Abstract
Lung cancer is the leading cause of cancer death in the world. However, there is large geographic variation internationally and within nations. Despite the fact that many causes of lung cancer have been established, cigarette smoking is the principal cause. Accounting for historical prevalence of cigarette smoking is a useful predictor of the lung cancer burden in most populations. The populations at high present risk of lung cancer can usually be predicted based on historical patterns of the prevalence of cigarette smoking, and the high risk populations of the future can be predicted based on the current prevalence of cigarette smoking. Lung cancer rates are consistently higher among men than women, and are particularly high among African American men, and among those of lower socioeconomic status.
At the individual level, some segments of the population (e.g., African Americans, females) have been hypothesized to have greater susceptibility to lung cancer for a given degree of cigarette smoking. Common variants in genes that encode for enzymes involved in carcinogen metabolism and detoxification and in repairing DNA damage are likely to be important determinants of inter-individual susceptibility to smoking-caused lung carcinogenesis.
Many lung cancer risk factors have been identified, but active cigarette smoking is the predominant cause of lung cancer and the principal marker of both high-risk populations and high-risk individuals. In the absence of cigarette smoking, lung cancer would be a rare disease. Strategies that effectively prevent youths from starting to smoke and to promote cessation among dependent smokers can transform populations from high-risk to low-risk.
I. Introduction
Lung cancer is the leading cause of cancer death globally, accounting for 18% of cancer deaths and more than one million deaths per year.1 Worldwide, in 2002 more than 1.3 million people were newly diagnosed with lung cancer, which comprise 12% of all new cancer diagnoses.2
The theme of this paper is to consider who is at high-risk for lung cancer from the epidemiologic perspective. Addressing this issue requires considering the concept of high-risk at the both the population level and the individual level. During the past six decades, epidemiologic studies have resulted in a well-established set of factors that are known to be causally associated with lung cancer. The framework for this discussion is provided by this large body of epidemiologic data, which provides sufficient evidence to accurately characterize populations and individuals who can be considered to be at high risk of lung cancer.
II. Defining high risk
Many causes of lung cancer have been identified, including: active cigarette smoking;3 exposure to secondhand cigarette smoke (passive smoking);4 pipe and cigar smoking;5 occupational exposure to agents such as asbestos, nickel, chromium, and arsenic;6 exposure to radiation, including radon gas in homes;7, 8 and exposure to air pollution.9–11 Despite this array of known causal factors, identifying populations and individuals at high risk of lung cancer is relatively straightforward. This is because from among these many well-established causes of lung cancer, active cigarette smoking is far and away the strongest determinant of lung cancer risk. Thus, defining populations and individuals at high risk for lung cancer is simplified by the fact that there is a single 4 factor that is established cause of most (approximately 85%) of the lung cancer burden. Viewed alternatively, lung cancer was a rare disease prior to the epidemic of cigarette smoking that occurred in many nations during the twentieth century and it would be a rare disease today in the absence of cigarette smoking.
II.A High risk populations
At the population level, age-adjusted lung cancer rates, incidence rates or mortality rates, can be compared across populations to identify which populations are at highest risk. The causal association between cigarette smoking and the occurrence of lung cancer is so strong that this link is even evident on the population level. Under these circumstances, even if the only data one had to compare populations was the prevalence of cigarette smoking, in most instances this would be ample information to distinguish high-risk from low-risk populations. Within this context, high risk populations by geographic region or within subgroups of a population can be identified.9
The population risk for lung cancer is thus largely a function of the prevalence of cigarette smoking. Furthermore, smoking-caused lung carcinogenesis is a multi-step process that occurs over decades.10 Therefore, due to the “latent period,” or “induction period” of lung cancer, the ensuing lung cancer epidemic that follows an increase in smoking prevalence in a population occurs approximately three decades later. Given that the risk of developing and dying from lung cancer is largely determined by active cigarette smoking, and that there is an approximately three decade lag time between smoking prevalence and lung cancer rates, the population patterns of the occurrence of lung cancer are largely determined by historical patterns in the prevalence of cigarette smoking.9
Specifically, the current population patterns in the occurrence of lung cancer represent the smoking patterns of the past.9 When looking to the future, the current smoking prevalence is a powerful predictor of lung cancer rates three decades from now. In practice, smoking prevalence and lung cancer mortality patterns show parallel trends and cigarette smoking data can be used to predict the future burden of lung cancer.11
The population prevalence of smoking can fluctuate widely over time. Given the tight link between cigarette smoking and lung cancer, it follows that oscillation in lung cancer rates will occur, commensurate with changes in smoking patterns decades earlier. Pronounced variation can be present in lung cancer rates even in the same geographic region across time. For example, in the United States the average per capita cigarette consumption rose steeply between 1910 and 1950. The impact of the increased cigarette smoking occurred when lung cancer deaths began to spiral upward in the 1930’s and continued unabated through the 1980’s. Following the 1964 publication of the Surgeon General’s report that documented a causal association between cigarette smoking and lung cancer, the prevalence of smoking among adults declined.11 This was followed approximately three decades later by a decline in lung cancer incidence rates. Since 1985 the age-standardized incidence rates have decreased by 3.3% in men whereas they have increased by 22% in women.2 Both these temporal trends in men and women are predictable based on historical patterns of smoking prevalence.9
II.B High risk individuals
At the level of individuals, we rarely refer to absolute risk of lung cancer but more commonly identify high risk relative to a low risk group using measures of association such as the relative risk. For example, current active cigarette smokers have commonly 6 been observed to have a risk of developing lung cancer that was 20-fold greater than among those who never smoked, or a relative risk of 20.
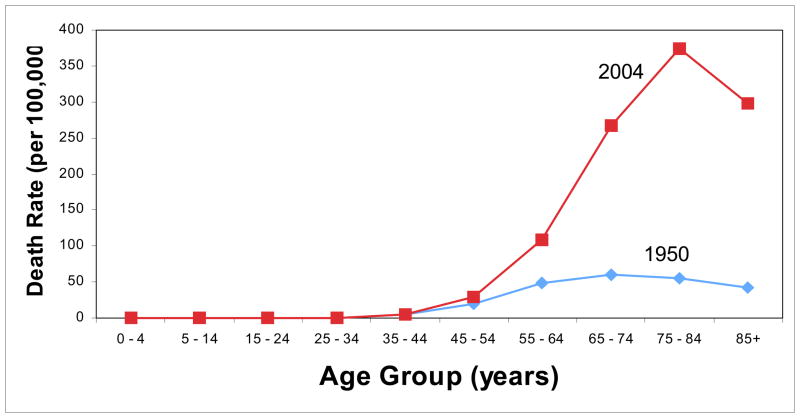
Characterizing individual risk of lung cancer is largely a function of two characteristics: personal history of active cigarette smoking and age. The concept of latent period between onset of cigarette smoking and lung cancer applies to individuals as well as populations. The pathway of smoking-caused lung carcinogenesis is a process that usually takes place during a period of several decades. Thus, even among those who begin smoking at very early ages, the majority of the burden of smoking-caused lung cancer occurs among the elderly. However, the risk associated with age alone, in the absence of personal active cigarette smoking, is very small compared to the impact of cigarette smoking on lung cancer risk in older ages. To illustrate this point, Figure 1 shows the U.S age-specific lung cancer death rates in 1950, when the U.S. lung cancer epidemic was in its infancy, and in 2004, a period near the peak of the lung cancer epidemic. The 1950 rates portray lung cancer rates by age group before the full impact of the smoking epidemic occurred, whereas in comparison the much higher rates in 2004 demonstrate that the high age-specific rates are largely attributable to the impact of the smoking epidemic.
Figure 1.
Age-specific death rates (per 100,000) in the Unites States in 1950 and 2004
Source: SEER Cancer Statistics Review
The major determinant of high-risk individuals is their personal history of active cigarette smoking. This information can be readily collected from individuals, and used to determine individual level risk of lung cancer in a more refined fashion than is possible at the population level. Given the clear monotonic dose-response relationships between cigarette smoking history and lung cancer, the individual risk determination is refined by not simply measuring whether or not an individual smokes cigarettes, but more fully accounting for variables related to the ages, duration, and intensity of smoking, such as the: age of initiation, number of cigarettes smoked per day, number of years smoked, and, if relevant, age of cessation.3, 12
III. Assessing population-level risk: Cigarette smoking prevalence and lung cancer rates
Worldwide, the populations at high-risk of lung cancer can be assessed based on current incidence rates. For example, in men the highest incidence rates of lung cancer occurred in Central and Eastern Europe and Northern America (65.7 and 61.2 per 100,000, respectively). In women, the highest incidence rates of lung cancer occurred in North America and Northern Europe (35.6 and 21.3 per 100,000, respectively).2 Conversely, among both men and women the lowest incidence rates are seen in Africa.
However, geographic regions that are considered to be at high risk of lung cancer can vary as global smoking patterns change. When considering population risks for lung cancer, it is thus useful to consider both the prevalence of cigarette smoking and the lung cancer rates. For example, a nation with a historically low cigarette smoking prevalence that has experienced a recent surge in smoking prevalence will have low current lung cancer rates, but is at risk for high lung cancer rates in the future.
To illustrate this principle, for selected countries we have summarized the joint patterns of cigarette smoking prevalence and lung cancer mortality rates for men (Table 1) and women (Table 2).13, 14 To do so, we categorized the cigarette smoking prevalence as low, medium, or high and lung cancer mortality rates as low, moderately low, moderately high, or high. Because both smoking prevalence and lung cancer mortality rates are uniformly lower in women than in men, different cut-off points were used for men and women.
Table 1.
Cigarette smoking prevalence and age-adjusted lung cancer mortality rates (per 100,000) in selected countries (men).
| Lung Cancer Mortality Rate | Smoking Prevalence (%) | ||
|---|---|---|---|
| LOW (<25) | MEDIUM (25–40) | HIGH (>40) | |
| LOW (<4.6) | Nigeria Rwanda Ghana Sudan Tanzania |
Djibouti Yemen |
|
| MODERATELY LOW (4.6 – 13) | Oman Saudi Arabia United Arab Emirates Iran |
India Egypt Zimbabwe |
El Salvador Namibia Guinea Ecuador Peru Nicaragua |
| MODERATELY HIGH (22.6 – 42.3) | Sweden Bahrain |
Thailand Israel Portugal Norway Iceland Finland Switzerland Ireland |
South Africa Viet Nam Mongolia China |
| HIGH (42.4 – 83.9) | Singapore Canada |
Germany UK USA Netherlands Belgium |
Korea Russian Federation Kazakhstan Poland Hungary |
Table 2.
Cigarette smoking prevalence and age-adjusted lung cancer mortality rates (per 100,00) in selected countries (women).
| Lung Cancer Mortality Rate | Smoking Prevalence (%) | ||
|---|---|---|---|
| LOW (<10) | MEDIUM (11–20) | HIGH (>20) | |
| LOW (<1.6) | Nigeria Swaziland Ghana |
Guinea | |
| MODERATELY LOW (1.6 – 3.3) | India Morocco Iran Djibouti Oman Pakistan |
Burundi Uganda Egypt |
Kenya Nepal Namibia Bangladesh |
| MODERATELY HIGH (6.0 – 10.0) | Costa Rica Russian Federation Iraq Indonesia Viet Nam Thailand |
Ecuador Mexico South Africa Peru Japan Finland |
Brazil Greece Chile France Romania Italy Israel |
| HIGH (10.1 – 27.8) | Malaysia Korea Bahrain Philippines China |
Canada Belgium |
Venezuela Germany Norway United States Denmark |
Nations with both a low current lung cancer burden and prevalence of cigarette smoking are those that currently appear to be immune from experiencing a lung cancer epidemic. Nations that fit this profile are exclusively from Africa; for men these are Nigeria, Rwanda, Ghana, Sudan, and Tanzania (Table 1), and for women these are Nigeria, Swaziland, and Ghana (Table 2). The rates of lung cancer in these countries likely depict the rates that would have been experienced globally in the absence of the epidemic of cigarette smoking.
Countries with low lung cancer mortality rates but high cigarette smoking prevalence are likely to be amidst the transition from historically low smoking prevalence to increased smoking prevalence. The increase in smoking prevalence in these nations portends a future lung cancer epidemic, but one that has not yet commenced. Nations that fit this profile for males are Djibouti and Yemen (Table 1) and for females, Guinea (Table 2). Countries that currently have moderately low lung cancer mortality rates that are likely to experience future shifts to high rates include for males El Salvador, Namibia, Guinea, Ecuador, Peru, and Nicaragua (Table 1) and for females Kenya, Nepal, Namibia, and Bangladesh (Table 2). Marked increases in the occurrence of lung cancer are expected from these countries from Africa, Asia, and Central and South America. This corroborates what is known about trends in the prevalence of cigarette smoking. For example, Africa has experienced a surge in cigarette consumption during recent years.15
Some countries are presently classified as high risk of lung cancer, but give rise to optimism that the population lung cancer risk will ebb downward into the lower risk categories in the future. These are countries with high lung cancer mortality rates currently, but whose smoking prevalence falls into the low or medium categories. Among men, these nations include Singapore, Canada, Germany, the United Kingdom, United States, Netherlands, and Belgium (Table 1). Among women, these nations include Malaysia, Korea, Bahrain, Philippines, China, Canada, and Belgium (Table 2). The situation among Chinese women is a notable exception to the general patterns that have been described. In China, the high current rates of lung cancer mortality among women are unlikely to be due to high rates of cigarette smoking prevalence, as is usually the case. Rather, the high lung cancer rates appear to be due to exposure to other risk factors that include indoor air pollution from cooking fumes.18–20
The co-occurrence of high current lung cancer mortality rates alongside high current cigarette smoking prevalence is a marker for the high-risk populations of greatest concern. These populations are at highest risk presently and also for the foreseeable future. Nations that fall into this category for males are Korea, the Russian Federation, Kazakhstan, Poland, and Hungary (Table 1) and for women are Venezuela, Germany, Norway, U.S.A., and Denmark (Table 2).
From the global perspective, a high-risk population of particular concern is Chinese males. This is due not only to the upward trends in the prevalence of cigarette smoking that have occurred, but also because the population of China is so large. The epidemic of cigarette smoking in China has been substantial,16 with per capita cigarette consumption in Chinese men having risen from one cigarette per day in 1952, to four in 1972, to ten in 1992. As a consequence, the lung cancer incidence rates have already increased and will continue to increase substantially.17 This represents such a large proportion of the world’s population that this will have a major impact on the global burden of lung cancer in the 21st century.
Whereas the majority of the world’s lung cancer burden used to be in predominantly developed countries, by 2002 the proportion of newly diagnosed lung cancers occurring in developed and developing countries was nearly equal.2 The overall pattern seen in Tables 1 and 2 is consistent with a continuing trend for the lung cancer burden to become increasingly concentrated in the developing world.18
III.A. Geographic variation within the United States
The preceding discussion focused on international comparisons to identify high risk populations, but the same principles apply to any geographic region. For example, in the United States, the age-adjusted lung cancer mortality rates vary more than 3-fold between the state with the highest rate (Kentucky) and the state with the lowest rate (Utah) (Table 3).19, 20 Consistent with the international patterns, the trends in lung cancer mortality rates tend to parallel statewide estimates of cigarette smoking prevalence for the selected states shown in Table 3.
Table 3.
Cigarette smoking prevalence and lung cancer mortality rates (age-adjusted per 100,000) in selected states.
| State | Smoking Prevalence (%) | Age-Adjusted Mortality Rate |
|---|---|---|
| Utah | 9.8 | 24.7 |
| Hawaii | 17.5 | 37.0 |
| New Mexico | 20.1 | 39.6 |
| Wyoming | 21.6 | 49.1 |
| Alaska | 24.0 | 56.5 |
| Indiana | 24.1 | 65.7 |
| West Virginia | 25.7 | 66.6 |
| Kentucky | 28.5 | 78.6 |
IV. Individual level risk: Host factors
Clinically, two characteristics largely determine an individual’s risk of lung cancer: cigarette smoking in combination with age. The importance of age reflects the fact that tobacco-caused lung carcinogenesis is cumulative process that usually occurs over decades. In nonsmokers, the risk of developing lung cancer by age alone is minimal compared to age-associated risk among smokers (Figure 1).
A lung cancer risk prediction model developed by Bach and colleagues helps to illustrate the impact of smoking plus age on lung cancer risk. In this model, age, packs of cigarettes smoked per day, and duration of smoking were used to determine the 10-year risk of developing lung cancer among current and former smokers. For example, a 55-year old person who has been smoking one pack per day for 25 years has a 1% chance of developing lung cancer in 10 years. A 75-year old person who has been smoking two packs per day for 50 years has a 15% chance of developing lung cancer in 10 years.21
At the individual level, the concept of high risk for lung cancer can also refer to differentially high risks of lung cancer for a specific level of exposure to cigarette smoking. Advancing understanding of this differential susceptibility to tobacco carcinogenesis is currently a major focus of lung cancer research.
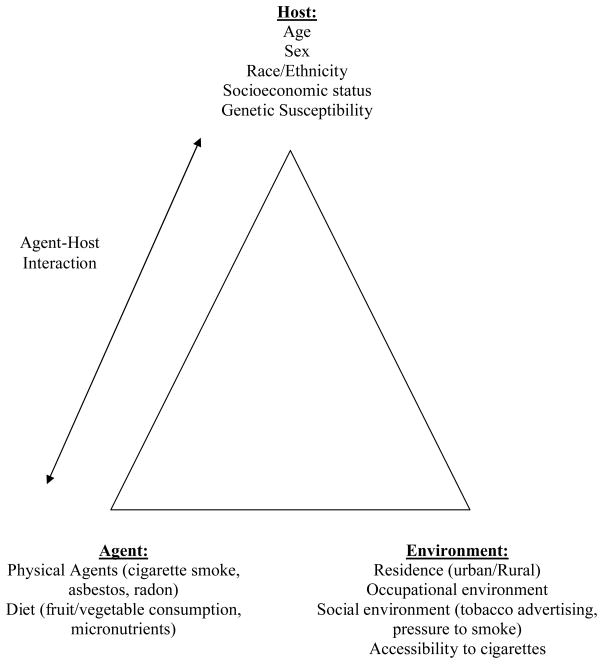
IV.A. Agent-Host-Environment Model Applied to Lung Cancer
The agents known to be causally associated with lung cancer, such as cigarette smoke, asbestos, chromium, nickel, and radon, can be integrated into an overarching agent-host-environment model (Figure 2). In this model, the environment refers broadly to factors that influence the likelihood that people will be exposed to causal agents. Host factors are those that affect susceptibility to these agents once exposed. The agent-host interaction refers to the complex interplay that can occur when there are important inter-individual differences in host susceptibility to agents that cause lung cancer. Host characteristics that can be of importance in this regard include demographic characteristics, such as age, race, and sex, as well as genetic variants that determine susceptibility to lung carcinogens at the molecular level.
Figure 2.
Agent-Host-Environment model applied to lung cancer
Two host factors that are demographic characteristics have attracted particular interest in this regard: race and sex. With respect to race, African Americans suffer a disproportionate share of the lung cancer burden. The rates are particularly high among African American men. In contrast, the rates of lung cancer have historically been higher in men than women. Hypotheses have been set forth postulating enhanced susceptibility to smoking-caused lung carcinogenesis in: 1) African Americans compared to other races and 2) women compared to men. The evidence concerning each of these hypotheses is considered separately below.
IV.B. Race/Ethnicity
Lung cancer is more common in African Americans than any other racial/ethnic group in the U.S., including European Americans, the group with the second high rates (76.9 versus 66.0 per 100,000). This disparity is due to the much higher incidence in African American men compared to European American men, as the incidence rate in African American women is actually lower than among European American women.20 Although historical differences in smoking prevalence are usually the primary determinants in high-risk populations, cigarette smoking prevalence does not by itself provide a viable explanation for the exceedingly high rates of lung cancer seen in African Americans.22
One possible explanation for this is that African American smokers are more susceptible to lung carcinogens from cigarette smoke than European American smokers. In the four studies that have examined the association between cigarette smoking and risk of lung cancer among African Americans compared to European Americans,24–27 there has been some evidence to support an increased risk of lung cancer among African American smokers compared to their white counterparts. However, the racial differences in smoking risk have not been uniformly observed across all categories of smoking history. For example, in one case-control study the greatest racial difference in risk was present among heavy smokers (≥21 cigarettes per day, ≥37.5 pack-years),23 whereas in a prospective cohort study the strongest racial difference in risk was seen among those who smoked fewer cigarettes per day.24
The majority of African American smokers smoke menthol brands of cigarettes, whereas the majority of European American smokers smoke nonmenthol brands of cigarettes. This has led to the hypothesis that the risk of lung cancer from menthol cigarettes is greater than for nonmenthol cigarettes; if so, menthol cigarettes would be a contributing factor to the excess risk of lung cancer experienced by African Americans. The results of some studies have provided support for this hypothesis,25 but to date the preponderance of the evidence does not indicate that the risk of lung cancer differs for menthol versus non-menthol cigarettes.26–28
If the racial disparity in lung cancer rates is not due primarily to cigarette smoking, research into other potential causes of this disparity is a priority. This is a situation where attention needs to be given to differential exposure to other known causes of lung cancer, such as occupational exposures to lung cancer causing agents. For example, occupational exposures including asbestos, nickel, chromium, and arsenic, are more common among African Americans than White Americans.29 Racial differences in exposure to air pollution in urban environment could be another contributing factor. Achieving definitive explanations for this important issue are more likely to be achieved with research approaches that are holistic, attempting to tease apart the complex interactions between this inter-related constellation of risk factors.
IV.C. Gender
The age-adjusted global incidence rate of lung cancer is much lower in women than men (12.1 versus 35.5 per 100,000).2 Within nations, lung cancer consistently occurs at higher rates in men than women. For example, in the United States the incidence rates from 2000–2004 were 81.2 and 52.3 per 100,000 in men and women, respectively.20 However, lung cancer incidence among males has been declining during the past several years, whereas this is not the case for females. Even though far more men than women are diagnosed and die from lung cancer at the present time, the mortality and incidence rates between the genders will converge in the future.11
At the population level, the high male-to-female ratio in the occurrence of lung cancer is primarily due to the historically higher prevalence of cigarette smoking in men than women. Therefore, the increase in lung cancer incidence and mortality among women in developed countries has been mostly explained by the fact that cigarette smoking uptake occurred later among women than men and the peak smoking prevalence in women is much lower than that among men.
At the level of individual risk, it has been hypothesized that for a given cigarette smoking history, women are more susceptible than men. Table 4 summarizes the studies that have examined the association of smoking and risk of lung cancer among women compared to men. Several case-control studies have supported the hypothesis that women are more susceptible to the carcinogenic effects of cigarette smoke than men.30–35 On the other hand, the results of other case-control studies suggest otherwise, with reports of no difference 36 or even that men had a higher smoking-related risk of lung cancer than women.37, 38 Furthermore, the results of three cohort studies indicate that women are not more susceptible to the carcinogenic effects of cigarette smoke than men. In the Nurse’s Health Study and the Health Professionals Follow-up Study there was no statistically significant difference between women and men.39 In two other cohort studies, the results suggested a stronger association between smoking and lung cancer in men than women.40, 41
Table 4.
Summary of studies that have examined the association of smoking and lung cancer by gender
| Study author, year | Population | Location | Odds Ratio/Relative Risk* | ||
|---|---|---|---|---|---|
|
| |||||
| Case-control studies | |||||
|
| |||||
| Lubin et al., 1984 | Hospital-based 7804 cases (11.3% F) 15,207 controls (11.5% F) |
Western Europe | |||
| Cig/day | SCC | AC | |||
| 10–19 | 1.6 | 1.1 | |||
| 20–29 | 2.5 | 0.44 | |||
| ≥30 | 1.4 | 0.66 | |||
|
| |||||
| Schoenberg et al., 1989 | Population-based 994 cases 995 controls |
New Jersey | |||
| Any cigarette smoking | 0.71 | ||||
|
| |||||
| Brownson et al, 1992 | Registry-based 14,596 cases (35.7% F) 36,438 controls |
Missouri | |||
| Current smoker | 1.2 | ||||
|
| |||||
| Harris et al., 1993 | Hospital-based 4423 cases (34.1% F) 4171 controls (37.3% F) |
New York, NY | |||
| Tar exposure (kg) | Ever smokers | Current smokers | |||
| 1–4 | 1.0 | ||||
| 5–8 | 2.0 | 1.8 | |||
| ≥8 | 1.9 | 1.7 | |||
| Combined | 1.7 | 1.5 | |||
|
| |||||
| Osann et al., 1993 | Population-based 1986 cases (41.9% F) 3507 controls (47.2% F) |
California | |||
| Ever smokers | 0.76 | ||||
| Current smokers | 0.74 | ||||
|
| |||||
| Risch et al., 1993 | Population-based 845 cases (52.3% F) 772 controls (53.1% F) |
Canada | |||
| Pack-years | |||||
| 0–30 | 1.4 | ||||
| 30–60 | 2.4 | ||||
| 60+ | 3.6 | ||||
|
| |||||
| Zang et al., 1996 | Hospital-based 1936 cases (41.9% F) 2070 controls (45.8% F |
United States | |||
| Cigarette Exposure | |||||
| Cigarettes per day | 1.4 | ||||
| Pack-years | 1.4 | ||||
|
| |||||
| Kreuzer et al., 1999 | Population and hospital-based 4623 cases (19.5% F) 5169 controls (21.2% F) |
Germany and Italy | |||
| Ever smokers | 0.26 | ||||
|
| |||||
| Cohort studies | |||||
|
| |||||
| Thun et al., 1995 | 786,387 participants (CPS-I) 711,363 participants (CPS-II) |
United States, Puerto Rico, and Guam | |||
| Current Smokers | Women | Men | |||
| CPS-I | 2.7 | 11.9 | |||
| CPS-II | 12.8 | 23.2 | |||
|
| |||||
| Prescott et al., 1998 | 30,874 participants (43.5% F) 867 cases (23.4% F) |
Copenhagen | |||
| Women | Men | ||||
| Ever smokers | 6.4 | 11.9 | |||
|
| |||||
| Bain et al., 2004 | 85,693 participants (70.4% F) 1266 cases |
United States | |||
| Former smokers | 1.18 | ||||
| Current smokers | 1.11 | ||||
Estimates of smoking-lung cancer association in women relative to men
IV.D. Socioeconomic status
Socioeconomic status (SES) is inversely associated with lung cancer risk.29, 42 Thus, persons of lower SES are a population at high risk for lung cancer. Many lung cancer risk factors are more concentrated among those of lower SES. A large proportion of the excess risk observed among low SES populations is likely attributable to cigarette smoking because lower SES is associated with higher smoking prevalence, greater use of non-filter, high-tar cigarettes, and lower quit rates.42, 43 However, those of lower SES also experience a greater likelihood of exposure to other lung cancer risk factors and this is likely to contribute at least a portion of the SES differential in lung cancer risk. For example, those of lower SES are also more likely to eat less healthful diets and be exposed to occupational and environmental carcinogens 42 and secondhand cigarette smoke.44–46
IV.E. Inherited Susceptibility
Despite the fact that cigarette smoking is the predominant cause of lung cancer, lung cancer occurs in only a minority of cigarette smokers. This suggests that inherited factors that affect inter-individual susceptibility to cigarette smoking play an important role in the etiology of lung carcinogenesis. In support of a role for inherited susceptibility are the many studies that have observed a family history of lung cancer is associated with elevated risk for lung cancer.47 A family history of lung cancer has even been observed to be a risk factor for lung cancer in a population of nonsmokers, and the association with family history was stronger among younger age groups.48
Research to pinpoint the specific source of inherited susceptibility has been ongoing in earnest. The identification of a major source of inherited susceptibility, i.e. a genetic mutation with high penetrance, had proven elusive until recently. A promising lead in this regard was reported from a linkage analysis of 52 extended pedigrees, in which a locus on chromosome 6q23-25 was associated with a major susceptibility to lung cancer.49
Another source of the inherited susceptibility to lung cancer is in the form of low penetrance, high prevalence common polymorphisms in genes that are involved in pathways that affect the likelihood that carcinogens in cigarette smoke will bind to and damage DNA. These pathways consist of genes that are involved in carcinogen metabolism and detoxification, as well as DNA repair genes.
Carcinogen metabolism generally takes place in two phases. In Phase I, unreactive, nonpolar compounds are converted to highly reactive intermediate compounds. In turn, these intermediates can form complexes in Phase II conjugation reactions, complexes that are less reactive and more easily excreted. However, prior to conjugation the intermediate metabolite may interact with other parts of the cell, such as DNA, and potentially contribute to progression on the pathway of lung carcinogenesis. Polycyclic aromatic hydrocarbons, and other carcinogenic compounds present in cigarette smoke are metabolically activated by Phase I enzymes of the cytochrome p450 system to form intermediates that bind to DNA. Some common variants in genes that encode for these enzymes have been studied in relation to lung cancer risk. For example, evidence suggests that two specific polymorphisms in the CYP1A1 gene, the MspI polymorphism50 and a polymorphism in exon 751 are associated with increased risk of lung cancer.
Clearly, a strong rationale also exists for variants in genes that encode for Phase II enzymes to account for inter-individual susceptibility to smoking-caused lung cancer. As an example, a large body of evidence has accumulated concerning the potential role of polymorphisms in the GSTM1 gene in relation to lung cancer risk. In summaries of results from many studies, the results indicate that those with the GSTM1 null genotype have a greater risk of lung cancer than those with the GSTM1 present genotype; however, among cigarette smokers differential risks according these genotypes have not been clearly discernable.52
Further downstream in the carcinogenic pathway, inter-individual variation in the capacity to repair DNA would affect the likelihood of progression in the carcinogenic pathway once a carcinogen bound to DNA. For this reason, substantial research has focused on sup-optimal DNA repair as a susceptibility factor for lung cancer. The underlying hypothesis is that the lesser capacity to repair DNA damage, the greater the susceptibility to smoking-caused lung cancer. Further research is needed to better understand the association between variation in DNA repair capacity and lung cancer risk, but so far the evidence indicates this is a promising lead.53 Phenotypic assays have been developed to attempt to measure susceptibility to DNA damage. Individuals with less proficient DNA repair capacity phenotype, as measured by a non-specific mutagen sensitivity assay, have been shown to have an increased risk of lung cancer in some studies.54, 55
V. Summary
From the epidemiologic perspective, conceptualizing populations at high risk of lung cancer benefits considerably from distinguishing population-level versus individual-level risk. At the level of both populations and individuals, the combination of cigarette smoking with ample time since initiation of the exposure serves as a strong marker of lung cancer risk.
The current research into lung cancer risk concerns the possibility of differential susceptibility to smoking-caused lung cancer. Of interest is this regard are potential racial and gender differences, and the overall question of the genetic markers that determine susceptibility to cigarette smoking caused lung cancer. These are important questions, but when considering tobacco-induced lung carcinogenesis, the variation in risk among smokers is likely to be subtle compared to the huge difference in risk between smokers and nonsmokers.
Many lung cancer risk factors have been identified, but active cigarette smoking is the predominant cause of lung cancer and the principal marker of both high-risk populations and high-risk individuals. Cigarette smoking is itself preventable, so the pathway toward preventing lung cancer is clear: preventing the uptake of smoking among youths and promoting cessation among dependent smokers. In the absence of cigarette smoking, lung cancer would be a rare disease. Strategies that effectively prevent youths from starting to smoke and to promote cessation among dependent smokers can transform populations from high-risk to low-risk. This positive transformation is underway in the United States and some other developed countries.
Acknowledgments
This research was carried out with funding from the National Cancer Institute (CA105069).
References
- 1.Rosen G. A history of public health. Expanded. Baltimore, MD: The Johns Hopkins University Press; 1993. [Google Scholar]
- 2.Parkin D, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. The Health Consequences of Smoing: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 4.U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- 5.National Cancer Institute. Cigars: Health Effects and Trends. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 1998. [Google Scholar]
- 6.Alberg AJ, Ford JG, Samet JM. Epidemiology of Lung Cancer: ACCP Evidence-Based Clinical Practice Guidelines (2nd Edition) Chest. 2007;132(3S):29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 7.Health effects of exposure to radon (BEIR VI) Washington, D.C: National Academy Press, National Research Council, Committee on Health Risks of Exposure to Radon, Board on Radiation Effects Research, Commission on Life Sciences; 1999. [Google Scholar]
- 8.Technical support document for the 1992 Citizen’s Guide to Radon. Washington, D.C: U.S. Government Printing Office, U.S. Environmental Protection Agency; 1992. [Google Scholar]
- 9.Shibuya K, Inoue M, Lopez AD. Statistical modeling and projections of lung cancer mortality in 4 industrialized countries. Int J Cancer. 2005;117:476–485. doi: 10.1002/ijc.21078. [DOI] [PubMed] [Google Scholar]
- 10.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Travis WD, Tarone RE, Travis L, Devesa SS. Lung Cancer Rates Convergence in Young Men and Women in the United States: Analysis by Birth Cohort and Histologic Type. Int J Cancer. 2003;105:101–107. doi: 10.1002/ijc.11020. [DOI] [PubMed] [Google Scholar]
- 12.Lubin JH, Caporaso N, Wichmann HE, Schaffrath-Rosario A, Alvanja MC. Cigarette smoking and lung cancer: modeling effect modification of total exposure and intensity. Epidemiology. 2007;18(5):639–648. doi: 10.1097/EDE.0b013e31812717fe. [DOI] [PubMed] [Google Scholar]
- 13.Tobacco Country Profiles 2003. Atlanta, GA: American Cancer Society; 2003. [Google Scholar]
- 14.Ferlay J, Bray F, Pisani P, Parkin DM. IARC Cancer Base No.5 version 2.0. IARC Press; Lyon: 2004. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. [Google Scholar]
- 15.Lam WK, White NW, Chan-Yeung MM. Lung cancer epidemiology and risk factors in Asia and Africa. Int J Tuberc Lung Dis. 2004;8(9):1045–1057. [PubMed] [Google Scholar]
- 16.Yang L, Parkin D, Ferlay J. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol Biomarkers Prev. 2005;14(1):243–250. [PubMed] [Google Scholar]
- 17.Liu B, Peto R, Chen Z. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. Br Med J. 1998;317(7170):1411–1422. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362:847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 20.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 21.Bach PB, Kattan MW, Thornquist MD, et al. Variations in Lung Cancer Risk Among Smokers. J Natl Cancer Inst. 2003;95(6):470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 22.Pinsky PF. Racial and ethnic differences in lung cancer incidence: how much is explained by differences in smoking patterns? (United States) Cancer Causes Control. 2006;17(8):1017–1024. doi: 10.1007/s10552-006-0038-2. [DOI] [PubMed] [Google Scholar]
- 23.Stellman SD, Chen Y, Muscat JE, et al. Lung Cancer Risk in White and Black Americans. Ann Epidemiol. 2003;13(4):294–302. doi: 10.1016/s1047-2797(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 24.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and Racial Differences in the Smoking-Related Risk of Lung Cancer. N Engl J Med. 2006;354(4):333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 25.Sidney S, Tekawa IS, Friedman GD, Sadler MC, Tashkin DP. Mentholated cigarette use and lung cancer. Arch Intern Med. 1995;155(7):727–732. [PubMed] [Google Scholar]
- 26.Kabat GC, Hebert JR. Use of mentholated Cigarettes and Lung Cancer Risk. Cancer Research. 1991;51(24):6510–6513. [PubMed] [Google Scholar]
- 27.Brooks DR, RPJ, Strom BL, Rosenberg L. Menthol Cigarettes and Risk of Lung Cancer. American Journal of Epidemiology. 2003;158(7):609–616. doi: 10.1093/aje/kwg182. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter CL, Jarvik ME, Morgenstern H, McCarthy WJ, London SJ. Mentholated Cigarette Smoking and Lung Cancer Risk. Annals of Epidemiology. 1999;9(2):114–120. doi: 10.1016/s1047-2797(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 29.Cooley ME, Jennings-Dozier K. Lung Cancer in African Americans: A Call for Action. Cancer Pract. 1998;6(2):99–106. doi: 10.1046/j.1523-5394.1998.1998006099.x. [DOI] [PubMed] [Google Scholar]
- 30.Lubin JH, Blot WJ. Assessment of lung cancer risk factors by histologic category. J Natl Cancer Inst. 1984;73(2):383–389. doi: 10.1093/jnci/73.2.383. [DOI] [PubMed] [Google Scholar]
- 31.McDuffie HH, Klaassen DJ, Dosman JA. Female-male differences in patients with primary lung cancer. Cancer. 1987;59(10):1825–1830. doi: 10.1002/1097-0142(19870515)59:10<1825::aid-cncr2820591024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Brownson RC, Chang JC, Davis JR. Gender and histologic type variations in smoking-related risk of lung cancer. Epidemiology. 1992;3(1):61–64. doi: 10.1097/00001648-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Harris RE, Zang EA, Anderson JI, Wynder EL. Race and Sex Differences in Lung Cancer Risk Associated with Cigarette Smoking. Int J Epidemiol. 1993;22(4):592–599. doi: 10.1093/ije/22.4.592. [DOI] [PubMed] [Google Scholar]
- 34.Risch HA, Howe GR, Jain M, Burch JD, Holoway EJ, Miller AB. Are female smokers at a higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol. 1993;138:281–293. doi: 10.1093/oxfordjournals.aje.a116857. [DOI] [PubMed] [Google Scholar]
- 35.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: Examination of the evidence. J Natl Cancer Inst. 1996;88(3/4):183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 36.Schoenberg JB, Wilcox HB, Mason TJ, Bill J, Stemhagen A. Variation in smoking-related lung cancer risk among New Jersey women. Am J Epidemiol. 1989;130(4):688–695. doi: 10.1093/oxfordjournals.aje.a115390. [DOI] [PubMed] [Google Scholar]
- 37.Osann KE, Anton-Culver H, Kurosaki T, Taylor T. Sex Differences in Lung-Cancer Risk Associated with Cigarette Smoking. Int J Cancer. 1993;54:44–48. doi: 10.1002/ijc.2910540108. [DOI] [PubMed] [Google Scholar]
- 38.Kreuzer M, Boffetta P, Whitley E, et al. Gender differences in lung cancer risk by smoking: a mutlicentre case-control study in Germany and Italy. Br J Cancer. 2000;82(1):227–233. doi: 10.1054/bjoc.1999.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bain C, Feskanich D, Speizer FE, et al. Lung Cancer Rates in Men and Women With Comparable Histories of Smoking. J Natl Cancer Inst. 2004;96(11):826–834. doi: 10.1093/jnci/djh143. [DOI] [PubMed] [Google Scholar]
- 40.Prescott E, Osler M, Hein HO, et al. Gender and smoking-related risk of lung cancer. The Copenhagen Center for Prospective Population Studies. Epidemiology. 1998;9(1):79–83. [PubMed] [Google Scholar]
- 41.Thun MJ, Day-Lally CA, Calle EE, Flanders D, Heath CW. Excess Mortality among Cigarette Smokers: Changes in a 20-Year Interval. Am J Public Health. 1995;85(9):1223–1230. doi: 10.2105/ajph.85.9.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gadgeel SM, Kalemkerian GP. Racial differences in lung cancer. Cancer Metastasis Rev. 2003;22:39–46. doi: 10.1023/a:1022207917249. [DOI] [PubMed] [Google Scholar]
- 43.Gadgeel SM, Severson RK, Kau Y, Graff J, Weiss LK, Kalemkerian GP. Impact of Race in Lung Cancer: analysis of temporal trends from a Surveillance, Epidemiology, and End Results Database. Chest. 2001;120(1):55–63. doi: 10.1378/chest.120.1.55. [DOI] [PubMed] [Google Scholar]
- 44.Borland R, Pierce JP, Burns DM, Gilpin E, Johnson M, Bal D. Protection from environmental tobacco smoke in California: the case for a smoke-free workplace. JAMA. 1992;268(6):749–752. [PubMed] [Google Scholar]
- 45.Curtin F, Morabia A, Bernstein M. Lifetime Exposure to Environmental Tobacco Smoke among Urban Women. Am J Epidemiol. 1998;148(11):1040–1047. doi: 10.1093/oxfordjournals.aje.a009580. [DOI] [PubMed] [Google Scholar]
- 46.Scarinci IC, Watson JM, Slawson DL, Klesges RC, Murray DM, Eck-Clemens LH. Socioeconomic status, ethnicity, and environmental tobacco exposure among non-smoking females. Nicotine Tob Res. 2000;2:355–361. doi: 10.1080/713688150. [DOI] [PubMed] [Google Scholar]
- 47.Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123(1 Suppl):21S–49S. doi: 10.1378/chest.123.1_suppl.21s. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz AG, Yang P, Swanson GM. Familial risk of lung cancer among nonsmokers and their relatives. Am J Epidemiol. 1996;144:554–562. doi: 10.1093/oxfordjournals.aje.a008965. [DOI] [PubMed] [Google Scholar]
- 49.Bailey-Wilson JE, Amos CI, Pinney SM, et al. A Major Lung Cancer Susceptibility Locus Maps to Chromosome 6q23-25. Am J Hum Genet. 2004;75:460–474. doi: 10.1086/423857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vineis P, Veglia F, Benhamou S, et al. CYP1A1 T3801 C polymorphism and lung cancer: a pooled analysis of 2451 cases and 3358 controls. Int J Cancer. 2003;104(5):650–657. doi: 10.1002/ijc.10995. [DOI] [PubMed] [Google Scholar]
- 51.Le Marchand L, Guo C, Benhamou S, et al. Pooled analysis of the CYP1A1 exon 7 polymorphism and lung cancer (United States) Cancer Causes Control. 2003;14(4):339–346. doi: 10.1023/a:1023956201228. [DOI] [PubMed] [Google Scholar]
- 52.Benhamou S, Lee WJ, Alexandrie AK, et al. Meta- and pooled analyses of the effects of glutathione S-transferase M1 polymorphisms and smoking on lung cancr risk. Carcinogenesis. 2002;23(8):1343–1350. doi: 10.1093/carcin/23.8.1343. [DOI] [PubMed] [Google Scholar]
- 53.Spitz MR, Wei Q, Dong Q, Amos CI, Wu X. Genetic susceptibility to lung cancer: the role of DNA damage and repair. Cancer Epidemiol Biomarkers Prev. 2003;12(8):689–698. [PubMed] [Google Scholar]
- 54.Wei Q, Cheng L, Amos CI, et al. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst. 2000;92(21):1764–1772. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- 55.Zheng YL, Loffredo CA, Yu Z, et al. Bleomycin-induced chromosome breaks as a risk marker for lung cancer: a case-control study with population and hospital controls. Carcinogenesis. 2003;24(2):269–274. doi: 10.1093/carcin/24.2.269. [DOI] [PubMed] [Google Scholar]