Abstract
The present study had two aims. First, to determine if bimodal audio–visual targets allow for greater inhibition of visual distractors, which in turn may lead to greater saccadic trajectory deviations away from those distractors. Second, to determine if bimodal targets can reduce age differences in the ability to generate deviations away, as older adults tend to benefit more from multisensory integration than younger adults. The results show that bimodal targets produced larger deviations away than unimodal targets, but only when the distractor preceded the target, and this effect was comparable across age groups. Furthermore, in contrast to previous research, older adults in this study showed similar deviations away from distractors to those of younger adults. These findings suggest that age differences in the production of trajectory deviations away are not inevitable and that multisensory integration may be an important means for increasing top–down inhibition of irrelevant distraction.
Keywords: Multisensory integration, Eye movements, Saccades, Curvature, Aging
Introduction
The visual field is often cluttered with irrelevant stimuli that must be avoided when making a saccadic eye movement to a particular target. One effect of these irrelevant distractors is to influence the trajectory of saccades, sometimes causing trajectories to deviate toward the distractor, and other times away (for a review, see Van der Stigchel et al. 2006). The particular direction of saccadic deviations can be explained by the averaging process that is thought to occur within the oculomotor map of the superior colliculus (SC; McPeek and Keller 2001; Port and Wurtz 2003). On this map, potential saccade goals are coded as vectors through spatially-specific peaks of activity, and, when a saccade is initiated, its trajectory reflects the average of these vectors. As a result, when a distractor causes a stimulus-driven peak of activity, this activity contributes to the final average vector causing the saccade to deviate toward the distractor’s location (Tipper et al. 2001; Godijn and Theeuwes 2002). If time allows, inhibition is applied to the distractor-related activity from a source external to the SC, most likely the frontal eye fields (FEFs; Schlag-Rey et al. 1992), which leads to below baseline activity at that location and thus, a saccade that veers away from the distractor. In either case, corrective processes, potentially arising from the cerebellum (Quaia et al. 1999), must then redirect the eyes back toward the saccade goal. Thus, deviations away tend to occur at longer saccadic latencies, as top–down inhibitory processes require time to dampen down bottom–up activation (McSorley et al. 2006; Ludwig and Gilchrist 2003; Campbell et al. 2009).
From an aging standpoint, the role of inhibitory processes in determining saccadic trajectories is especially interesting given that there is a wide body of literature showing age-related declines in inhibitory control (for a review, see Hasher et al. 1999). For instance, in studies of oculomotor capture that use an abrupt onset to disrupt search performance, older adults demonstrate disproportionately longer search times (Cassavaugh et al. 2003) and often make more erroneous saccades toward the onset than younger adults (Kramer et al. 2000; Ryan et al. 2007; Campbell and Ryan 2009; but see Kramer et al. 1999; Colcombe et al. 2003), suggesting an age-related decline in oculomotor inhibition. In line with this work, a recent study from our laboratories (Campbell et al. 2009) has demonstrated that older adults show a substantial reduction in deviations away. In this study, older and younger adults moved their eyes to a target which appeared concurrently with an irrelevant distractor. While younger adults showed the expected pattern of deviating toward the distractor for short-latency saccades, and away from the distractor at longer latencies (for which inhibition had time to accrue), older adults simply showed a decrease in the magnitude of deviations toward the distractor over time. Even at very long saccadic latencies (i.e., ~375 ms), they did not show deviations away. This result suggests that older adults lack the inhibitory ability to produce saccadic deviations away from distractors.
The main goal of this study was inspired by the recent surge of research reporting that age-related enhancements occur with multisensory integration (Laurienti et al. 2006; Peiffer et al. 2007; Diederich et al. 2008). For instance, Laurienti et al. (2006) gave older and younger adults a speeded discrimination task using visual (red and blue filled circles), auditory (the spoken words “red” and “blue”) and bimodal stimuli (the auditory and visual cues together). Although both groups were faster to respond in the multi-sensory condition, this benefit was significantly larger in the older group. A follow-up study (Peiffer et al. 2007) further demonstrated that this effect is not attributable to general cognitive slowing, as older adults continued to show greater multisensory enhancement than younger adults on a simple reaction time (RT) task which equated the two groups on unimodal responding. More recently, Diederich et al. (2008) extended these findings to saccadic reaction times (SRTs) by having older and younger adults saccade to a visual target which onset with or without a co-occurring auditory stimulus. While older adults’ SRTs were slower overall, their performance gain on bimodal trials was greater than that of younger adults. Thus, older adults appear to show greater integration between the senses than younger adults, although the precise cause of this age difference remains a matter of debate (Diederich et al. 2008; Hugenschmidt et al. 2009).
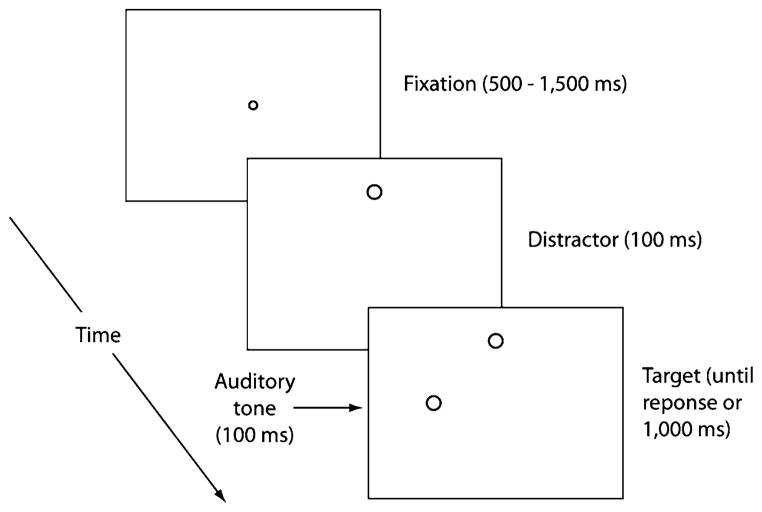
If multisensory targets can have such robust effects on the initiation time of saccades, especially in older adults, such targets may well have effects on other aspects of saccadic programing. The question we ask in this study is whether a multisensory target can help older adults inhibit irrelevant visual distractors when generating a saccade to the target. If multisensory targets are processed more quickly, then this should allow the FEFs to more quickly and accurately distinguish between the target and distractor stimuli, resulting in greater top–down inhibition of the distractor location at the time the saccadic movement is initiated and, therefore, greater curvature away from the distractor. Interestingly, the question of how multisensory targets affect trajectory deviations has yet to be addressed even in younger adults, although related studies have shown that younger adults do show deviations toward (Frens et al. 1995) and away from (Doyle and Walker 2002) auditory and tactile distractors. Thus, our study will, for the first time, examine the effects of a bimodal auditory–visual target on saccadic trajectory deviations in general and, in addition, will determine if age differences in the ability to generate deviations away can be ameliorated by the inclusion of bimodal targets. The basic task we will use requires younger and older adults to fixate a central fixation dot and then move their eyes to an “O” target which will appear to the left or right of fixation. Visual targets will either occur alone (unimodal condition) or accompanied by a spatially compatible tone (bimodal condition). A single “O” distractor will also be present on each trial, appearing above or below fixation, and it will occur either 100 ms before the target stimulus (early distractor condition) or 100 ms after it (late distractor condition; see Fig. 1 for a typical trial sequence). If bimodal targets allow for greater inhibition of a competing distractor, then both older and younger adults should show larger deviations away from the distractor in the bimodal condition. As older adults tend to benefit more from multisensory targets, this effect may be larger in the older group. In addition, the early and late distractor conditions will provide information on whether or not older adults benefit from having more time to inhibit an irrelevant distractor prior to target onset.
Fig. 1.
Example of a typical trial sequence (early distractor, bimodal target condition)
Method
Participants
Participants were 14 younger (18–29; M = 20.92, SD = 2.89) and 14 older adults (61–73; M = 67.07, SD = 4.21). Younger adults were undergraduate students at the University of Toronto and received partial course credit for their participation. Older adults were recruited from the community and received monetary compensation for their participation. Data from two younger adults and one older adult were replaced because their eyes could not be tracked reliably. All participants reported of having normal or corrected to normal vision and hearing.
Younger adults had an average of 14.14 (SD = 1.79) years of education and a mean score of 31.19 (SD = 3.34) on the Shipley Vocabulary Test (Shipley 1946). Older adults did not differ from younger adults in years of education (M = 15.04, SD = 3.14), t(26) = 0.92, p = 0.36, but they did score higher on the vocabulary test (M = 35.20, SD = 3.17), t(26) = 3.25, p < 0.01.
Apparatus
Eye movements were recorded by monitoring pupil position and corneal reflectance using a camera-based eye tracker (SR Research Eyelink 1000) with a temporal resolution of 1,000 Hz and an RMS spatial resolution of 0.01° of visual angle. Gaze position was established using a nine-point calibration and validation procedure. The beginning and end of each saccade was determined using a 30°/s threshold, with the additional criteria that the eye exceeded an acceleration of 8,000°/s during the movement. Experimental displays were presented on a 19-inch flat CRT at a refresh rate of 85 Hz and a resolution of 1,024 × 768 pixels. Two speakers were located 35-cm away from either side of the monitor at middle height. A chin rest was used to fix participants’ heads 80 cm from the monitor.
Procedure
Each experimental session began with a sound localization test during which a series of ten 400-Hz tones were randomly presented from the left and right speakers for 100 ms each. Participants were to indicate the location of the tone by saying “left” or “right,” at which point the experimenter pressed the space bar to advance to the next trial. All participants successfully completed this task without error on their first attempt at a volume of 70 db. The eye tracker setup followed the sound localization test. Calibration and validation were performed repeatedly until a minimum average accuracy of 0.5° was attained, and between blocks the experimenter could elect to recalibrate the eye tracker if necessary.
Figure 1 depicts a typical trial sequence for the early distractor, bimodal target condition. Each trial began with a fixation stimulus (a white ring with an outer diameter of 0.35° and an inner diameter of 0.16°) that was presented in the center of the display on a light-gray background. Once participants moved their gaze to within 1.5° of the fixation stimulus (all reported distances are from the center of the stimulus), they were required to maintain fixation within this region for a randomly determined duration between 500 and 1,500 ms, at which point, depending on the experimental condition, either the target or distractor first appeared. On early distractor trials, the distractor appeared first in isolation for 100 ms before the visual target onset and both stimuli remained on the screen for a maximum duration of 1,000 ms. On late distractor trials, the visual target appeared first for 100 ms, followed by the distractor for a maximum duration of 900 ms. A white ring—subtending 1.0° horizontally and vertically and drawn with line widths of 0.1°—served as the target and distractor on every trial. Distractors always appeared 8.0° above or below the fixation stimulus, whereas visual targets always appeared 8.0° to the left or right of the fixation stimulus. Moreover, to examine the influence of a multisensory target, the visual target was simultaneously paired with a spatially compatible tone (400 Hz, 70 db) for 100 ms on half of all experimental trials. The remaining experimental trials were unimodal, in that the visual target appeared without the spatially compatible tone.
Once the target appeared, participants were required to move their gaze to within 2° of the target stimulus using a single saccade. If participants failed to maintain fixation before the target appeared, a 200-Hz error tone sounded from both speakers for 100 ms, the display items were extinguished for 750 ms, and then the trial recommenced. If fixation failed three times consecutively, the experimenter could choose to recalibrate the eye tracker. After the target was presented, if participants failed to initiate a saccade within 1,000 ms, or failed to move their eyes to the target location first, then an error tone sounded and the trial was counted as an error. At the end of each trial, the display items remained on the display for 250 ms and were then extinguished for an inter-trial interval of 600 ms.
Design
The design was a 2 (age) × 2 (distractor onset) × 2 (sound) mixed factorial, with age (younger, older) as a between-subjects factor, and both distractor onset (early, late) and sound (unimodal, bimodal) as within-subjects factors. Both within-subject factors were completely randomized within each of the eight 41-trial long experimental blocks. In addition, participants completed eight practice trials at the start of the experiment.
Measures
Two dependent measures were used in this experiment: SRT and saccadic curvature. SRT was calculated as the latency between the onset of the target stimulus and the onset of the target directed saccade. Saccadic curvature was calculated using the quadratic method outlined by Ludwig and Gilchrist (2002). Namely, the trajectory of each saccade was scaled and translated to travel a common absolute distance, and the best-fitting quadratic polynomial to the trajectory was determined. The coefficient of the quadratic term of the resulting polynomial provides the measure of the amplitude of curvature (i.e., deviation), which is reported in hundredths of a degree of visual angle. Positive values indicate deviations toward the distractor, while negative values indicate deviations away from the distractor.
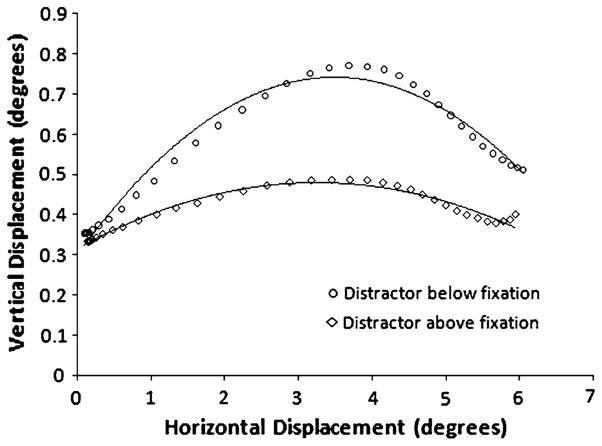
Figure 2 gives an example of the average saccade trajectory as a function of distractor location for one participant in the reported experiment. As is typically observed, idiosyncratic biases can be seen in this participant’s eye movements. For example, this participant tended to fixate slightly above the fixation point, undershoot the target location by approximately 2° (Becker 1972), and initiate most saccades with a trajectory that was biased toward the top of the display. To account for individual differences in saccade start and end positions, the measure of saccade curvature was designed to first translate and then scale the trajectories before finding the best fitting polynomial (Ludwig and Gilchrist 2002). There are two methods that have been previously employed to account for individual biases in saccade curvature. Many previous studies have included a no-distractor condition as a baseline against which distractor-induced curvature can be compared (e.g., Al-Aidroos and Pratt 2009; Doyle and Walker 2002; Godijn and Theeuwes 2002; McSorley et al. 2006). Alternatively, some studies account for natural variations in saccade curvature by placing distractors (or attention) at mirror locations on both sides of the target, and thus curvature is compared against the location of the distractor, rather than the no-distractor baseline (e.g., Campbell et al. 2009; Sheliga et al. 1995a, b; Van der Stigchel et al. 2007; Van der Stigchel and Theeuwes 2005). In the present study, we employed this second solution. Participants made saccades to a left or right target, distractors appeared above or below the fixation point on equal numbers of trials, and curvature was computed relative to the location of the distractor. While this solution does not allow us know how distractors influence curvature relative to when no distractor is present, measuring curvature as a function of distractor position does increase our power (by eliminating no-distractor trials) to measure the effect of distractors on saccade trajectories. Looking again at Fig. 2, the effect of distractors on curvature can be seen clearly as the two lines are different. Further, although this subject’s saccades tended to curve toward the top of the display (even when the distractor was in that direction), curvature was greatest when the distractor was on the bottom of the display and, therefore, on average the participant’s saccades deviated away from the distractor’s location.
Fig. 2.
The average saccade trajectory as a function of distractor location for one participant in the reported experiment (lines represent the best fit quadratic polynomials for each trajectory). The eye is rotating from the location of the fixation point to a rightward target (trajectories for left target trials have been reflected across the vertical access). This example of a younger adult’s performance clearly demonstrates an effect of distractors on saccade trajectories. Of note, the horizontal and vertical axes are not drawn on equal scales
Results
Error trials of younger (M = 4.16%, SD = 3.93) and older (M = 8.17%, SD = 5.12) participants were excluded from further analyses. Also, trials were recursively trimmed from each participant’s dataset using a three standard deviation cut-off, first based on SRT and then curvature, for both younger (3.81%) and older (4.88%) participants. Means and standard errors for both SRTs and trajectory curvature are shown in Table 1.
Table 1.
Mean saccadic reaction times (SRT; ms) and trajectory curvature (degrees)
| Distractor | Target | Younger
|
Older
|
||
|---|---|---|---|---|---|
| SRT | Curvature | SRT | Curvature | ||
| Early | Unimodal | 224.29 (9.20) | −0.013 (0.006) | 268.95 (11.65) | −0.007 (0.003) |
| Bimodal | 210.29 (7.03) | −0.025 (0.007) | 239.79 (10.77) | −0.017 (0.006) | |
| Late | Unimodal | 255.36 (7.66) | −0.016 (0.007) | 293.98 (14.39) | −0.002 (0.002) |
| Bimodal | 234.43 (6.92) | −0.015 (0.006) | 254.27 (12.67) | 0.001 (0.004) | |
Note: standard errors are given in parentheses
Saccadic reaction time
To examine the effects of the sound and distractor-onset manipulations on the SRTs of younger and older adults, SRTs were first submitted to a 2 (age) × 2 (distractor onset) × 2 (sound) mixed analysis of variance (ANOVA). Participants were faster to move their eyes when the distractor appeared before the target rather than afterwards, F(1,26) = 70.17, MSE = 15,841.29, p < 0.001, possibly because of warning effects in the early distractor condition (Ross and Ross 1980; Taylor et al. 1998). Furthermore, SRTs were faster when the target was bimodal rather than unimodal, F(1,26) = 136.92, MSE = 18,876.04, p < 0.001. Overall, older adults were slower to move their eyes than younger adults, F(1,26) = 5.56, MSE = 30,889.29, p < 0.05, although their SRTs were speeded to a greater extent by bimodal targets than those of younger adults, F(1,26) = 14.67, MSE = 2023.00, p < 0.01. Furthermore, the sound benefited both groups’ performance more in the late distractor condition than the early distractor condition, F(1,26) = 5.53, MSE = 531.57, p < 0.05, possibly because SRTs were slower in this condition and thus, allowed more room for improvement by the sound. None of the other interactions reached significance, Fs < 2.
Saccadic trajectory deviations
Trajectory curvature was also submitted to a 2 (age) × 2 (distractor onset) × 2 (sound) mixed ANOVA (see, Fig. 3). There was a trend toward a main effect of age, F(1,26) = 2.88, MSE = 0.003, p = 0.10, and participants’ trajectory deviations were affected by the timing of the distractor onset, F(1,26) = 10.45, MSE = 0.002, p < 0.01. Furthermore, there was a trend toward an interaction between age and distractor onset, F(1,26) = 2.88, MSE = 0.001, p = 0.10. Because of the findings of Campbell et al. (2009), planned comparisons were conducted and these showed that although older and younger adults demonstrated similar deviations away from the distractor when it appeared before the target, t(26) = 0.92, only younger adults continued to deviate away from the distractor when it appeared after the target, t(26) = 2.37, p < 0.05. This latter effect is consistent with the age-related reductions in deviations away reported by Campbell et al.
Fig. 3.
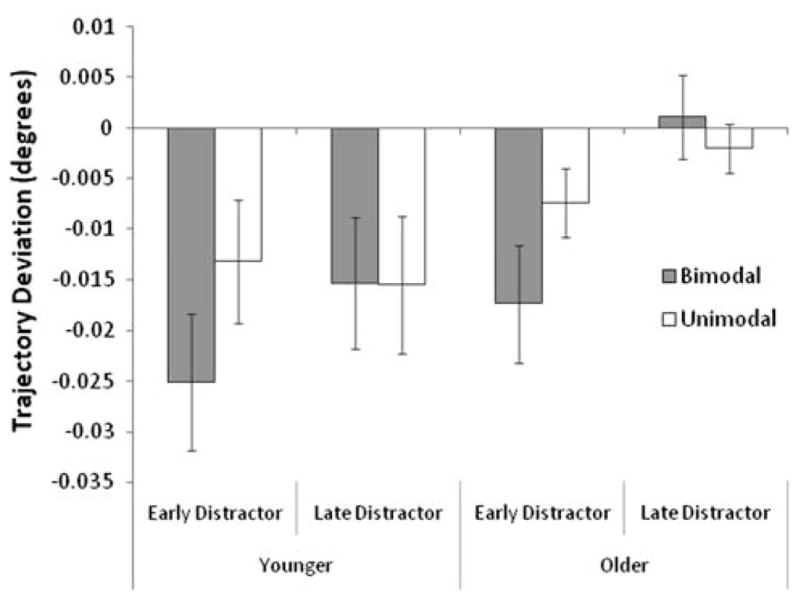
Mean trajectory curvature (degrees) for older and younger adults across sound and distractor-onset manipulations. Error bars represent standard errors of the mean
Turning to the main purpose of our study, determining if bimodal targets would lead to greater deviations away from distractors and whether this effect would be greater in the older group, the main effect of sound was only marginally significant, F(1,26) = 3.58, MSE = 0.001, p = 0.07. Importantly, however, there was a significant interaction between sound and distractor onset, F(1,26) = 5.78, MSE = 0.001, p < 0.05. As can be seen in Fig. 3, bimodal targets led to greater deviations away in the early distractor condition, t(27) = 3.11, p < 0.01, but not in the late distractor condition, t(27) = 0.47. Furthermore, the magnitude of this effect was similar for both age groups, as the three-way interaction between age, sound, and distractor onset was not significant, F < 1.
Discussion
The present study had a twofold purpose. The first purpose was to determine if the multisensory integration that occurs with bimodal saccade targets allows irrelevant visual distractors to be more effectively inhibited, which in turn leads to greater saccadic trajectory deviations away from their locations. The second purpose examined whether age-related differences in trajectory deviations could be ameliorated by bimodal targets, as older adults tend to benefit more from multisensory integration than younger adults. Results showed that participants’ trajectory deviations were affected by the sound manipulation, but only when the distractor preceded the target. Bimodal targets produced larger deviations away than unimodal targets and, contrary to our prediction, they did not differentially affect older adults. These effects can be contrasted, however, with those seen for SRTs which, in accordance with previous research (Diederich et al. 2008), were speeded to a greater extent by bimodal targets in the older group. Older adults in our study were also greatly affected by the distractor-onset manipulation, showing deviations away in the early distractor condition that were of similar magnitude to those of younger adults. This was in contrast to the late distractor condition (where the distractor appeared after the target) for which older adults no longer deviated away from the distractor while younger adults continued to do so.
The main finding of this study is that people show greater deviations away from the distractor when the target is accompanied by a spatially compatible tone. Neurophysiological work with cats (Meredith and Stein 1986) and monkeys (Wallace et al. 1996) has shown that some neurons within the SC respond maximally to multisensory stimuli, particularly when the individual stimuli themselves (e.g., a light and a sound) are weak. These neurons, in turn, project to premotor output neurons in the deep layers of the SC which directly affect orienting responses, such as saccadic eye movements (Wallace et al. 1993). Numerous studies with humans have shown that SRTs to visual targets are significantly faster when accompanied by auditory stimuli in close spatial and temporal contiguity (for a review, see Colonius and Diederich 2004). Although the precise mechanisms underlying this effect remain unknown (Colonius and Diederich 2004; Pouget et al. 2002), it is thought that greater activation in the SC in response to bimodal targets leads to inhibition of omnipause neurons in the brainstem which normally serve to maintain fixation (Munoz and Wurtz 1993). To our knowledge, the present study is the first to show that trajectory deviations away from distractors can also be affected by bimodal targets, potentially because of similar enhancements to saccade goal activity within the oculomotor map of the SC. Intriguingly, projections from the FEFs have been shown to preferentially target multisensory neurons within the SC (Meredith 1999), suggesting a potential interface for multisensory interactions in the production of trajectory deviations. Importantly, while bimodal targets did lead to greater top–down inhibition of irrelevant distractors, we only observed this effect in the early distractor condition. This difference may be attributable to the timing of the distractor, although it may also be attributable to the latencies of the saccades (saccade latencies in the late-onset condition were longer than in the early-onset condition). Nevertheless, the results of the present study clearly demonstrate that multisensory integration is capable of increasing saccadic trajectory deviations away from distractors.
While our results show that strengthening the target signal with a coincident tone can lead to an increase in deviations away from distractors, a recent study by van Zoest et al. (2008) found the opposite effect; weaker deviations away for stronger target signals. In that study, when the strength of the stimulus-driven target signal was increased by presenting a visual stimulus at the target location (rather than having participants perform antisaccades or memory-guided saccades), deviations away from distractors were reduced. However, in both the antisaccade and memory-guided saccade conditions of that study, participants had knowledge of the target location for greater amounts of time before saccade onset than in the prosaccade condition (due to longer SRTs in the antisaccade condition, and a 500 ms preview in the memory-guided saccade condition). Knowledge of the target location for greater amounts of time may have allowed for a stronger internal representation of the target which, despite the lack of an external target stimulus, may have bolstered top–down inhibition of the distractor location in a similar manner to the multisensory targets used in our study. Thus, while the question of how target strength impacts upon trajectory deviations remains unresolved, our results suggest that any advance knowledge of either the target or distractor location should bolster top–down inhibition of the distractor, resulting in greater deviations away.
Based on previous research showing enhanced multisensory integration with age (Laurienti et al. 2006; Peiffer et al. 2007; Diederich et al. 2008), we predicted that older adults’ trajectory deviations would be more greatly affected by bimodal targets than those of younger adults. This did not prove to be the case. While older adults did show a greater gain in SRT than younger adults, there were no age differences in how trajectory deviations were affected by the sound. With respect to reaction times, greater multisensory integration among older adults is thought to be due to either differences in baseline neural activity or impaired sensory processing (Diederich et al. 2008; Hugenschmidt et al. 2009). As previously discussed, weaker peripheral signals can lead to even greater neural activity in response to multi-sensory stimuli (Meredith and Stein 1986; Frens et al. 1995) and thus, older adults’ enhanced integration could be a result of their poorer peripheral processing. It is unclear why similar benefits would not be afforded to older adults’ trajectory deviations. One thing to note is that SRTs and trajectory deviations away tend to have an inverse relationship: the faster the eye movement, the less it deviates away from a distractor (McSorley et al. 2006). In this study, older adults were speeded to a greater extent by bimodal targets, and this may have reduced the magnitude of their deviations. Their overall SRTs remained, however, longer than the younger adults’ and the older adults consistently showed less deviation in the unimodal condition.
While a recent study demonstrated an inability on the part of older adults to generate deviations away from distractors across a range of saccadic latencies (Campbell et al. 2009), older participants in the present study managed to show deviations away when the distractor preceded the target. Younger adults, on the other hand, showed significant deviations away from the distractor in both the early and late distractor conditions, demonstrating that deviations away from the late distractor were possible within this paradigm. This raises the question: why were older adults only able to deviate away in the early distractor condition? Perhaps they benefited from having more time to inhibit the distractor prior to target onset. In support of this possibility, aging research on inhibition of return (IOR), the phenomenon whereby a target is detected more slowly if it appears in a previously attended location, has shown that the onset of IOR is delayed in older adults (Castel et al. 2003), suggesting that even when inhibition is applied successfully, it tends to be more sluggish in the elderly (Gazzaley et al. 2008).
In addition to time, the present results suggest another criterion for successful inhibition: that the to-be-inhibited distractor appears in isolation first before the onset of a target. In the study by Campbell et al., the target and distractor appeared at exactly the same time and thus, older adults may have been unable to simultaneously dampen distractor-related activity and select the correct saccade target signal, even when they took a long time (~375 ms) to move their eyes. Importantly, in the bimodal early-onset condition of the present study, the average latency between distractor onset and saccade initiation was only 344 ms (SRT plus a 100 ms SOA) for older adults, and yet deviations away were produced. If it were only a matter of having enough time, this result suggests that older adults in the study by Campbell et al. should have shown deviations away at very long saccadic latencies, yet they did not. Therefore, it may be that older adults need to observe the distractor in isolation for it to be successfully inhibited.1
Although older adults’ trajectory deviations were not differentially affected by the sound, both older adults and younger adults did show greater deviations away in the bimodal condition, at least for early distractors. Thus, multisensory integration provides a useful means for increasing top–down inhibition of irrelevant distraction. Taken together with the RT findings from both this study and others (e.g., Laurienti et al. 2006; Peiffer et al. 2007; Diederich et al. 2008), these results illustrate the potential benefits that can be afforded to older adults’ performance by providing them with multisensory cues. Recent applied work demonstrates the potential value of multisensory enhancement, such as in-car warning signals that improve braking time (Ho et al 2007; Spence and Ho 2008) and handrails that use audio–visual cues to improve balance control in older adults (Maki et al. 2008). Given evidence of greater distractibility shown by older adults (e.g., Healey et al. 2008), the current study suggests another interesting direction for applied work: that is, exploring how multisensory targets can decrease the influence of irrelevant distraction on older adults’ performance across a wide range of tasks.
Acknowledgments
This work was supported by Natural Sciences and Engineering Council of Canada Grant 482547 to Jay Pratt and by Canadian Institutes of Health Research Grant MOP89769 and U.S. National Institute on Aging Grant R37 AGO4306 to Lynn Hasher.
Footnotes
Such a conclusion, however, is qualified by additional differences between these experiments. For example, targets and distractors were much closer together in the study of Campbell et al. than in the present paper.
Contributor Information
Karen Lucia Campbell, Department of Psychology, University of Toronto, 100 St. George Street, Toronto, ON M5S 3G3, Canada, Rotman Research Institute, Baycrest Centre, Toronto, Canada.
Naseem Al-Aidroos, Department of Psychology, University of Toronto, 100 St. George Street, Toronto, ON M5S 3G3, Canada.
Robert Fatt, Department of Psychology, University of Toronto, 100 St. George Street, Toronto, ON M5S 3G3, Canada.
Jay Pratt, Department of Psychology, University of Toronto, 100 St. George Street, Toronto, ON M5S 3G3, Canada.
Lynn Hasher, Department of Psychology, University of Toronto, 100 St. George Street, Toronto, ON M5S 3G3, Canada, Rotman Research Institute, Baycrest Centre, Toronto, Canada.
References
- Al-Aidroos N, Pratt J. Top–down control in time and space: evidence from saccadic latencies and trajectories. Vis Cogn. 2009 doi: 10.1080/13506280802456939. [DOI] [Google Scholar]
- Becker W. The control of eye movements in the saccadic system. In: Dichgans J, Bizzi E, editors. Cerebral control of eye movements. Karger; New York: 1972. pp. 308–316. [Google Scholar]
- Campbell KL, Ryan JD. The effects of practice and external support on older adults’ control of reflexive eye movements. Aging Neuropsychol Cogn. 2009 doi: 10.1080/13825580902926846. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Al-Aidroos N, Pratt J, Hasher L. Repelling the young and attracting the old: examining age-related differences in saccadic trajectory deviations. Psychol Aging. 2009;24:163–168. doi: 10.1037/a0014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassavaugh ND, Kramer AF, Irwin DE. Influence of task-irrelevant onset distractors on the visual search performance of young and old adults. Aging Neuropsychol Cogn. 2003;10:44–60. [Google Scholar]
- Castel AD, Chasteen AL, Scialfa CT, Pratt J. Adult age differences in the time course of inhibition of return. J Gerontol Psychol Sci. 2003;58B:256–259. doi: 10.1093/geronb/58.5.p256. [DOI] [PubMed] [Google Scholar]
- Colcombe AM, Kramer AF, Irwin DE, Peterson MS, Colcombe S, Hahn S. Age-related effects of attentional and oculomotor capture by onsets and color singletons as a function of experience. Acta Psychol. 2003;113:205–225. doi: 10.1016/s0001-6918(03)00019-2. [DOI] [PubMed] [Google Scholar]
- Colonius H, Diederich A. Multisensory interaction in saccadic reaction time: a time-of-window-of-integration model. J Cogn Neurosci. 2004;16:1000–1009. doi: 10.1162/0898929041502733. [DOI] [PubMed] [Google Scholar]
- Diederich A, Colonius H, Schomburg A. Assessing age-related multisensory enhancement with the time-window-of-integration model. Neuropsychologia. 2008;46:2556–2562. doi: 10.1016/j.neuropsychologia.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Doyle MC, Walker R. Multisensory interactions in saccade target selection: curved saccade trajectories. Exp Brain Res. 2002;142:116–130. doi: 10.1007/s00221-001-0919-2. [DOI] [PubMed] [Google Scholar]
- Frens MA, Van Opstal AJ, Van der Willigen RF. Spatial and temporal factors determine auditory-visual interactions to human saccade eye movements. Percept Psychophys. 1995;57:802–816. doi: 10.3758/bf03206796. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D’Esposito M. Age-related top–down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godijn R, Theeuwes J. Programming of endogenous and exogenous saccades: evidence for a competitive integration model. J Exp Psychol Hum Percept Perform. 2002;28:1039–1054. doi: 10.1037//0096-1523.28.5.1039. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. In: Gopher D, Koriat A, editors. Attention and performance, XVII. MIT Press; Cambridge, MA: 1999. pp. 653–675. [Google Scholar]
- Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: costs and potential benefits. In: Sossin WS, Lacaille J-C, Castellucci VF, Belleville S, editors. Progress in brain research. Vol. 169. Elsevier; Amsterdam: 2008. pp. 353–363. [DOI] [PubMed] [Google Scholar]
- Ho C, Reed N, Spence C. Multisensory in-car warning signals for collision avoidance. Hum Factors. 2007;49:1107–1114. doi: 10.1518/001872007X249965. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Mozolic JL, Laurienti PJ. Suppression of multisensory integration by modality-specific attention in aging. NeuroReport. 2009;20:349–353. doi: 10.1097/WNR.0b013e328323ab07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Irwin DE, Theeuwes J. Attentional capture and aging: implications for visual search performance and oculo-motor control. Psychol Aging. 1999;14:135–154. doi: 10.1037//0882-7974.14.1.135. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Irwin DE, Theeuwes J. Age differences in the control of looking behavior: do you know where your eyes have been? Psychol Sci. 2000;11:210–217. doi: 10.1111/1467-9280.00243. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Burdette JH, Maldjian JA, Wallace MT. Enhanced multisensory integration in older adults. Neurobiol Aging. 2006;27:1155–1163. doi: 10.1016/j.neurobiolaging.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Ludwig CJH, Gilchrist ID. Measuring saccade curvature: a curve-fitting approach. Behav Res Methods Instrum Comput. 2002;34(4):618–624. doi: 10.3758/bf03195490. [DOI] [PubMed] [Google Scholar]
- Ludwig CJH, Gilchrist ID. Target similarity affects saccade curvature away from irrelevant onsets. Exp Brain Res. 2003;152:60–69. doi: 10.1007/s00221-003-1520-7. [DOI] [PubMed] [Google Scholar]
- Maki BE, Perry SD, Scovil CY, Peters AL, McKay SM, Lee TA, Corbeil P, Fernie GR, McIlroy WE. Interventions to promote more effective balance-recovery reactions in industrial settings: new perspectives on footwear and handrails. Ind Health. 2008;46:40–50. doi: 10.2486/indhealth.46.40. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Short-term priming, concurrent processing, and saccade curvature during a target selection task in the monkey. Vis Res. 2001;41:785–800. doi: 10.1016/s0042-6989(00)00287-x. [DOI] [PubMed] [Google Scholar]
- McSorley E, Haggard P, Walker R. Time course of oculomotor inhibition revealed by saccade trajectory modulation. J Neurophysiol. 2006;96:1420–1424. doi: 10.1152/jn.00315.2006. [DOI] [PubMed] [Google Scholar]
- Meredith AM. The frontal eye fields target multisensory neurons in the cat superior colliculus. Exp Brain Res. 1999;128:460–470. doi: 10.1007/s002210050869. [DOI] [PubMed] [Google Scholar]
- Meredith AM, Stein BE. Visual, auditory, and somatosensory convergence on cells in superior colliculus result in multisensory integration. J Neurophysiol. 1986;56:640–662. doi: 10.1152/jn.1986.56.3.640. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RB. Fixation cells in monkey superior colliculus: II. Reversible activation and deactivation. J Neurophysiol. 1993;70:576–589. doi: 10.1152/jn.1993.70.2.576. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Mozolic JL, Hugenschmidt CE, Laurienti PJ. Age-related multisensory enhancement in a simple audiovisual detection task. Neuroreport. 2007;18:1077–1081. doi: 10.1097/WNR.0b013e3281e72ae7. [DOI] [PubMed] [Google Scholar]
- Port NL, Wurtz RH. Sequential activity of simultaneously recorded neurons in the superior colliculus during curved saccades. J Neurophysiol. 2003;79:1887–1903. doi: 10.1152/jn.01151.2002. [DOI] [PubMed] [Google Scholar]
- Pouget A, Deneve S, Duhamel J. A computational perspective on the neural basis of multisensory spatial representations. Nat Rev Neurosci. 2002;3:741–747. doi: 10.1038/nrn914. [DOI] [PubMed] [Google Scholar]
- Quaia C, Lefèvre P, Optican LM. Model of the control of saccades by superior colliculus and cerebellum. J Neurophysiol. 1999;82:999–1018. doi: 10.1152/jn.1999.82.2.999. [DOI] [PubMed] [Google Scholar]
- Ross LE, Ross SM. Saccade latency and warning signals: stimulus onset, offset, and change as warning effects. Percept Psychophys. 1980;27(3):251–257. doi: 10.3758/bf03204262. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Leung G, Turk-Browne NB, Hasher L. Assessment of age-related changes in inhibition and binding using eye movement monitoring. Psychol Aging. 2007;22:239–250. doi: 10.1037/0882-7974.22.2.239. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Schlag J, Dassonville P. How the frontal eye field can impose a saccade goal on superior colliculus neurons. J Neurophysiol. 1992;67:1003–1005. doi: 10.1152/jn.1992.67.4.1003. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Craighero L, Rizzolatti G. Spatial attention-determined modifications in saccade trajectories. NeuroReport. 1995a;6:585–588. doi: 10.1097/00001756-199502000-00044. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G. Spatial attention and eye movements. Exp Brain Res. 1995b;105:261–275. doi: 10.1007/BF00240962. [DOI] [PubMed] [Google Scholar]
- Shipley WC. Institute of living scale. Western Psychological Services; Los Angeles: 1946. [Google Scholar]
- Spence C, Ho C. Multisensory warning signals for event perception and safe driving. Theor Issues Ergon Sci. 2008;9:523–554. [Google Scholar]
- Taylor T, Kingstone AF, Klein RM. Visual offsets and oculomotor disinhibition: endogenous contributions to the gap effect. Can J Exp Psychol. 1998;52:192–200. [Google Scholar]
- Tipper SP, Howard LA, Paul MA. Reaching affects saccade trajectories. Exp Brain Res. 2001;136:241–249. doi: 10.1007/s002210000577. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S, Theeuwes J. Relation between saccade trajectories and spatial distractor locations. Cogn Brain Res. 2005;25:579–582. doi: 10.1016/j.cogbrainres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S, Meeter M, Theeuwes J. Eye movement trajectories and what they tell us. Neurosci Biobehav Rev. 2006;30:666–679. doi: 10.1016/j.neubiorev.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S, Meeter M, Theeuwes J. The spatial coding of the inhibition evoked by distractors. Vis Res. 2007;47:210–218. doi: 10.1016/j.visres.2006.11.001. [DOI] [PubMed] [Google Scholar]
- van Zoest W, Van der Stigchel S, Barton JJS. Distractor effects on saccade trajectories: a comparison of prosaccades, antisaccades, and memory-guided saccades. Exp Brain Res. 2008;186:431–442. doi: 10.1007/s00221-007-1243-2. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Converging influences from visual, auditory, and somatosensory cortices onto output neurons of the superior colliculus. J Neurophysiol. 1993;69:1797–1809. doi: 10.1152/jn.1993.69.6.1797. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Wilkinson LK, Stein BE. Representation and integration of multiple sensory inputs in primate superior colliculus. J Neurophysiol. 1996;76:1246–1266. doi: 10.1152/jn.1996.76.2.1246. [DOI] [PubMed] [Google Scholar]