Abstract
AIM: To compare resistin mRNA expression in subcutaneous adipose tissue (SAT) and its correlation with insulin resistance (IR) in postmenopausal obese women.
METHODS: A total of 68 postmenopausal women (non obese = 34 and obese = 34) were enrolled for the study. The women of the two groups were age matched (49-70 years). Fasting blood samples were collected at admission and abdominal SAT was obtained during surgery for gall bladder stones or hysterectomy. Physical parameters [age, height, weight, body mass index (BMI)] were measured. Biochemical (plasma insulin and plasma glucose) parameters were estimated by enzymatic methods. RNA was isolated by the Trizol method. SAT resistin mRNA expression was done by real time- reverse transcription polymerase chain reaction (RT-PCR) by using Quanti Tect SYBR Green RT-PCR master mix. Data was analyzed using independent Student’s t test, correlation and simple linear regression analysis.
RESULTS: The mean weight (52.81 ± 8.04 kg vs 79.56 ± 9.91 kg; P < 0.001), BMI (20.23 ± 3.05 kg/m2 vs 32.19 ± 4.86 kg/m2; P < 0.001), insulin (8.47 ± 3.24 μU/mL vs 14.67 ± 2.18 μU/mL; P < 0.001), glucose (97.44 ± 11.31 mg/dL vs 109.67 ± 8.02 mg/dL; P < 0.001) and homeostasis model assessment index (2.01 ± 0.73 vs 3.96 ± 0.61; P < 0.001) were significantly higher in postmenopausal obese women compared to postmenopausal non obese women. The mean serum resistin level was also significantly higher in postmenopausal obese women compared to postmenopausal non obese women (9.05 ± 5.15 vs 13.92 ± 6.32, P < 0.001). Furthermore, the mean SAT resistin mRNA expression was also significantly (0.023 ± 0.008 vs 0.036 ± 0.009; P < 0.001) higher and over expressed 1.62 fold (up-regulated) in postmenopausal obese women compared to postmenopausal non obese women. In postmenopausal obese women, the relative SAT resistin mRNA expression showed positive (direct) and significant correlation with BMI (r = 0.78, P < 0.001) and serum resistin (r = 0.76, P < 0.001). Furthermore, the SAT resistin mRNA expression in postmenopausal obese women also showed significant and direct association (r = 0.45, P < 0.01) with IR, while in postmenopausal non obese women it did not show any association (r = -0.04, P > 0.05).
CONCLUSION: Increased SAT resistin mRNA expression probably leads to inducing insulin resistance and thus may be associated with obesity-related disorders in postmenopausal obese women.
Keywords: Resistin, Subcutaneous adipose tissue, Insulin resistance, Obesity, Body mass index
INTRODUCTION
Obesity is one of the most common metabolic disorders in developing countries and is characterized by a reduction in insulin sensitivity, both in animal models and in humans[1]. The incidence of obesity worldwide has increased drastically during recent decades. Consequently, obesity and associated disorders now constitute a serious threat to the current and future health of the population. The World Health Organization (WHO) estimates that more than 1 billion adults worldwide are overweight, 300 million of whom are clinically obese, defined as having a body mass index (BMI) equal to or greater than 30 kg/m2[2]. Obesity is associated with an array of additional health problems, including increased risk of insulin resistance, type 2 diabetes (T2D), fatty liver disease, atherosclerosis, degenerative disorders including dementia, airway disease and some cancers[3]. The molecular mechanisms involved in obesity-related insulin resistance are not yet well understood.
Postmenopausal obese women may be at increased risk for metabolic syndrome (MetS) because of the increase in total and central adiposity after menopause transition[4]. The emergence of these risk factors may be a direct result of ovarian failure or alternatively an indirect result of metabolic consequences of central fat redistribution with estrogen deficiency[5]. Adipose tissue is an active metabolic tissue that secretes multiple metabolically important proteins known as “adipokines” (leptin, adiponectin, tumor necrosis factor (TNF)-α, interleukin (IL)-6 and resistin etc.)[6,7]. These adipocyte derived products are presently subject to intensive research concerning their involvement in the regulation of adipose tissue physiology and in particular their potential implication in insulin resistance (IR), obesity and diabetes[8,9].
Plasma concentration of resistin is elevated in obesity compared to a healthy person. In humans and rodents, plasma resistin concentrations are positively correlated with BMI[10]. It is well documented that accumulation of visceral fat is associated with a higher risk for development of obesity-related diseases such as T2D, cardiovascular disease, hypertension and hyperlipidemia[11,12]. However, the role of subcutaneous adipose tissue (SAT) dysregulation could be a potential mechanism but its role in the pathogenesis of MetS has been conflicting[13]. The SAT, which comprises approximately 80% of adipose tissue and the major source of fatty acids for the liver, has metabolically correlated to indices of insulin resistance as well as to visceral adipose tissue (VAT)[14-17].
In addition to intra-abdominal fat, Salmenniemi et al[18] have shown that the amount of SAT in subjects with MetS positively correlates with increasing MetS and negatively correlates with circulating adiponectin levels. Carr et al[19] have also reported that SAT is significantly associated with MetS and increases with increasing number of MetS features, independent of age and sex.
Several studies have shown that both VAT and SAT are associated with adverse cardio metabolic risk factors[20,21]. Regardless of these observations and continuing debate regarding metabolic importance of VAT and SAT, it appears that both contribute significantly to metabolic and cardiovascular risks. However, due to higher mass of SAT than VAT, it may affect metabolic factors more significantly[22] and thus may have more clinical importance. Furthermore, subcutaneous adipocytes are larger than omental, have higher lipoprotein lipase activity and are more lipolytic on an absolute basis, which reflect a higher fat storage capacity in this depot, especially in women[23].
Recently, a significant link between SAT and dyslipidemia in patients with T2D was reported[24]. Visceral and subcutaneous adipocytes have different capacities to produce hormones and enzymes with variation in mRNA expressions of adipokines (resistin, adiponectin, TNF-α, IL-6 etc.) from various depots of adipose tissue[25].
In an animal study, expression of resistin in subcutaneous adipose tissue was controversial[26]. In humans, data on resistin is conflicting. Some authors have shown that it is elevated in individuals with obesity and diabetes[27-29], whereas others have shown that it is not elevated[30-32]. A recent study reported that human resistin induced insulin resistance and Metformin reversed the effect of resistin in hepatocytes[33]. Taken together, these studies suggest that the depot-specific expression of resistin at the mRNA level could be relevant to insulin sensitivity.
The literature regarding the SAT resistin expression in Asian populations, especially Indians, is lacking. Thus, with best our knowledge, the present study was undertaken with the objective to investigate SAT resistin mRNA expression in postmenopausal Indian women and its association with insulin resistance.
MATERIALS AND METHODS
Subjects
A total of 68 postmenopausal (non obese = 34 and obese = 34) age matched (49-70 years) women were recruited at CSM Medical University, Lucknow, India who underwent elective abdominal surgery for gall bladder stones or hysterectomy. Respective abdominal SAT was obtained during the surgery. During surgery, no specific standard diet and hormonal therapy were given to the patients, which ensured and ruled out the effect of hormones or diet on fat deposition. All tissue samples were stored in RNAlatar (Sigma-Aldrich) for RNA extraction. The study was approved by the Institutional Ethics Committee. Subjects were classified as obese according to BMI > 25 kg/m2, as per WHO’s guideline for Asians[34], and BMI < 25 kg/m2 as non obese served as control. Informed consent was obtained from each patient.
Biochemical estimation
Blood samples were taken the morning after their admission to the hospital for surgery. Plasma insulin concentrations were determined using immune radiometric assay (immunotech). Plasma glucose concentrations were determined by glucose oxidase-peroxidase method (Merck) using semi automated glucose analyzer (Microlab 300, Merck). Serum resistin level was measured by enzyme-linked immunosorbent assay (Quantikine Human Resistin Version 16 190607 15, Biovendor).
Calculation: Insulin resistance
Homeostasis model assessment (HOMA), an index of IR[35] based on plasma levels of fasting glucose and insulin, has been widely applied for quantifying insulin resistance and β-cell function in an Asian population was evaluated as [HOMA-IR = fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5].
RNA extraction
Total RNA was isolated using Tri-Reagent (Sigma Chemical Co., St. Louis, MO). RNA was measured spectrophotometrically at 260 and 280 nm, while RNA integrity was checked by visual inspection of the two ribosomal RNAs 18S and 28S on agarose gel.
Real-time polymerase chain reaction measurement of resistin mRNA
One-step reverse transcription polymerase chain reaction (RT-PCR) was carried out using Quanti Tect SYBR Green RT-PCR master mix kit (Qiagen). PCR amplification was carried out in Light Cycler 480 (Roche, real time thermal cycler). Ninety-six well PCR plate using following temperature profile of 50 °C, 30 min, (Reverse transcription) 95 °C, 15 min, (initial denaturation) followed by 40 cycles of 94 °C, 15 s, 59 °C, 30 s and 72 °C, 30 s for denaturation, annealing and extension steps respectively. Primer sequence of human resistin was 5’-GCTGTTGGTGTCTAGCAAGAC-3’ (forward) 5’-CATCATCATCATCATCTCCAG-3’ (reverse). The following primer sequence of β-actin as internal control with following sequence was 5’-GTGGCATCCACGAAACTACCTT-3’ (forward) and 5’-GGACTCCTGATACTCCTGCTTG-3’ (reverse). The PCR primers were synthesized by Agile Life Science Technologies India. Expression of glyceraldehyde-3-phosphate dehydrogenase or β-actin was used to normalize resistin expression values. There was no difference in glyceraldehyde-3-phosphate dehydrogenase or β-actin expression between adipocytes from non obese and obese subjects or between the omental and subcutaneous depots.
Statistical analysis
Data were expressed as mean ± SD. Anthropometric measurements and biochemical parameters and SAT resistin mRNA expression of the two independent groups were compared by Student’s t test. Correlation and simple linear regression analysis was done to assess association of resistin SAT mRNA expressions with BMI and HOMA index, considering BMI and HOMA index an independent variable and SAT resistin mRNA expression, the dependent variable. A two-sided (α = 2) conventional P < 0.05 was considered statistically significant.
RESULTS
Basic characteristics
The basic characteristics viz. physical (age, weight, height and BMI) and biochemical parameters (insulin, glucose and HOMA index) of the two groups are summarized in Table 1. Table 1 shows that the mean weight, BMI, insulin, glucose and HOMA index were significantly (P < 0.001) higher in the obese compared to non obese. However, the age and height were found to be similar (P > 0.05) between the two groups, indicating the subjects of two groups were age matched.
Table 1.
Physical and biochemical parameters summary (mean ± SD) of two groups (n = 34)
| Variables | Non obese | Obese | P value |
| Age (yr) | 54.94 ± 6.87 | 54.06 ± 6.71 | 0.594 |
| Weight (kg) | 52.81 ± 8.04 | 79.56 ± 9.91 | < 0.001 |
| Height (cm) | 161.76 ± 8.17 | 157.74 ± 9.09 | 0.059 |
| BMI (kg/m2) | 20.23 ± 3.05 | 32.19 ± 4.86 | < 0.001 |
| Insulin (µU/mL) | 8.47 ± 3.24 | 14.67 ± 2.18 | < 0.001 |
| Glucose (mg/dL) | 97.44 ± 11.31 | 109.67 ± 8.02 | < 0.001 |
| HOMA | 2.01 ± 0.73 | 3.96 ± 0.61 | < 0.001 |
BMI: Body mass index; HOMA: Homeostasis model assessment.
Resistin mRNA expression in SAT
The SAT resistin mRNA expression of non obese and obese is summarized graphically in Figure 1. Figure 1 shows that the mean SAT resistin mRNA expression of obese was significantly higher compared to non obese (0.023 ± 0.008 vs 0.036 ± 0.009, P < 0.001). The relative mean SAT resistin mRNA expression up regulated 1.62 fold (56.1%) in the obese than non obese. Furthermore, mean serum resistin level were also significantly higher in obese compared to non obese (9.05 ± 5.15 vs 13.92 ± 6.32, P < 0.001) (Figure 2).
Figure 1.
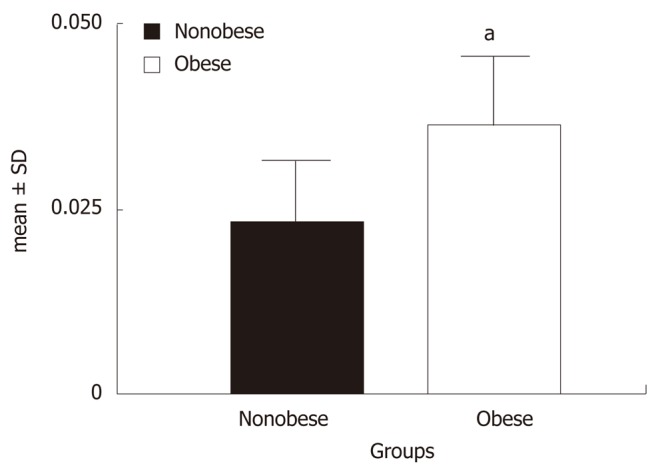
Relative subcutaneous adipose tissue resistin mRNA expressions of two groups. aP < 0.001 vs non obese (by Student’s t test).
Figure 2.
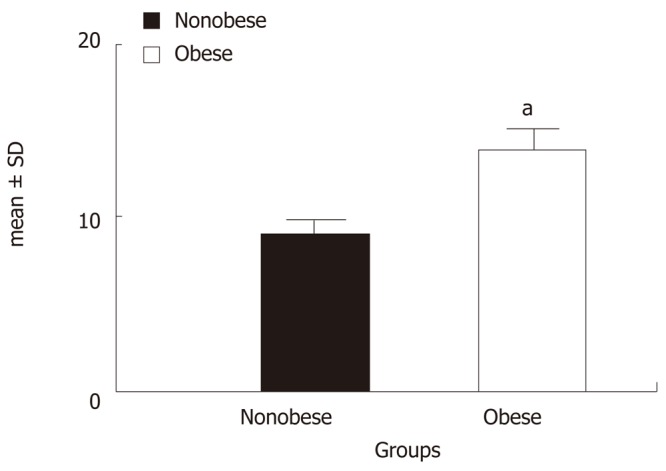
Mean serum resistin levels (ng/mL) of two groups. aP < 0.001 vs non obese (by Student’s t test).
Correlations of resistin mRNA expression with BMI, serum resistin and insulin resistance
The association of relative SAT resistin mRNA expression of obese women with their respective BMI, serum resistin and insulin resistance (HOMA) are shown graphically in Figure 3. Figure 3 shows that the relative SAT resistin mRNA expression positively (directly) and significantly correlated with BMI (r = 0.78, P < 0.001), HOMA (r = 0.45, P < 0.01) and serum resistin (r = 0.76, P < 0.001).
Figure 3.
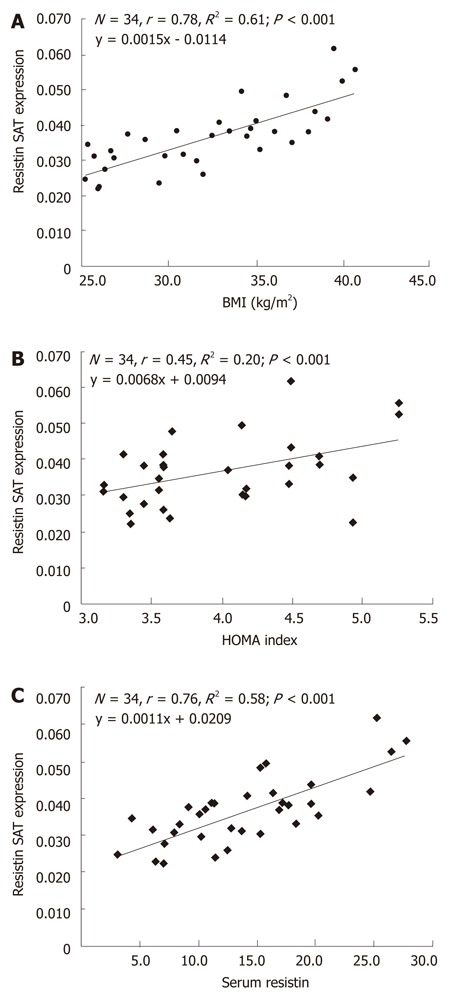
Correlation and simple linear regression of subcutaneous adipose tissue resistin mRNA expression with body mass index (A), homeostasis model assessment index (B) and serum resistin levels (C) in postmenopausal obese women. HOMA: Homeostasis model assessment; BMI: Body mass index.
DISCUSSION
The physiological functions of resistin in humans have been mostly investigated at the levels of DNA polymorphisms and plasma proteins[36,37]. However, only a few studies have tried to relate the resistin mRNA levels to some physiological functions, such as obesity, diabetes and insulin sensitivity[29,38]. Furthermore, most of the mRNA studies consist of small sample sizes with controversial findings. Although the limitation of sample size prevented these mRNA reports from more comprehensive analyses compared with those studies using plasma resistin, the value of these studies should not be overlooked.
The literature regarding SAT resistin mRNA expression in Asians, especially Indians, is lacking. With the best of our knowledge, for the first time, the present study evaluates SAT resistin mRNA expression in postmenopausal Indian women and its association with insulin resistance. In the present study, we found significantly higher SAT resistin mRNA expression in the obese compared to non obese. Our finding is in contrast with Baranova et al[39] who reported no difference in SAT resistin mRNA expression in the obese with and without insulin resistance. However, a few reports show decreased mRNA and protein expression in isolated subcutaneous and omental adipocytes[40,41].
Findings of resistin gene expression in humans are controversial. Some reports have shown mRNA or protein expression in human adipose tissue, while others have reported the absence/poor mRNA expression in this tissue[42,43]. Our findings corroborate with a cohort study by Smith et al[37] who reported higher expression in obese women. Moreover, a recent report showed higher resistin mRNA expression of both visceral and subcutaneous adipose tissues in obese than non obese women[25].
It was also shown that serum resistin had significant effects on insulin action, potentially linking obesity with IR[44]. Previous studies examined showed that serum resistin concentrations are raised in high-fat-induced obese and obese mice models[45]. It is well documented that administration of anti resistin antibody improves insulin action and glucose metabolism in mice with diet-induced obesity, suggesting a role for resistin in the development of insulin resistance[45]. In addition, a recent study showed that administration of recombinant resistin causes insulin resistance in mice[46]. It is also well documented that resistin decreases insulin-stimulated glucose uptake in 3T3-L1 adipocytes and the inhibitory effect is prevented by anti resistin antibody[45]. These findings strengthen the hypothesis that resistin may be a link between obesity and its associated risks.
In the present study, we also determined the level of serum resistin in obese and non obese postmenopausal women. Serum resistin was significantly higher in the obese than non obese. Our finding is in accordance with Degawan et al[29] who reported significantly higher resistin protein in the serum of obese than non obese. However, several studies showed that serum resistin level do not correlate well with markers of adiposity[8,31]. Savage et al[40] also reported no correlation between insulin resistance and resistin gene expression in whole abdominal adipose tissue. In contrast, we found positive and significant correlation of SAT resistin mRNA expression with BMI, HOMA and serum resistin levels in obese women. The finding is consistent with a recent finding which showed a strong correlation between serum resistin and resistin mRNA expression of abdominal SAT in the obese[30]. However, Yang et al[38] reported that resistin was undetectable in SAT and unrelated to insulin resistance. One study[29] reported higher SAT resistin expression in the obese and positive association with BMI but not related to insulin resistance. We hypothesized that the conflicting results regarding SAT resistin mRNA expression in humans may be due to race differences and environmental conditions.
In conclusion, we found higher and significant positive correlation of SAT resistin mRNA expression with serum resistin, BMI and insulin resistance (HOMA index), suggesting its potential role in development of insulin resistance and thus associated with obesity-related disorders. The findings of this study may have clinical implications but may be validated further on varied populations with large sample sizes.
ACKNOWLEDGMENTS
We thank Dr. Shailendra Kumar Yadav and Dr. Surendra Kumar for assistance with subject recruitment and the staff of Arushi Hospital, Lucknow. We also thank the patients who participated in the study and donated their valuable adipose tissue.
COMMENTS
Background
The incidence of obesity has dramatically increased in recent years. Consequently, disturbances in secretion of resistin caused by obesity have an influence on the development of metabolic complications. It regulates insulin sensitivity but the role of resistin in insulin sensitivity is not yet determined. Many studies have reported that resistin decreases insulin sensitivity in rat models but it is unclear in humans. The aim of the present study was to investigate the relationship of subcutaneous adipose tissue (SAT) resistin mRNA expression with body mass index (BMI), homeostasis model assessment (HOMA) and serum resistin in obese postmenopausal Indian women.
Research frontiers
Adipose tissue produces resistin which may play an influential role in energy homeostasis, triglyceride storage and mobilization of fat with increased adiposity, specifically central adiposity. Furthermore, it seems apparent that the pathogenesis of type 2 diabetes mellitus is mediated through the concurrent progression of insulin resistance and subclinical inflammation. The molecular mechanisms for this are less understood. The research hotspot is to establish the relevance of resistin to human diabetes, particularly its effects on the central nervous system and β-cell function.
Innovations and breakthroughs
Previous reports suggested that resistin secreted by visceral adipose tissue is responsible for development of obesity-related problems but we cannot ignore the role of subcutaneous adipose tissue. Most studies have been done in cultured cells but lack fresh adipose tissue. Furthermore, the literature regarding SAT resistin mRNA expression in Asians, especially Indians, is lacking. Our results showed a direct association of SAT resistin mRNA expression with BMI, HOMA and serum resistin levels in postmenopausal obese women.
Applications
The study suggests that resistin secreted by adipose tissue may be responsible for insulin resistance. Selective manipulation of adipocyte hormones offers a dimension to treat insulin resistance.
Terminology
Resistin is a member of a class of cysteine-rich proteins collectively termed resistin-like molecules. Subcutaneous adipose tissue: Most of the remaining non visceral fat is found just below the skin in a region called the hypodermis.
Peer review
The paper investigates SAT resistin mRNA expression in postmenopausal Indian women and its association with insulin resistance. It is well and clearly written.
Footnotes
Supported by Indian Council of Medical Research, New Delhi, India and Central Council Research in Yoga and Naturopathy, New Delhi, India, to Sadashiv and Tiwari S
Peer reviewer: Rajagopalan Sriraman, BSc, MD, MRCP, PhD, FRCP, Lincoln County Hospital, Greetwell Road, Lincoln LN2 5QY, United Kingdom
S- Editor Wu X L- Editor Roemmele A E- Editor Wu X
References
- 1.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145–1158. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. The World Health Report. Reducing Risks, Promoting Healthy Life. Geneva: 2002. [Google Scholar]
- 3.Nigro J, Osman N, Dart AM, Little PJ. Insulin resistance and atherosclerosis. Endocr Rev. 2006;27:242–259. doi: 10.1210/er.2005-0007. [DOI] [PubMed] [Google Scholar]
- 4.You T, Yang R, Lyles MF, Gong D, Nicklas BJ. Abdominal adipose tissue cytokine gene expression: relationship to obesity and metabolic risk factors. Am J Physiol Endocrinol Metab. 2005;288:E741–E747. doi: 10.1152/ajpendo.00419.2004. [DOI] [PubMed] [Google Scholar]
- 5.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 6.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 7.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 8.Silha JV, Krsek M, Skrha JV, Sucharda P, Nyomba BL, Murphy LJ. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol. 2003;149:331–335. doi: 10.1530/eje.0.1490331. [DOI] [PubMed] [Google Scholar]
- 9.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 10.Lu HL, Wang HW, Wen Y, Zhang MX, Lin HH. Roles of adipocyte derived hormone adiponectin and resistin in insulin resistance of type 2 diabetes. World J Gastroenterol. 2006;12:1747–1751. doi: 10.3748/wjg.v12.i11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson B, Bengtsson C, Björntorp P, Lapidus L, Sjöström L, Svärdsudd K, Tibblin G, Wedel H, Welin L, Wilhelmsen L. Is abdominal body fat distribution a major explanation for the sex difference in the incidence of myocardial infarction? The study of men born in 1913 and the study of women, Göteborg, Sweden. Am J Epidemiol. 1992;135:266–273. doi: 10.1093/oxfordjournals.aje.a116280. [DOI] [PubMed] [Google Scholar]
- 12.Poirier P, Després JP. Waist circumference, visceral obesity, and cardiovascular risk. J Cardiopulm Rehabil. 2003;23:161–169. doi: 10.1097/00008483-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 15.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Adams-Huet B, Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes. 1996;45:1684–1693. doi: 10.2337/diab.45.12.1684. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira I, Henry RM, Twisk JW, van Mechelen W, Kemper HC, Stehouwer CD. The metabolic syndrome, cardiopulmonary fitness, and subcutaneous trunk fat as independent determinants of arterial stiffness: the Amsterdam Growth and Health Longitudinal Study. Arch Intern Med. 2005;165:875–882. doi: 10.1001/archinte.165.8.875. [DOI] [PubMed] [Google Scholar]
- 18.Salmenniemi U, Ruotsalainen E, Pihlajamäki J, Vauhkonen I, Kainulainen S, Punnonen K, Vanninen E, Laakso M. Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation. 2004;110:3842–3848. doi: 10.1161/01.CIR.0000150391.38660.9B. [DOI] [PubMed] [Google Scholar]
- 19.Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 20.Oka R, Miura K, Sakurai M, Nakamura K, Yagi K, Miyamoto S, Moriuchi T, Mabuchi H, Koizumi J, Nomura H, et al. Impacts of visceral adipose tissue and subcutaneous adipose tissue on metabolic risk factors in middle-aged Japanese. Obesity (Silver Spring) 2010;18:153–160. doi: 10.1038/oby.2009.180. [DOI] [PubMed] [Google Scholar]
- 21.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 22.Bhardwaj S, Misra A, Misra R, Goel K, Bhatt SP, Rastogi K, Vikram NK, Gulati S. High prevalence of abdominal, intra-abdominal and subcutaneous adiposity and clustering of risk factors among urban Asian Indians in North India. PLoS One. 2011;6:e24362. doi: 10.1371/journal.pone.0024362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchernof A, Bélanger C, Morisset AS, Richard C, Mailloux J, Laberge P, Dupont P. Regional differences in adipose tissue metabolism in women: minor effect of obesity and body fat distribution. Diabetes. 2006;55:1353–1360. doi: 10.2337/db05-1439. [DOI] [PubMed] [Google Scholar]
- 24.Goel K, Misra A, Vikram NK, Poddar P, Gupta N. Subcutaneous abdominal adipose tissue is associated with the metabolic syndrome in Asian Indians independent of intra-abdominal and total body fat. Heart. 2010;96:579–583. doi: 10.1136/hrt.2009.183236. [DOI] [PubMed] [Google Scholar]
- 25.Terra X, Auguet T, Porras JA, Quintero Y, Aguilar C, Luna AM, Hernández M, Sabench F, del Castillo D, Richart C. Anti-inflammatory profile of FTO gene expression in adipose tissues from morbidly obese women. Cell Physiol Biochem. 2010;26:1041–1050. doi: 10.1159/000323979. [DOI] [PubMed] [Google Scholar]
- 26.Way JM, Görgün CZ, Tong Q, Uysal KT, Brown KK, Harrington WW, Oliver WR, Willson TM, Kliewer SA, Hotamisligil GS. Adipose tissue resistin expression is severely suppressed in obesity and stimulated by peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2001;276:25651–25653. doi: 10.1074/jbc.C100189200. [DOI] [PubMed] [Google Scholar]
- 27.Fujinami A, Obayashi H, Ohta K, Ichimura T, Nishimura M, Matsui H, Kawahara Y, Yamazaki M, Ogata M, Hasegawa G, et al. Enzyme-linked immunosorbent assay for circulating human resistin: resistin concentrations in normal subjects and patients with type 2 diabetes. Clin Chim Acta. 2004;339:57–63. doi: 10.1016/j.cccn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Youn BS, Yu KY, Park HJ, Lee NS, Min SS, Youn MY, Cho YM, Park YJ, Kim SY, Lee HK, et al. Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89:150–156. doi: 10.1210/jc.2003-031121. [DOI] [PubMed] [Google Scholar]
- 29.Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, Zhu Q, Considine RV. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452–5455. doi: 10.1210/jc.2002-021808. [DOI] [PubMed] [Google Scholar]
- 30.Heilbronn LK, Rood J, Janderova L, Albu JB, Kelley DE, Ravussin E, Smith SR. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab. 2004;89:1844–1848. doi: 10.1210/jc.2003-031410. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, Orlova C, Mantzoros CS. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848–4856. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- 32.Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27:2450–2457. doi: 10.2337/diacare.27.10.2450. [DOI] [PubMed] [Google Scholar]
- 33.Sheng CH, Di J, Jin Y, Zhang YC, Wu M, Sun Y, Zhang GZ. Resistin is expressed in human hepatocytes and induces insulin resistance. Endocrine. 2008;33:135–143. doi: 10.1007/s12020-008-9065-y. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. Geneva: 2002. [Google Scholar]
- 35.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 36.Singh AK, Tiwari S, Gupta A, Natu SM, Mittal B, Pant AB. Association of Resistin with Metabolic Syndrome in Indian Subjects. Metab Syndr Relat Disord. 2012:[Epub ahead of print]. doi: 10.1089/met.2011.0128. [DOI] [PubMed] [Google Scholar]
- 37.Smith SR, Bai F, Charbonneau C, Janderová L, Argyropoulos G. A promoter genotype and oxidative stress potentially link resistin to human insulin resistance. Diabetes. 2003;52:1611–1618. doi: 10.2337/diabetes.52.7.1611. [DOI] [PubMed] [Google Scholar]
- 38.Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, Alkan S, Gong DW. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–935. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 39.Baranova A, Gowder SJ, Schlauch K, Elariny H, Collantes R, Afendy A, Ong JP, Goodman Z, Chandhoke V, Younossi ZM. Gene expression of leptin, resistin, and adiponectin in the white adipose tissue of obese patients with non-alcoholic fatty liver disease and insulin resistance. Obes Surg. 2006;16:1118–1125. doi: 10.1381/096089206778392149. [DOI] [PubMed] [Google Scholar]
- 40.Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O'Rahilly S. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 41.Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun. 2001;285:561–564. doi: 10.1006/bbrc.2001.5173. [DOI] [PubMed] [Google Scholar]
- 42.McTernan CL, McTernan PG, Harte AL, Levick PL, Barnett AH, Kumar S. Resistin, central obesity, and type 2 diabetes. Lancet. 2002;359:46–47. doi: 10.1016/s0140-6736(02)07281-1. [DOI] [PubMed] [Google Scholar]
- 43.Le Lay S, Boucher J, Rey A, Castan-Laurell I, Krief S, Ferré P, Valet P, Dugail I. Decreased resistin expression in mice with different sensitivities to a high-fat diet. Biochem Biophys Res Commun. 2001;289:564–567. doi: 10.1006/bbrc.2001.6015. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee RR, Lazar MA. Resistin: molecular history and prognosis. J Mol Med (Berl) 2003;81:218–226. doi: 10.1007/s00109-003-0428-9. [DOI] [PubMed] [Google Scholar]
- 45.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 46.Rajala MW, Obici S, Scherer PE, Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest. 2003;111:225–230. doi: 10.1172/JCI16521. [DOI] [PMC free article] [PubMed] [Google Scholar]


