively low levels of stress in our animals. In the study by Ring et al. (7), mice most likely had a heightened degree of stress given their previous surgery for i.c.v. treatment. A range of behavioural and emotional stressors are known to increase the activity of the endogenous oxytocin system (75) and this may have been important for the anxiolytic-like effects reported for oxytocin (7). Other research has shown that i.c.v. infusion of an OTR antagonist blocks the anxiolytic-like effects of brain oxytocin in mated male rats that have heightened endogenous oxytocin release, although it is without effect in unmated males (76) (and virgin females) (73). Moreover, there is evidence that the effects of oxytocin on anxiety are dependent upon the location of activation in the brain, and occur only after local administration into the central amygdala in females (9) or the paraventricular hypothalamic nucleus in males (32).
Relative to Experiments 1A and 1B, strong and consistent locomotor suppression was observed in Experiment 2 after WAY 267,464 (100 mg/kg) and oxytocin (1 mg/kg). The earlier timing of the test and the extensive habituation rats received to the locomotor test chambers in Experiment 2 may have been critical. The mild, sedative-like effect of WAY 267,464 and oxytocin was characterised by a relaxed low body posture, hypoexploration and decreased locomotor activity that lasted for the entire 60-min assay, consistent with previous reports with oxytocin (34).
Pattern of c-Fos expression produced by WAY 267,464 and oxytocin
Administration of WAY 267,464 or oxytocin potently increased c-Fos expression in the PVN, CEA, LBN and NTS in the present study, and these effects are consistent with previous literature (34,77,78). This indicates a commonality of action of WAY 267,464 and oxytocin at the OTR with the differential doses employed (e.g. 100 : 1 mg/kg). Importantly, however, only WAY 267,464 significantly increased the number of c-Fos positive cells in the PrL, MnPO, SON, MePD and VLPAG, whereas oxytocin uniquely elevated c-Fos expression in the LC and BSTld and attenuated expression in the LHb, perhaps reflecting their possible differential actions at vasopressin receptors.
Some of the differences in c-Fos expression produced by WAY 267,464 and oxytocin in the present study might conceivably be attributable to dose factors. The higher dose of oxytocin (2 mg/kg) used in the study by Carson et al. (34) caused a greater c-Fos response in the SON, PrL and MnPO than was seen here with oxytocin at 1 mg/kg, and similar to that found here with WAY 267,464. However, Carson et al. (34) used adult rats in contrast to the adolescent rats that were used in the present study, and an increase and decrease in V1aR and OTR binding, respectively, occurs in various brain regions with age (50).
The neural mechanisms underlying affiliative behaviour are exceedingly complex and involve distributed neural circuitry and diverse neurotransmitter systems. The neural correlates of the prosocial effect of WAY 267,464 are therefore difficult to precisely identify. Increased c-Fos expression in the MePD after WAY 267,464 identifies this region as a possible correlate of the compound’s acute prosocial effect in rats. Our group showed that 3,4-methylenedioxymethamphetamine (MDMA) (47), a drug which produces acute prosocial effects and stimulates the endogenous oxytocin system, increases c-Fos in this region (79). Moreover, the OTR system in the MePD is important for normal social recognition (80,81) and the processing of biologically relevant chemosensory signals (82).
WAY 267,464 increased the number of c-Fos positive cells in the PrL, a region that regulates the formation of mating-induced partner preference in female prairie voles through modulation of oxytocin transmission in the nucleus accumbens (NAcc) (83). The PrL and NAcc interact in the processing of reward-related information, such that WAY 267,464 may have facilitated social preference by increasing the incentive properties of social interactions. An analogous mechanism has been suggested to underlie the prosocial effect of MDMA that preferentially activates the NAcc during social engagement (79).
The LHb innervates dopaminergic (DA) cells in the substantia nigra compacta and ventral tegmental area (84,85). By acting on mesolimbic DA systems, the LHb may regulate incentive motivational processes (86). Specifically, the activity of LHb neurones increases in response to negative reward-related information and this causes an inhibition of DA cells, whereas reward-predicting stimuli are associated with a reduction in LHb neuronal activity, and a subsequent increase in mesolimbic DA activity (87). The extent that oxytocin attenuated c-Fos expression in the LHb highlights its potential to modulate the motivational component of drug-seeking behaviour (88) and natural rewards by modulation of DA.
We observed that oxytocin, but not WAY 267,464, significantly increased c-Fos expression in the BSTld and LC that likely reflects the V1aR agonist properties of oxytocin versus the possible V1aR antagonist action of WAY 267,464, given the presence of vasopressin and its corresponding receptors in both regions (62). The behavioural significance of the c-Fos produced by oxytocin in the BSTld may relate to maternal and intermale aggression, anxiety and stress (63,89,90), whereas the increased c-Fos expression in the LC following oxytocin is in line with previous reports that it can interact with cardiovascular regions to regulate blood pressure and heart rate (52).
In agreement with our previous work (34), oxytocin produced a significant increase in the number of c-Fos labelled neurones in the LBN and CEA in the present study. We also report a significant increase in c-Fos expression in the NTS after oxytocin. The oxytocin-induced increase in c-Fos expression in the LBN, CEA and NTS was also observed with WAY 267,464, which suggests a commonality of action at the OTR between the high dose of WAY 267,464 and oxytocin used in the present study. The LBN, CEA and NTS are closely interconnected and play an important role in the behavioural and autonomic response to environmental stress. Previous studies have shown that exogenous oxytocin administration into the NTS attenuates the tachycardic response in sedentary and trained rats (91), and potentiates bradycardic function in response to a pressor challenge (92). The ability of WAY 267,464 and oxytocin to stimulate c-Fos in the CEA is also in line with the anxiolytic-like effects reported for WAY 267,464 (25), and past studies that have identified the CEA in the prosocial (81,93) and anxiolytic-like (9,23) effects of oxytocin, as well as its inhibitory action on fear-conditioned-induced freezing (94).
It is also notable that WAY 267,464 increased the number of c-Fos positive cells in the MnPO and VLPAG, which are two areas that closely interact to modulate thermoregulatory processes (95). Ring et al. (25) report that WAY 267,464, when administered through i.c.v. infusion, significantly reduced the hyperthermic response shown by vehicle-treated mice in response to stress. Similar reductions in stress-induced hyperthermia have been reported for both oxytocin (7) and vasopressin (96), and therefore it will be important to address in future studies the mechanism through which WAY 267,464 is able to regulate body temperature in response to stress.
WAY 267,464 significantly increased c-Fos expression in the SON of which a large proportion represented oxytocin-releasing neurones. The greater c-Fos expression produced by WAY 267,464 in the SON may reflect its possible antagonist action at the V1aR in contrast to the agonist effect of oxytocin at this receptor. The inhibition of vasopressin neurones by WAY 267,464 through V1aR antagonism may have disinhibited oxytocin neurones thereby contributing to the enhanced activity of oxytocin-expressing cells. A similar mechanism has been proposed in the CEA where there is a strong inhibitory connection between vasopressin and oxytocin cells, such that inhibiting vasopressin would effectively ‘boost’ oxytocin transmission (97) in this brain region.
Further evidence of the possible differential activity of WAY 267,464 and oxytocin at the V1a receptor is shown by the regional distribution of c-Fos produced by each compound in the SON. The V1aR and OTR are concentrated in two distinct regions of the SON, with the V1aR predominating in the ventral zone (98) and the majority of OTRs being located in the dorsal region (74). Examination of the distribution of c-Fos produced by WAY 267,464 (Fig. 7c) in the SON shows expression in both the ventral and dorsal zones consistent with the hypothesis that it may stimulate oxytocinergic cells through inhibition of vasopressin neurones, and this can be compared to the c-Fos expression produced by oxytocin (Fig. 7b) that is restricted to the dorsal oxytocin region. Our group has also shown that a 2 mg/kg dose of oxytocin preferentially stimulates c-Fos expression in the dorsal SON where oxytocin neurones predominate (34), and similar effects were also reported for MDMA (99).
By contrast to the SON, there was a significant increase in c-Fos expression, as well as an increase in the percentage of oxytocin-synthesising neurones that were c-Fos positive in the PVN after WAY 267,464 and oxytocin. The large increase in the number of c-Fos labelled neurones with both compounds may be explained by the presence of c-Fos in the dorsolateral and medial regions of the PVN (Fig. 7f,e) after WAY 267,464 and oxytocin, respectively, that suggests vasopressin and CRF neurones were also recruited (100). It has been well documented that oxytocin modulates CRF transmission in the PVN that likely occurs through activation of the OTR because this receptor has been localised on CRF neurones (101). The c-Fos expression produced by WAY 267,464 in the region of the PVN where vasopressin neurones predominate may reflect neuronal ‘deactivation’ because c-Fos immunohistochemistry cannot distinguish between neuronal ‘activation’ or ‘deactivation’ (79).
Conclusions
To summarise, we have shown that peripheral administration of the nonpeptide OTR agonist WAY 267,464 produces an acute prosocial effect under basal conditions, an effect that is not found with oxytocin itself, at the doses tested in the present study. This effect appears independent of a reduction in nonsocial anxiety or motor impairment, and may reflect the possible V1aR antagonist action of WAY 267,464 given some reports of prosocial effects through this mechanism. The ability of WAY 267,464 and oxytocin to increase the number of c-Fos positive cells in the CEA, LBN, NTS and PVN may suggest a common action at the OTR in these regions. However, the differential c-Fos expression produced by WAY 267,464 and oxytocin in the BSTld, LC, MePD and SON may reflect their contrasting actions at the V1aR. The mutual inhibitory link between oxytocin and vasopressin neurones in some brain regions may cause V1aR antagonists to release oxytocin cells from inhibition, and the present study suggests that vasopressin antagonism may be a promising target for new psychotropic drugs that are aimed at psychiatric disorders where social deficits are prominent (e.g. autism). However, more work is clearly needed to understand the complex interaction between oxytocin and vasopressin systems in the regulation of affiliative behaviour.
Acknowledgements
We would like to thank Marion Miazzo and Huang Xiang for their help with the binding experiments; Markus Muttenthaler for his help with preparation of compounds; and Michael Freissmuth for useful discussion. The cDNA clones for receptor sequences were obtained as a gift from Ralf Schülein. We are also grateful to Michael Bowen for his assistance with the data analysis. C.W.G. is supported by a grant from the Austrian Science Foundation (FWF-P22889). I.S.M. and G.E.H. received support for this project from a National Health and Medical Research Council grant. I.S.M. is supported by a fellowship from the Australian Research Council.
References
- 1.Lee HJ, Macbeth AH, Pagani JH, Young WS., III Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., III A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biol Psychiatry. 2001;50:609–613. doi: 10.1016/s0006-3223(01)01139-8. [DOI] [PubMed] [Google Scholar]
- 6.Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S. Modulary effects of oxytocin and carbetocin on stress-induced changes in rat behavior in the open-field. J Physiol Pharmacol. 2009;60:57–62. [PubMed] [Google Scholar]
- 7.Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology. 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- 8.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 9.Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 1997;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 11.Witt DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol Biochem Behav. 1992;43:855–861. doi: 10.1016/0091-3057(92)90418-f. [DOI] [PubMed] [Google Scholar]
- 12.Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS ONE. 2011;6:e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves ‘mind-reading’ in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green JJ, Hollander E. Autism and oxytocin: new developments in translational approaches to therapeutics. Neurotherapeutics. 2010;7:250–257. doi: 10.1016/j.nurt.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34:917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 21.Gimpl G. Oxytocin receptor ligands: a survey of the patent literature. Expert Opin Ther Pat. 2008;18:1239–1251. [Google Scholar]
- 22.Chini B, Manning M. Agonist selectivity in the oxytocin/vasopressin receptor family: new insights and challenges. Biochem Soc Trans. 2007;35:737–741. doi: 10.1042/BST0350737. [DOI] [PubMed] [Google Scholar]
- 23.Viviani D, Stoop R. Opposite effects of oxytocin and vasopressin on the emotional expression of the fear response. Prog Brain Res. 2008;170:207–218. doi: 10.1016/S0079-6123(08)00418-4. [DOI] [PubMed] [Google Scholar]
- 24.Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- 25.Ring RH, Schechter LE, Leonard SK, Dwyer JM, Platt BJ, Graf R, Grauer S, Pulicicchio C, Resnick L, Rahman Z, Rizzo SJ Sukoff, Luo B, Beyer CE, Logue SF, Marquis KL, Hughes ZA, Rosenzweig-Lipson S. Receptor and behavioral pharmacology of WAY-267464, a non-peptide oxytocin receptor agonist. Neuropharmacology. 2010;58:69–77. doi: 10.1016/j.neuropharm.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 26.de Wied D, Diamant M, Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol. 1993;14:251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]
- 27.Ferris CF. Functional magnetic resonance imaging and the neurobiology of vasopressin and oxytocin. Prog Brain Res. 2008;170:305–320. doi: 10.1016/S0079-6123(08)00425-1. [DOI] [PubMed] [Google Scholar]
- 28.Bleickardt C, Mullins D, Macsweeney C, Werner B, Pond A, Guzzi M, Martin FDC, Varty G, Hodgson R. Characterization of the V1a antagonist, JNJ-17308616, in rodent models of anxiety-like behavior. Psychopharmacology. 2009;202:711–718. doi: 10.1007/s00213-008-1354-x. [DOI] [PubMed] [Google Scholar]
- 29.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 31.Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol Psychiatry. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- 33.Slattery D, Neumann I. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology. 2010;58:56–61. doi: 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, Boucher AA, McGregor IS. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addict Biol. 2010;15:448–463. doi: 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- 35.Uvnas-Moberg K, Ahlenius S, Hillegaart V, Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochem Behav. 1994;49:101–106. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 36.Uvnas-Moberg K, Alster P, Hillegaart V, Ahlenius S. Oxytocin reduces exploratory motor behaviour and shifts the activity towards the centre of the arena in male rats. Acta Physiol Scand. 1992;145:429–430. doi: 10.1111/j.1748-1716.1992.tb09385.x. [DOI] [PubMed] [Google Scholar]
- 37.Sumner B, Cruise L, Slattery D, Hill D, Shahid M, Henry B. Testing the validity of c-fos expression profiling to aid the therapeutic classification of psychoactive drugs. Psychopharmacology. 2004;171:306–321. doi: 10.1007/s00213-003-1579-7. [DOI] [PubMed] [Google Scholar]
- 38.Hudson P, Pitt G, Batt R, Roe M. Piperazines as Oxytocin Agonists. In: fetcher Patent., editor. PCT. International Application; Ferring BV: 2005. [Google Scholar]
- 39.Batt AR, Ashworth DM, Baxter AJ, Haigh RM, Hudson P, Laurent C, Penson A, Pitt GRW, Robson PA, Rooker DP, Tartar AL, Yea CM, Roe MB. Non-Peptide Piperazine Ureas as Oxytocin Agonists. 226th ACS National Meeting; New York, NY. 2003. [Google Scholar]
- 40.Muttenthaler M, Andersson A, de Araujo AD, Dekan Z, Lewis RJ, Alewood PF. Modulating oxytocin activity and plasma stability by disulfide bond engineering. J Med Chem. 2010;53:8585–8596. doi: 10.1021/jm100989w. [DOI] [PubMed] [Google Scholar]
- 41.Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology. 2010;58:38–43. doi: 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Klinger M, Kuhn M, Just H, Stefan E, Palmer T, Freissmuth M, Nanoff C. Removal of the carboxy terminus of the A2A-adenosine receptor blunts constitutive activity: differential effect on cAMP accumulation and MAP kinase stimulation. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:287–298. doi: 10.1007/s00210-002-0617-z. [DOI] [PubMed] [Google Scholar]
- 43.Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 44.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 45.Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 1990;46:321–340. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- 46.McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (‘ecstasy’) Neuroscience. 2007;146:509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney: 1997. [Google Scholar]
- 49.van Nieuwenhuijzen PS, McGregor IS, Hunt GE. The distribution of gamma-hydroxybutyrate-induced Fos expression in rat brain: comparison with baclofen. Neuroscience. 2009;158:441–455. doi: 10.1016/j.neuroscience.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Lukas M, Bredewold R, Neumann ID, Veenema AH. Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology. 2010;58:78–87. doi: 10.1016/j.neuropharm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 51.Neumann ID. Brain oxytocin mediates beneficial consequences of close social interactions: from maternal love and sex. In: Pfaff DW, Kordon C, Chanson P, Christen Y, editors. Hormones and Social Behavior. Springer; Heidelberg: 2008. pp. 81–101. [Google Scholar]
- 52.Grippo AJ, Trahanas DM, Zimmerman RR, II, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152:629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Litvin Y, Murakami G, Pfaff DW. Effects of chronic social defeat on behavioral and neural correlates of sociality; vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol Behav. 2011;103:393–403. doi: 10.1016/j.physbeh.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Ferris CF, Delville Y. Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinology. 1994;19:593–601. doi: 10.1016/0306-4530(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 56.Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav. 2010;58:273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Thompson RR, Walton JC. Peptide effects on social behavior: effects of vasotocin and isotocin on social approach behavior in male goldfish (Carassius auratus) Behav Neurosci. 2004;118:620–626. doi: 10.1037/0735-7044.118.3.620. [DOI] [PubMed] [Google Scholar]
- 58.Insel TR, Winslow JT, Wang Z, Young LJ. Oxytocin, vasopressin, and the neuroendocrine basis of pair bond formation. Adv Exp Med Biol. 1998;449:215–224. doi: 10.1007/978-1-4615-4871-3_28. [DOI] [PubMed] [Google Scholar]
- 59.Engelmann M, Ludwig M, Landgraf R. Simultaneous monitoring of intracerebral release and behavior: endogenous vasopressin improves social recognition. J Neuroendocrinol. 1994;6:391–395. doi: 10.1111/j.1365-2826.1994.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 60.Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc Natl Acad Sci U S A. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. Eur J Neurosci. 2010;31:883–891. doi: 10.1111/j.1460-9568.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- 62.Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- 63.Caldwell HK, Lee HJ, Macbeth AH, Young WS., III Vasopressin: behavioral roles of an ‘original’ neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Semsar K, Godwin J. Multiple mechanisms of phenotype development in the bluehead wrasse. Horm Behav. 2004;45:345–353. doi: 10.1016/j.yhbeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Toyoda F, Yamamoto K, Ito Y, Tanaka S, Yamashita M, Kikuyama S. Involvement of arginine vasotocin in reproductive events in the male newt Cynops pyrrhogaster. Horm Behav. 2003;44:346–353. doi: 10.1016/j.yhbeh.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 66.Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, Tsujimoto G, Mishima K, Iwasaki K, Fujiwara M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res. 2007;178:123–127. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Shimazaki T, Iijima M, Chaki S. The pituitary mediates the anxiolytic-like effects of the vasopressin V1B receptor antagonist, SSR149415, in a social interaction test in rats. Eur J Pharmacol. 2006;543:63–67. doi: 10.1016/j.ejphar.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 68.Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., III Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 69.Lolait SJ, Stewart LQ, Jessop DS, Young WS, III, O’Carroll AM. The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology. 2007;148:849–856. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu T, Mori T, Tsujimoto G. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest. 2004;113:302–309. doi: 10.1172/JCI19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur J Neurosci. 2007;26:3597–3605. doi: 10.1111/j.1460-9568.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- 73.Neumann I, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–244. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 74.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 75.Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- 76.Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kita I, Yoshida Y, Nishino S. An activation of parvocellular oxytocinergic neurons in the paraventricular nucleus in oxytocin-induced yawning and penile erection. Neurosci Res. 2006;54:269–275. doi: 10.1016/j.neures.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 78.Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG. c-Fos expression in rat brain and brainstem nuclei in response to treatments that alter food intake and gastric motility. Mol Cell Neurosci. 1993;4:93–106. doi: 10.1006/mcne.1993.1011. [DOI] [PubMed] [Google Scholar]
- 79.Thompson MR, Hunt GE, McGregor IS. Neural correlates of MDMA (‘Ecstasy’)-induced social interaction in rats. Soc Neurosci. 2009;4:60–72. doi: 10.1080/17470910802045042. [DOI] [PubMed] [Google Scholar]
- 80.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW. Micro-particle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci USA. 2007;104:4670–4675. doi: 10.1073/pnas.0700670104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Samuelsen CL, Meredith M. Oxytocin antagonist disrupts male mouse medial amygdala response to chemical-communication signals. Neuroscience. 2011;180:96–104. doi: 10.1016/j.neuroscience.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol. 2010;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006;494:75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABAA receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, Ben-Tzion M, Yadid G. Electrical stimulation of the lateral habenula produces an inhibitory effect on sucrose self-administration. Neuropharmacology. 2011;60:381–387. doi: 10.1016/j.neuropharm.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bosch OJ, Pfortsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J Neuroendocrinol. 2010;22:420–429. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- 90.Wigger A, Sánchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neumann ID, Holsboer F, Plotsky PM. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology. 2004;29:1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- 91.Braga DC, Mori E, Higa KT, Morris M, Michelini LC. Central oxytocin modulates exercise-induced tachycardia. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1474–R1482. doi: 10.1152/ajpregu.2000.278.6.R1474. [DOI] [PubMed] [Google Scholar]
- 92.Higa KT, Mori E, Viana FF, Morris M, Michelini LC. Baroreflex control of heart rate by oxytocin in the solitary-vagal complex. Am J Physiol Regul Integr Comp Physiol. 2002;282:R537–R545. doi: 10.1152/ajpregu.00806.2000. [DOI] [PubMed] [Google Scholar]
- 93.Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 95.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 96.Terlouw E Claudia, Kent S, Cremona S, Dantzer R. Effect of intracere-broventricular administration of vasopressin on stress-induced hyperthermia in rats. Physiol Behav. 1996;60:417–424. [PubMed] [Google Scholar]
- 97.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 98.Gouzènes L, Sabatier N, Richard P, Moos FC, Dayanithi G. V1a-and V2-type vasopressin receptors mediate vasopressin-induced Ca2+ responses in isolated rat supraoptic neurones. J Physiol. 1999;517:771–779. doi: 10.1111/j.1469-7793.1999.0771s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hunt GE, McGregor IS, Cornish JL, Callaghan PD. MDMA-induced c-Fos expression in oxytocin-containing neurons is blocked by pretreatment with the 5-HT-1A receptor antagonist WAY 100635. Brain Res Bull. 2011;86:65–73. doi: 10.1016/j.brainresbull.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 100.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 101.Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]