Abstract
The formation of healthy gametes depends on programmed DNA double strand breaks (DSBs), which are each repaired as a crossover (CO) or non-crossover (NCO) from a homologous template. Although most of these DSBs are repaired without giving COs, little is known about the genetic requirements of NCO-specific recombination. We show that Fml1, the Fanconi anemia complementation group M (FANCM)-ortholog of Schizosaccharomyces pombe, directs the formation of NCOs during meiosis in competition with the Mus81-dependent pro-CO pathway. We also define the Rad51/Dmc1-mediator Swi5-Sfr1 as a major determinant in biasing the recombination process in favour of Mus81, to ensure the appropriate amount of COs to guide meiotic chromosome segregation. The conservation of these proteins from yeast to Humans suggests that this interplay may be a general feature of meiotic recombination.
Keywords: Homologous recombination, Meiosis, Fml1, Mus81, Schizosaccharomyces pombe
Faithful chromosome segregation during meiosis depends on the establishment of chiasmata through recombinational repair of programmed DNA double-strand breaks (DSBs) to produce crossovers (COs) between homologous chromosomes (homologs). However, in most cases only a minority of the DSBs are earmarked to form COs, and therefore the majority have to be repaired by using either the homolog without CO formation or the sister chromatid (1).
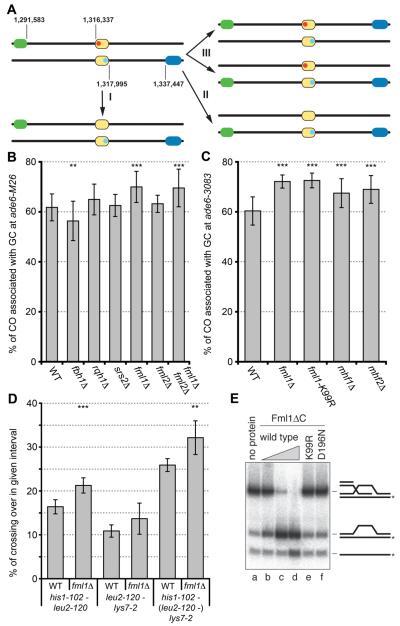
In order to identify helicase activities involved in non-crossover (NCO)-recombination during meiosis in the fission yeast Schizosaccharomyces pombe, we screened for helicases potentially capable of D loop unwinding during synthesis-dependent strand annealing (SDSA), which is thought to be a major pathway of NCO recombination (1). To this end, we used a genetic recombination assay consisting of a meiotic recombination hotspot at the ade6 gene and two flanking scorable markers (Fig. 1A). We hypothesized that at least one of the helicases promoting NCO recombination pathways in mitotic cells would also have a role during meiosis. From our candidate list – fbh1, srs2, rqh1, fml1 and fml2 – only the deletion of fml1 gave the expected increase in CO formation associated with a meiotic gene conversion (GC) event at two different hotspot alleles, ade6-M26 and ade6-3083, and at a non-hotspot allele ade6-M375 (Fig. 1, B and C, and tables S1 to S3) (2–5). Increases in COs were also observed on a different chromosome (Fig. 1D and table S4) and by a physical assay at the mbs1 locus (fig. S1), indicating that Fml1’s role in suppressing CO formation is not restricted to a single locus.
Fig. 1.
Fml1-Mhf is required for wild-type levels of NCO during meiosis. (A) Schematic of the meiotic recombination assay indicating the positions (in base pairs) of ura4+-aim2 (green), his3+-aim (blue) and ade6 (yellow) on chromosome 3. The point mutations in the ade6-3083/-M26 hotspot and ade6-469 coldspot alleles are labelled in red and light blue, respectively. The common types of outcomes of the assay are shown: (I) GC at ade6 without CO, (II) GC at ade6 with CO of the flanking markers, and (III) CO without GC at ade6. (B and C) Frequency of CO associated with GC events at ade6 hotspots in wild type and mutants (tables S1 and S2) (2). (D) Frequency of CO in two neighbouring intervals in wild type and the fml1Δ mutant (table S4). In (B) to (D), statistical significance in comparison with wild type indicated as *P <0.1, **P <0.05, and ***P <0.01 (for P values, see corresponding tables in the supplementary materials). (E) D loop unwinding by Fml1ΔC (lanes b to d: 0.05 nM, 0.5 nM, and 5 nM), Fml1ΔC-K99R (lane e: 5 nM) and Fml1ΔC-D196N (lane f: 5 nM). The schematics represent the D loop and its dissociation products, with the asterisk indicating the position of the 5′ end 32P label.
In vitro purified Fml1, like its budding yeast ortholog Mph1, unwinds D loops and is therefore suited to promoting SDSA (Fig. 1E) (6, 7). The fml1-K99R mutant, which encodes protein that retains full DNA binding activity but is unable to unwind D loops (Fig. 1E and fig. S2), exhibits the same hyper-CO phenotype as the null mutant indicating that Fml1’s helicase function is required for NCO formation (Fig. 1C). A significant increase in CO is also observed by deleting Fml1’s cofactors Mhf1 and Mhf2, whose orthologs in humans promote the DNA binding and catalytic activities of Fanconi anemia complementation group M (FANCM) (Fig. 1C and table S2) (8, 9).
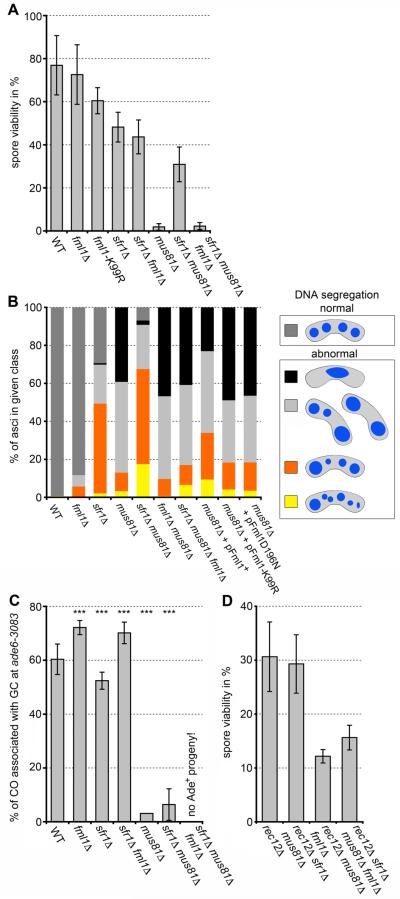
In fission yeast the formation of CO products from joint DNA molecules depends on the endonuclease Mus81-Eme1 (10). The deletion of mus81 causes joint DNA molecules to remain unresolved, which prevents chromosome segregation and results in a reduction in the viability of progeny (Fig. 2, A and B, fig. S3 and table S5) (10-12). The mating efficiency of mus81Δ fml1Δ double mutants is very low (table S6), preventing comprehensive genetic analysis; however, visual inspection of mus81Δ fml1Δ asci showed a higher incidence of clumped DNA masses than in mus81Δ single mutants, indicating an aggravation of the chromosome segregation problem (Fig. 2B and table S7). These data indicate that at best, Fml1 only poorly substitutes for the loss of the CO recombination pathway by feeding joint molecules into a NCO pathway. The meiosis-specific Rad51-paralogue Dmc1 has been shown to form D loops, which are more resistant to dismantling by DNA translocases than those formed by Rad51 (13); however, in fission yeast deletion of dmc1 does not change the level of COs associated with GCs (table S2). The Rad51/Dmc1-mediator complex Swi5-Sfr1 (14) is required for wild-type levels of CO and its deletion ameliorates the defects seen in a mus81Δ mutant (Fig. 2, A, B and C) (15). This rescue of mus81Δ by sfr1Δ and the reduction of CO formation associated with GC in a sfr1Δ single mutant depend on the presence of fml1 (Fig. 2, A, B and C). This suggests that Swi5-Sfr1 protects D loops from being unwound by Fml1 and in doing so promotes Mus81-mediated CO formation. In accordance with this, we see a reduction in Mus81 foci in sfr1Δ meiotic nuclei compared with wild type (fig. S4 and table S8).
Fig. 2.
Fml1 is able to drive a NCO pathway of meiotic recombination in the absence of Mus81. (A) Viability of progeny from wild-type and mutant crosses (table S5). (B) Distribution of DNA masses in wild-type and mutant asci with or without overexpression of wild-type and mutant Fml1 (fig. S3). (C) Frequency of CO associated with GC events at ade6-3083 from wild-type and mutant crosses. Statistical significance in comparison with wild type is shown as *P <0.1, **P <0.05, and ***P <0.01 (table S2). (D) Abolishing meiotic DSB formation by deleting rec12 partially rescues the spore viability defect of mus81Δ fml1Δ mutants (table S5).
Under vegetative growth conditions mus81Δ fml1Δ strains display synthetic sickness (6), and therefore to confirm that the phenotypes we observe during meiosis are caused by the failure to process meiotic recombination intermediates, we abrogated meiotic DSB formation by deleting rec12 (also termed spo11) in mus81Δ sfr1Δ, mus81Δ fml1Δ and mus81Δ fml1Δ sfr1Δ strains. The spore viabilities of the mutant combinations were higher than or similar to the 12.5% expected from random segregation of three chromosome pairs (Fig. 2D). Although the spore viability in the mus81Δ fml1Δ rec12Δ and mus81Δ fml1Δ sfr1Δ rec12Δ crosses is not completely restored to rec12Δ levels, the rescue is robust enough to attribute much of the meiotic failure of these mutant combinations to a breakdown in processing meiotic recombination intermediates.
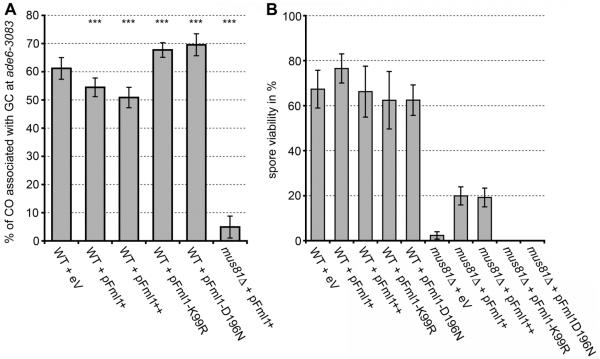
The transcription of mus81, eme1, swi5 and sfr1 is upregulated (by two-to sixfold) at the start of meiosis, whereas that of fml1 is not (16). Therefore, we wondered whether relative changes in the amounts of these proteins could influence whether DSBs are repaired as COs or NCOs. Indeed, Fml1 over-expression in wild type reduces COs at ade6-3083 in a dosage-dependent manner (Fig. 3A and table S9). This effect depends on Fml1’s helicase activity because overexpression of Fml1-K99R or Fml1-D196N, which can bind but not unwind D-loops (Fig. 1E and fig. S2), causes a significant increase in COs akin to fml1Δ (Fig. 3A and table S9). Overexpression of these mutants also confers fml1Δ-like sensitivity to genotoxins (fig. S5). Most likely, these mutant proteins impede endogenous wild-type Fml1 and thereby generate a fml1Δ-like phenotype.
Fig. 3.
Overexpression of Fml1 suppresses COs and partially rescues the poor spore viability of a mus81Δ mutant. (A and B) Frequency of CO associated with GC events at ade6-3083 (A) and viability of progeny (B) in wild-type and mus81Δ crosses overexpressing wild-type and mutant Fml1 (tables S5 and S9). Statistical significance in comparison with wild type in (A) is shown as *P <0.1, **P <0.05, and ***P <0.01 (for exact P values, see table S9).
Further evidence that the relative amount of Fml1 and Swi5-Sfr1 is a determinant in Fml1’s ability to unwind D loops in vivo comes from analyzing the effect of Fml1 overexpression in mus81Δ crosses. Here both the spore viability and chromosome segregation defects of mus81Δ crosses are ameliorated in a helicase-dependent manner and in a similar way as deleting sfr1: without producing COs (Figs. 2B and 3, A and B). As in wild-type crosses, overexpression of mutant Fml1 probably impedes endogenous wild-type Fml1, worsening the already poor spore viability and chromosome segregation of a mus81Δ cross (Figs. 2B and 3B and table S7). The partial rescue of spore viability and chromosome segregation in mus81Δ crosses is specific to Fml1 because none of the other candidate DNA helicases (Rqh1, Srs2, Fbh1, and Fml2) when overexpressed could do this (table S5).
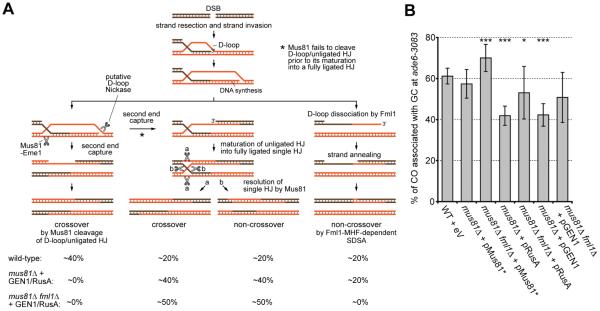
Swapping exogenous Holliday junction (HJ) resolvases, namely bacterial RusA and human GEN1, for Mus81 results in a reduction of CO associated with GC at an ade6 hot spot from ~60% down to ~40% (17, 18). Our explanation was that these HJ resolvases (in contrast to Mus81-Eme1) cleave recombination intermediates in an unbiased manner producing COs and NCOs in a 1:1 ratio. We hypothesized that the remaining 20% NCO recombination events stem from SDSA (Fig. 4A). If this is true, then exchanging Mus81 for RusA or GEN1 in a fml1Δ background, in which SDSA is abolished, would result in 50% COs and NCOs via unbiased HJ resolution (Fig. 4A). Indeed, 50% COs is what we find when RusA or GEN1 are expressed in mus81Δ fml1Δ strains (Fig. 4B).
Fig. 4.
Meiotic interhomologue recombination pathways in S. pombe. (A) The respective contribution of recombination pathways to the CO/NCO outcome and the changes observed when a pathway is deactivated. This model accounts for the fact that in a mus81Δ strain, only single HJs are observed to accumulate (10), but therefore it needs to invoke a D loop nickase activity (18). (B) Frequency of CO associated with GC events at ade6-3083 from wild-type, mus81Δ, and mus81Δ fml1Δ crosses expressing Mus81-Eme1, RusA or GEN1(1-527). Statistical significance in comparison with wild type is shown as *P <0.1, **P <0.05, and ***P <0.01 (table S9) (18).
It is conceivable that the Fml1-dependent NCO pathway proceeds via biased HJ cleavage rather than SDSA. However, deletion of the two known junction-specific nucleases (Slx1 and the XPF ortholog Rad16), which could potentially fulfill this function, has no effect on CO formation or spore viability in a mus81Δ sfr1Δ mutant (tables S2 and S5).
Our data show that Fml1-Mhf works in parallel with Mus81-Eme1 to process meiotic joint DNA molecules, and that Fml1’s ability to produce NCOs is mitigated by a relative up-regulation of a Swi5-Sfr1 and Mus81-Eme1-dependent pathway, in which Swi5-Sfr1 may stabilize Rad51/Dmc1-mediated single-end invasions so that they can be preferentially cleaved by Mus81-Eme1. Fml1 represents the only factor directly driving a meiotic NCO-specific pathway; however, other DNA helicases, such as RTEL-1 in C. elegans, apparently can direct the recombination outcome via template choice, creating an additional level of regulation (19, 20).
Supplementary Material
Acknowledgements
We thank R. Mercier for discussions and sharing data before publication. We are grateful to A. Decottignies, J. Kohli, R. J. McFarlane, P. Russell, G. R. Smith and W. W. Steiner for providing strains/reagents and to C. Bryer and J. Witzki for technical assistance. This work was supported by a Wellcome Trust Programme Grant 090767/Z/09/Z. A.L. was funded in part by an Erwin Schrödinger Fellowship (J 2489-B03) from the Austrian Science Fund (FWF).
Footnotes
Materials and Methods
Figs S1 to S5
Tables S1 to S10
References (21-36)
References and Notes
- 1.McMahill MS, Sham CW, Bishop DK. PLoS Biol. 2007;5:e299. doi: 10.1371/journal.pbio.0050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun W, Lorenz A, Osman F, Whitby MC. Nucleic Acids Res. 2011;39:1718. doi: 10.1093/nar/gkq977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cromie GA, Hyppa RW, Smith GR. Genetics. 2008;179:1157. doi: 10.1534/genetics.108.088955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuchert P, Kohli J. Genetics. 1988;119:507. doi: 10.1093/genetics/119.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steiner WW, Smith GR. Genetics. 2005;169:1973. doi: 10.1534/genetics.104.039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun W, et al. Mol Cell. 2008;32:118. doi: 10.1016/j.molcel.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prakash R, et al. Genes Dev. 2009;23:67. doi: 10.1101/gad.1737809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh TR, et al. Mol Cell. 2010;37:879. doi: 10.1016/j.molcel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Z, et al. Mol Cell. 2010;37:865. doi: 10.1016/j.molcel.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cromie GA, et al. Cell. 2006;127:1167. doi: 10.1016/j.cell.2006.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boddy MN, et al. Cell. 2001;107:537. doi: 10.1016/s0092-8674(01)00536-0. [DOI] [PubMed] [Google Scholar]
- 12.Osman F, Dixon J, Doe CL, Whitby MC. Mol Cell. 2003;12:761. doi: 10.1016/s1097-2765(03)00343-5. [DOI] [PubMed] [Google Scholar]
- 13.Bugreev DV, et al. Nat Struct Mol Biol. 2011;18:56. doi: 10.1038/nsmb.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haruta N, et al. Nat Struct Mol Biol. 2006;13:823. doi: 10.1038/nsmb1136. [DOI] [PubMed] [Google Scholar]
- 15.Ellermeier C, Schmidt H, Smith GR. Genetics. 2004;168:1891. doi: 10.1534/genetics.104.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mata J, Lyne R, Burns G, Bähler J. Nat Genet. 2002;32:143. doi: 10.1038/ng951. [DOI] [PubMed] [Google Scholar]
- 17.Gaskell LJ, Osman F, Gilbert RJ, Whitby MC. EMBO J. 2007;26:1891. doi: 10.1038/sj.emboj.7601645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenz A, West SC, Whitby MC. Nucleic Acids Res. 2010;38:1866. doi: 10.1093/nar/gkp1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youds JL, et al. Science. 2010;327:1254. doi: 10.1126/science.1183112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosu S, Libuda DE, Villeneuve AM. Science. 2011;334:1286. doi: 10.1126/science.1212424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabatinos SA, Forsburg SL. Methods Enzymol. 2010;470:759. doi: 10.1016/S0076-6879(10)70032-X. [DOI] [PubMed] [Google Scholar]
- 22.Smith GR. Methods Mol Biol. 2009;557:65. doi: 10.1007/978-1-59745-527-5_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein AL, McCusker JH. Yeast. 1999;15:1541. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.Maundrell K. Gene. 1993;123:127. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- 25.Lorenz A, Osman F, Folkyte V, Sofueva S, Whitby MC. Mol Cell Biol. 2009;29:4742. doi: 10.1128/MCB.00471-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doe CL, Dixon J, Osman F, Whitby MC. EMBO J. 2000;19:2751. doi: 10.1093/emboj/19.11.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ip SC, et al. Nature. 2008;456:357. doi: 10.1038/nature07470. [DOI] [PubMed] [Google Scholar]
- 28.Rass U, et al. Genes Dev. 2010;24:1559. doi: 10.1101/gad.585310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loidl J, Lorenz A. Methods Mol Biol. 2009;558:15. doi: 10.1007/978-1-60761-103-5_2. [DOI] [PubMed] [Google Scholar]
- 30.Lorenz A, et al. J Cell Sci. 2004;117:3343. doi: 10.1242/jcs.01203. [DOI] [PubMed] [Google Scholar]
- 31.Hyppa RW, Smith GR. Methods Mol Biol. 2009;557:235. doi: 10.1007/978-1-59745-527-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grishchuk AL, Kohli J. Genetics. 2003;165:1031. doi: 10.1093/genetics/165.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyppa RW, Smith GR. Cell. 2010;142:243. doi: 10.1016/j.cell.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osman F, Dixon J, Barr AR, Whitby MC. Mol Cell Biol. 2005;25:8084. doi: 10.1128/MCB.25.18.8084-8096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimoda C. J Cell Sci. 2004;117:389. doi: 10.1242/jcs.00980. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz A, Estreicher A, Kohli J, Loidl J. Chromosoma. 2006;115:330. doi: 10.1007/s00412-006-0053-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.