Abstract
Background
Rhabdoid tumors are a rare and aggressive cancer subtype which is usually diagnosed in early childhood. Little is known about their etiology. The purpose of this study was to describe the epidemiology of rhabdoid tumors and examine their relation to perinatal characteristics.
Methods
We identified 44 atypical teratoid/rhabdoid tumors (AT/RT) of the central nervous system (CNS) and 61 rhabdoid sarcomas (renal and extra-renal non-CNS tumors) from California Cancer Registry records of diagnoses 1988-2007 among children <6 years of age. We randomly selected 208,178 controls from California birthrolls. Multivariable logistic regression was used to examine associations between rhabdoid tumors and perinatal characteristics.
Results
After adjustment for demographic characteristics, low birthweight (<2500g) strongly increased risk for developing both rhabdoid sarcomas (OR=2.43, 95% CI 1.09, 5.41) and AT/RT (OR=2.99, 95% CI 1.31, 6.84). Both preterm delivery (<37 weeks gestation, OR=2.63, 95% CI 1.34, 5.17) and late term delivery (>42 weeks, OR=3.66, 95% CI 1.54, 8.71) also increased risk of rhabdoid sarcomas. Rhabdoid sarcoma cases (OR=3.08, 95% CI 1.11, 8.55) and AT/RT cases (OR=3.16, 95% CI 1.23, 8.13) also were more likely to be multiple births.
Conclusion
The excess of twin pregnancies may suggest an association with infertility treatments. This is the first population-based epidemiologic study to examine these rare tumors.
Keywords: rhabdoid tumors, birth characteristics, birthweight, social class, maternal age, multiple birth offspring, premature labor, neonatal intensive care
Introduction
Rhabdoid tumors are rare and aggressive embryonal tumors which are typically diagnosed in early childhood. Rhabdoid tumor of the kidney was first identified as a distinct histopathologic entity in 1978 [1]. Subsequent studies identified tumors with similar histology at other sites, particularly soft tissues and the central nervous system (CNS).[2] Atypical teratoid/rhabdoid tumor (AT/RT) of the central nervous system was first identified in 1995 and was classified by WHO shortly thereafter [3,4]. Often diagnosed at stage III or higher [5-8], these cancers have a poor prognosis, with a 5-year survival of 33% [9].
Rhabdoid tumors are quite rare. In the US, annual incidence among children less than 15 is 0.19 per million for renal tumors, 0.89 per million for AT/RT and 0.32 per million for tumors of other sites [10]. Rhabdoid tumors account for an estimated 0.9-2% of renal cancers and 1.5-2.1% of childhood CNS tumors in children, although these may be underestimates due to misdiagnosis [4,11-13]. Diagnoses have increased in recent years likely due to growing recognition of this cancer subtype [8].
The loss or inactivation of the SMARCB1/hSNF5/INI tumor suppressor gene has been identified as the hallmark genetic defect in these tumors [14,15]. This mutation can arise somatically or be inherited in the germline (35% of tumors), with germline mutations more often seen among patients with multiple primary tumors [15,16]. It has been hypothesized that this cancer, similar to retinoblastoma, may follow Knudson’s two hit hypothesis [17].
Despite calls for research [18-20], little is known on rhabdoid tumor etiology. Here we capitalize on the availability of a large statewide database to examine the epidemiology of rhabdoid tumors and their association with perinatal characteristics. This is the first population-based epidemiologic study to examine these cancers.
Methods
This study was conducted as part of the Air Pollution and Childhood Cancers (APCC) study, a case-control investigation of California children. Cases were identified from records of all neoplasms diagnosed 1988-2007 in the California Cancer Registry among children younger than age 6. Inclusion criteria for the APCC study were that all cases must have been born in California in order to match subjects with their birth certificate. We were able to match 89% of cases to a California birth certificate using first and last names and date of birth. Controls, who were children who had not received a cancer diagnosis in California before age 6, were frequency-matched by year of birth to all childhood cancer cases during the study period and randomly selected from California birthrolls.
We defined rhabdoid tumor cases as International Classification of Diseases for Oncology version 3 (ICD-O-3) code 8963 (rhabdoid sarcomas) and 9508 (atypical teratoid/rhabdoid tumor). Rhabdoid sarcomas included renal tumors and extra-renal, non-CNS cancers. We additionally checked death records to identify and exclude controls who died of other causes in childhood (< 6 years). After exclusion of 1,522 controls who had died, the final dataset included 105 rhabdoid tumor cases (including 44 AT/RT and 61 rhabdoid sarcomas) and 208,178 controls.
We examined California Cancer Registry records to determine if cases had ever been diagnosed with a second primary tumor; none of the cases had any second cancer diagnosis.
As this was a record-based study, we did not seek informed consent from individual subjects. The study received approvals from the human subject protection boards of the University of California Los Angeles and the California Committee for the Protection of Human Subjects.
Apart from cancer registry records, our main source of study data was birth certificates. As the child’s race was not collected on birth certificates for the entire time period under study, we report maternal and paternal race only. Socioeconomic status was measured using maternal and paternal education as well as the method of payment for prenatal care (private insurance vs. Medi-Cal, other governmental care or self-pay), which we have found to be a good predictor of income in other studies [21]. California birth certificates report estimates for gestational age based on the date of last menses. When this length of time was implausibly long (>45 weeks) we considered it as missing. We defined low birthweight as <2500g and high birthweight as > 4000g. Size for gestational age was defined as small if birth weight was less than the 10th percentile and defined as large if greater than the 90th percentile of the birthweight standards for a given gestational age. The 10th and 90th percentile values were obtained for each gestational week (20-45 weeks) by maternal race/ethnicity (non-Hispanic white, Hispanic of any race, black, Asian/Pacific Islander, and other) and child’s sex based on the total singleton live births in California between 1988 and 2006 using the method described by Alexander et al [22].
California birth certificates further collect data on complications in pregnancy and in labor and delivery, concurrent illnesses of the mother, clinical procedures done in the perinatal period, and abnormal conditions of the child. Not all variables were collected throughout the period under study. With regards to pregnancy and labor complications and clinical procedures, we reported upon those variables seen among at least 5 cases.
We calculated odds ratios (ORs) and 95% confidence intervals using unconditional logistic regression with SAS 9.1 (SAS, Cary, NC). We report unadjusted and adjusted results, with adjusted regressions controlling for birth year, maternal age, maternal race/ethnicity, and the method of payment for prenatal care. We considered additional adjustment for paternal race/ethnicity, but its inclusion in the model made little difference in effect estimates. Due to the later identification and classification of AT/RT as a distinct histopathologic entity, all analyses of this subtype were limited to children born after Jan 1, 1997.
Results
The demographic distribution of subjects is provided in Table I. As a whole, rhabdoid tumor cases were demographically similar to controls, with the exception that fathers of cases included larger proportions of White non-Hispanics than other races. The mothers of AT/RT cases were older, were more likely to be non-Hispanic white, and had greater years of education than controls. The fathers of AT/RT cases included larger proportions of white non-Hispanics and had greater years of education than controls. In comparison to controls, AT/RT cases were considerably more likely to have had their prenatal care paid by private insurance versus Medi-Cal or other government programs (OR=3.06, 95% CI 1.52, 6.23), a marker of higher income.
Table I.
Demographic characteristics in relation to rhabdoid tumors
| Characteristic | Controls (n=208,178) n (%) |
All cases (n=105) n (%) |
p- value a |
Rhabdoid sarcoma b (n=61) n (%) |
p- value |
AT/RT (n=44) n (%) |
p- value |
|---|---|---|---|---|---|---|---|
| Tumor stage | --- | --- | --- | ||||
| Localized | --- | 38 (36.9) | 16 (27.1) | 22 (50.0) | |||
| Regional | --- | 25 (24.3) | 15 (25.4) | 10 (22.7) | |||
| Distant | --- | 40 (38.8) | 28 (47.5) | 12 (27.3) | |||
| Sex | 0.4 | 0.6 | 0.4 | ||||
| Male | 106196 (51.0) | 58 (55.2) | 33 (54.1) | 25 (56.8) | |||
| Female | 101982 (49.0) | 47 (44.8) | 28 (45.9) | 19 (43.2) | |||
| Maternal age at birth | 0.6 | 0.2 | 0.06 | ||||
| <20 | 22670 (10.9) | 9 (8.6) | 7 (11.5) | 2 (4.5) | |||
| 20-29 | 108838 (52.3) | 51 (48.6) | 34 (55.7) | 17 (38.6) | |||
| 30-34 | 48180 (23.1) | 29 (27.6) | 17 (27.9) | 12 (27.3) | |||
| 35+ | 28452 (13.7) | 16 (15.2) | 3 (4.9) | 13 (29.5) | |||
| Mother’s race/ethnicity | 0.7 | 0.5 | 0.05 | ||||
| White non-Hispanic | 75677 (36.4) | 42 (40.0) | 22 (36.1) | 20 (45.5) | |||
| Hispanic of any race | 93563 (44.9) | 45 (42.9) | 31 (50.8) | 14 (31.8) | |||
| Other/not specified | 38938 (18.7) | 18 (17.1) | 8 (13.1) | 10 (22.7) | |||
| Maternal birthplace | 0.8 | 0.5 | 0.2 | ||||
| Born in US | 117430 (56.5) | 63 (60.0) | 35 (57.4) | 28 (63.6) | |||
| Mexico | 53011 (25.5) | 25 (23.8) | 18 (29.5) | 7 (15.9) | |||
| Other Foreign | 37495 (18.0) | 17 (16.2) | 8 (13.1) | 9 (20.5) | |||
| Father’s age | 0.9 | 0.6 | 0.6 | ||||
| <20 | 8058 (4.1) | 4 (4.0) | 3 (5.2) | 1 (2.3) | |||
| 20-29 | 87273 (44.8) | 44 (43.6) | 29 (50.0) | 15 (34.9) | |||
| 30-34 | 50099 (25.7) | 27 (26.7) | 16 (27.6) | 11 (25.6) | |||
| 35+ | 49185 (25.3) | 26 (25.7) | 10 (17.2) | 16 (37.2) | |||
| Father’s race | 0.04 | 0.06 | 0.004 | ||||
| White non-Hispanic | 72066 (34.6) | 47 (44.8) | 24 (39.3) | 23 (52.3) | |||
| Hispanic of any race | 89530 (43.0) | 43 (41.0) | 31 (50.8) | 12 (27.3) | |||
| Other/not specified | 46582 (22.4) | 15 (14.3) | 6 (9.8) | 9 (20.5) | |||
| Maternal education (years) c | 0.05 | 0.9 | 0.04 | ||||
| 8 or fewer | 24485 (13.6) | 7 (6.9) | 6 (10.5) | 1 (2.3) | |||
| 9-<12 | 33121 (18.4) | 16 (15.8) | 10 (17.5) | 6 (13.6) | |||
| 12 | 52427 (29.1) | 25 (24.8) | 16 (28.1) | 9 (20.5) | |||
| 13-15 | 35590 (19.8) | 25 (24.8) | 11 (19.3) | 14 (31.8) | |||
| 16+ | 34514 (19.2) | 28 (27.7) | 14 (24.6) | 14 (31.8) | |||
| Paternal education (years) c | 0.3 | 0.9 | 0.1 | ||||
| 8 or fewer | 24685 (14.6) | 8 (8.2) | 6 (11.1) | 2 (4.7) | |||
| 9-<12 | 25145 (14.9) | 14 (14.4) | 10 (18.5) | 4 (9.3) | |||
| 12 | 52104 (30.9) | 28 (28.9) | 17 (31.5) | 11 (25.6) | |||
| 13-15 | 29800 (17.7) | 21 (21.6) | 10 (18.5) | 11 (25.6) | |||
| 16+ | 36966 (21.9) | 26 (26.8) | 11 (20.4) | 15 (34.9) | |||
| Source of payment for prenatal care c |
0.006 | 0.6 | 0.001 | ||||
| Private | 91651 (50.8) | 65 (64.4) | 31 (54.4) | 34 (77.3) | |||
| Medi-Cal/other governmental/self-pay | 88918 (49.2) | 36 (35.6) | 26 (45.6) | 10 (22.7) |
P-values, computed by chi-square, compare each group of cases to controls. All p-values are two sided.
Rhabdoid sarcomas include renal tumors and extra-renal non-CNS tumors.
Not reported in all years of the study.
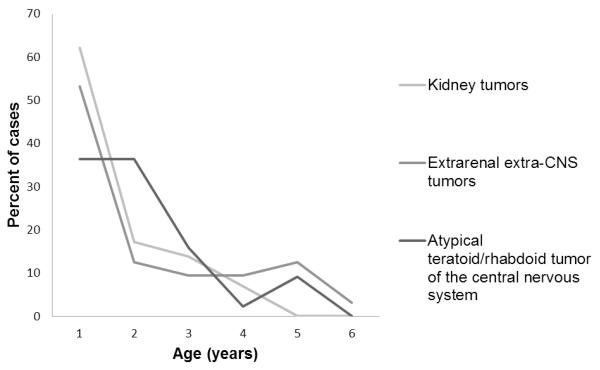
Figure I shows the age at diagnosis of cases. The median age at diagnosis for all cases was 12.3 months (mean=18.3 months). For rhabdoid sarcomas, it was 9.5 months [mean=17.1 months, standard deviation (SD)=17.0 months] and for AT/RT cases, 16.9 months (mean=19.5 months, SD=14.3 months).
Figure 1.
Age at diagnosis of rhabdoid tumors
Gestational characteristics are listed in Table II. In comparison to controls, rhabdoid tumor cases had a higher odds of low birthweight (OR=2.68, 95% CI 1.51, 4.75) and of preterm birth (OR=2.32, 95% CI 1.40, 3.87). Rhabdoid sarcoma and AT/RT cases both had a higher prevalence of low birthweight, and rhabdoid sarcoma cases had a higher prevalence of both preterm and late term delivery. The increased risk of cancer among children with low birthweight persisted when looking at singleton births only. Elevated point estimates for low birthweight among singletons were observed among all cases (adjusted OR=2.10, 95% CI 1.01, 4.37), and among rhabdoid sarcomas (adjusted OR=1.33, 95% CI 0.92, 5.89) and AT/RT (adjusted OR=1.85, 95% CI 0.56, 6.12) separately.
Table II.
Gestational factors in relation to rhabdoid tumors
| Control | All cases (n=105) | Rhabdoid sarcomasa (n=61) | AT/RT (n=44) | ||||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | Adjusted OR (95% CI) b |
n (%) | Adjusted OR (95% CI) b |
n (%) | Adjusted OR (95% CI) b |
|
| Birthweight | |||||||
| <2500g | 12171 (5.9) | 14 (13.3) | 2.68 (1.51, 4.75) | 7 (11.5) | 2.43 (1.09, 5.41) | 7 (15.9) | 2.99 (1.31, 6.84) |
| 2500-3999g | 173415 (83.4) | 78 (74.3) | Referent | 48 (78.7) | Referent | 30 (68.2) | Referent |
| 4000g+ | 22397 (10.8) | 13 (12.4) | 1.41 (0.78, 2.54) | 6 (9.8) | 1.09 (0.46, 2.57) | 7 (15.9) | 1.93 (0.85, 4.43) |
| Gestational age | |||||||
| Preterm (<37 weeks) | 20240 (10.3) | 20 (20.4) | 2.32 (1.40, 3.87) | 12 (21.1) | 2.63 (1.34, 5.17) | 8 (19.5) | 1.97 (0.90, 4.27) |
| Term (37-42 weeks) | 168708 (85.6) | 72 (73.5) | Referent | 39 (68.4) | Referent | 33 (80.5) | Referent |
| Postterm (43+ weeks) | 8141 (4.1) | 6 (6.1) | 2.19 (0.95, 5.06) | 6 (10.5) | 3.66 (1.54, 8.71) | 0 (0) | ------- |
| Size for gestational age c | |||||||
| Small | 20645 (10.3) | 10 (10.2) | 1.08 (0.57, 2.03) | 6 (10.5) | 0.84 (0.33, 2.11) | 4 (9.8) | 1.46 (0.61, 3.50) |
| Normal | 156189 (78.2) | 76 (77.6) | Referent | 45 (78.9) | Referent | 31 (75.6) | Referent |
| Large | 22908 (11.5) | 12 (12.2) | 1.05 (0.54, 2.03) | 6 (10.5) | 1.08 (0.46, 2.54) | 6 (14.6) | 1.00 (0.35, 2.85) |
| Method of delivery | |||||||
| Vaginal | 158789 (76.3) | 73 (69.5) | Referent | 44 (72.1) | Referent | 29 (65.9) | Referent |
| Cesarean | 49259 (23.7) | 32 (30.5) | 1.25 (0.81, 1.92) | 17 (27.9) | 1.28 (0.71, 2.29) | 15 (34.1) | 1.19 (0.63, 2.23) |
| Multiple birth | |||||||
| Singleton | 203010 (97.5) | 96 (91.4) | Referent | 57 (93.4) | Referent | 39 (88.6) | Referent |
| Twins or more | 5167 (2.5) | 9 (8.6) | 3.25 (1.63, 6.49) | 4 (6.6) | 3.08 (1.11, 8.55) | 5 (11.4) | 3.16 (1.23, 8.13) |
| Start of prenatal care | |||||||
| 1st trimester | 164462 (80.0) | 89 (85.6) | Referent | 50 (83.3) | Referent | 39 (88.6) | Referent |
| No care or after 1st trimester |
41029 (20.0) | 15 (14.4) | 1.02 (0.57, 1.83) | 10 (16.7) | 0.90 (0.43, 1.89) | 5 (11.4) | 1.13 (0.44, 2.94) |
| Parity | |||||||
| 0 | 81857 (39.3) | 43 (41.0) | Referent | 23 (37.7) | Referent | 20 (45.5) | Referent |
| 1 | 65078 (31.3) | 32 (30.5) | 0.90 (0.56, 1.45) | 21 (34.4) | 1.18 (0.63, 2.22) | 11 (25.0) | 0.63 (0.30, 1.32) |
| 2 or more | 61103 (29.4) | 30 (28.6) | 0.96 (0.57, 1.60) | 17 (27.9) | 1.04 (0.51, 2.13) | 13 (29.5) | 0.90 (0.43, 1.90) |
Rhabdoid sarcomas include renal tumors and extra-renal non-CNS tumors.
Odds ratios adjust for maternal age, maternal race/ethnicity, year of birth, and method of payment for prenatal care.
Size for gestational age was defined as small if birth weight was less than the 10th percentile and defined as large if greater than the 90th percentile of the birthweight standards for a given gestational age.
Gestation period was slightly shorter for AT/RT cases (mean=38.0 weeks, SD=3.5 weeks) in comparison to controls (mean=38.9 weeks, SD=2.5 weeks) and rhabdoid sarcoma cases (mean=38.6 weeks, SD=3.2 weeks; 2-sided p=0.02). There was no difference between cases and controls in size for gestational age.
Both rhabdoid sarcoma (OR=3.08, 95% CI 1.11, 8.55) and AT/RT (OR=3.16, 95% CI 1.23, 8.13) were more likely than controls to be twin or higher births. We did not observe differences in method of delivery, the start of prenatal care, or of parity.
Pregnancy or labor complications were rare in the dataset as a whole (Table III). In comparison to controls, rhabdoid tumor cases had a higher incidence of previous preterm births and had more reports of precipitous labor (<3 hours). Cases had higher rates of medical procedures at birth, and a greater likelihood of neonatal intensive care unit (NICU) admission than controls.
Table III.
Rhabdoid tumor in relation to pregnancy and labor complications, and clinical procedures relating to the newborn
| Controls | Rhabdoid tumor cases (n=105) |
||
|---|---|---|---|
| n (%) | n (%) | Adjusted ORa (95% CI) | |
| Previous preterm birth | 2016 (1.1) | 5 (4.9) | 4.49 (1.82, 11.09) |
| Precipitous labor (< 3 hours) | 2198 (1.2) | 5 (4.9) | 4.42 (1.80, 10.89) |
| Any medical procedure at birth | 6808 (4.2) | 9 (9.3) | 2.30 (1.16, 4.58) |
| NICU admission | 5185 (2.8) | 7 (6.9) | 2.19 (1.01, 4.73) |
Odds ratios adjust for maternal age, maternal race/ethnicity, year of birth, and method of payment for prenatal care. Variables were not reported in all years of the study.
Discussion
We observed a number of gestational characteristics to differ between rhabdoid tumor cases and our population controls. Rhabdoid tumor cases had high rates of low birthweight and preterm birth, and rhabdoid sarcoma (renal and extra-renal non-CNS) cases also had a higher likelihood of later gestational age. The shorter gestation of rhabdoid tumor cases that we observed has been previously reported [23]. The pregnancy and labor complications we observed, including increased odds of having any medical procedure at birth and having NICU admission, are not surprising given the greater likelihood of cases having shorter gestations and lower birthweight.
The larger numbers of multiple births among case families was striking. In our dataset, all instances of multiple pregnancies among cases were of twins, with no reports of triplets or higher; none of these twins were concordant for rhabdoid tumors. A number of case reports have documented rhabdoid tumors among both monozygotic and dizygotic twins [24-29]. In some instances, rhabdoid tumor cases were reported to be conceived by in vitro fertilization [27,28,30]. Researchers have previously speculated that there may be a link between assisted reproduction technologies (ART) and childhood cancers. The use of ART, which began to be reported on California birth certificates in 2006, was not reported for any rhabdoid case. However, ART is expected to be underreported on birth certificates [31]. It is not known whether multiple pregnancies are associated with other cancer risk factors. Previous studies which examined cancer in twins have not found increased risks for childhood cancers, although no study has reported specifically on rhabdoid tumors [32,33].
While rhabdoid sarcoma cases did not differ markedly from controls in demographic characteristics with the exception of paternal race/ethnicity, we observed considerable differences between AT/RT cases and controls in maternal age, maternal and paternal race/ethnicity and in several socioeconomic indices. We observed an increased risk for AT/RT among older mothers, as has been seen elsewhere [23]. Older maternal age has been linked to several other childhood cancers, including retinoblastoma, leukemia, and astrocytoma [34,35]. This may be explained by increases in chromosomal aberrations in maternal germ cells with aging [36]. Additionally there are changes in placental function and the uterine environment with older maternal age which may influence cancer risk [37-39].
Although other studies have reported either a female or a male predominance of tumors [8,20,23,40,41], we did not observe a differential risk in relation to the child’s sex. SEER has also reported no difference in risk between male and female children [42].
These results must be interpreted carefully, given the likelihood of underdiagnosed cases, particularly in the earlier years of the study [43]. Further, the accuracy of birth certificates varies by data item. Birthweight, type of insurance, and method of delivery tend to be highly valid, while length of gestation tends to have moderate to high validity [44-46]. As we did not have access to medical records, we were lacking additional information that may have shed light on etiology. Although we were interested to examine the unique characteristics of patients with multiple primary tumors, we could not do so because rhabdoid tumors have no special treatment in TNM staging that would indicate the presence of multiple tumors.
In conclusion, we observed rhabdoid tumors to be associated with low birthweight, preterm labor, and twin pregnancies. This is the first population-based epidemiologic study for this rare tumor.
Acknowledgements
This study was supported by grants from the National Institutes of Health (R21ES018960, R21ES019986).
References
- 1.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: results from the First National Tumor’ Wilms Study. Cancer. 1978;41:1937–1948. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Fanburg-Smith JC, Hengge M, Hengge UR, et al. Extrarenal rhabdoid tumors of soft tissue: a clinicopathologic and immunohistochemical study of 18 cases. Ann Diagn Pathol. 1998;2:351–362. doi: 10.1016/s1092-9134(98)80038-5. [DOI] [PubMed] [Google Scholar]
- 3.Rorke LB, Packer R, Biegel J. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood. J Neurooncol. 1995;24:21–28. doi: 10.1007/BF01052653. [DOI] [PubMed] [Google Scholar]
- 4.Kleihues P, Cavenee WK, International Agency for Research on Cancer . Pathology and genetics of tumours of the nervous system. IARC Press; Lyon: 2000. p. 314. [Google Scholar]
- 5.Tomlinson GE, Breslow NE, Dome J, et al. Rhabdoid tumor of the kidney in the National Wilms’ Tumor Study: age at diagnosis as a prognostic factor. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:7641–7645. doi: 10.1200/JCO.2004.00.8110. 10.1200/JCO.2004.00.8110. [DOI] [PubMed] [Google Scholar]
- 6.Zhuge Y, Cheung MC, Yang R, et al. Pediatric non-Wilms renal tumors: subtypes, survival, and prognostic indicators. The Journal of surgical research. 2010;163:257–263. doi: 10.1016/j.jss.2010.03.061. 10.1016/j.jss.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 7.Madigan CE, Armenian SH, Malogolowkin MH, et al. Extracranial malignant rhabdoid tumors in childhood: the Childrens Hospital Los Angeles experience. Cancer. 2007;110:2061–2066. doi: 10.1002/cncr.23020. 10.1002/cncr.23020. [DOI] [PubMed] [Google Scholar]
- 8.Woehrer A, Slavc I, Waldhoer T, et al. Incidence of atypical teratoid/rhabdoid tumors in children: a population-based study by the Austrian Brain Tumor Registry, 1996-2006. Cancer. 2010;116:5725–5732. doi: 10.1002/cncr.25540. 10.1002/cncr.25540. [DOI] [PubMed] [Google Scholar]
- 9.Sultan I, Qaddoumi I, Rodriguez-Galindo C, et al. Age, stage, and radiotherapy, but not primary tumor site, affects the outcome of patients with malignant rhabdoid tumors. Pediatr Blood Cancer. 2010;54:35–40. doi: 10.1002/pbc.22285. 10.1002/pbc.22285. [DOI] [PubMed] [Google Scholar]
- 10.Annual incidence of rhabdoid tumors among children <15, 2000-2008. Surveillance, Epidemiology, and End Results (SEER) Program; Bethesda, MD: 2011. Volume SEER 17. SEER*Stat version 7.0.6. [Google Scholar]
- 11.Vujanic GM, Sandstedt B, Harms D, et al. Rhabdoid tumour of the kidney: a clinicopathological study of 22 patients from the International Society of Paediatric Oncology (SIOP) nephroblastoma file. Histopathology. 1996;28:333–340. doi: 10.1046/j.1365-2559.1996.d01-436.x. [DOI] [PubMed] [Google Scholar]
- 12.Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17:503–511. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed HU, Arya M, Levitt G, et al. Part I: Primary malignant non-Wilms’ renal tumours in children. Lancet Oncol. 2007;8:730–737. doi: 10.1016/S1470-2045(07)70241-3. 10.1016/S1470-2045(07)70241-3. [DOI] [PubMed] [Google Scholar]
- 14.Versteege I, Sevenet N, Lange J, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203–206. doi: 10.1038/28212. 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 15.Biegel JA, Zhou JY, Rorke LB, et al. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–79. [PubMed] [Google Scholar]
- 16.Eaton KW, Tooke LS, Wainwright LM, et al. Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer. 2011;56:7–15. doi: 10.1002/pbc.22831. 10.1002/pbc.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson J. Malignant rhabdoid tumors: a familial condition? Pediatr Blood Cancer. 2011;56:1–2. doi: 10.1002/pbc.22834. 10.1002/pbc.22834. [DOI] [PubMed] [Google Scholar]
- 18.Swinney RM, Bowers DC, Chen TT, et al. Rhabdoid tumors in a shared parental environment. Pediatr Blood Cancer. 2006;47:343–344. doi: 10.1002/pbc.20846. 10.1002/pbc.20846. [DOI] [PubMed] [Google Scholar]
- 19.Brennan BM, Foot AB, Stiller C, et al. Where to next with extracranial rhabdoid tumours in children. Eur J Cancer. 2004;40:624–626. doi: 10.1016/j.ejca.2003.11.014. 10.1016/j.ejca.2003.11. [DOI] [PubMed] [Google Scholar]
- 20.Packer RJ, Biegel JA, Blaney S, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: report on workshop. J Pediatr Hematol Oncol. 2002;24:337–342. doi: 10.1097/00043426-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ritz B, Wilhelm M, Hoggatt KJ, et al. Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. Am J Epidemiol. 2007;166:1045–1052. doi: 10.1093/aje/kwm181. [DOI] [PubMed] [Google Scholar]
- 22.Alexander GR, Himes JH, Kaufman RB, et al. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 23.Isaacs H., Jr. Fetal and neonatal rhabdoid tumor. J Pediatr Surg. 2010;45:619–626. doi: 10.1016/j.jpedsurg.2009.12.011. 10.1016/j.jpedsurg.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Sahdev I, James-Herry A, Scimeca P, et al. Concordant rhabdoid tumor of the kidney in a set of identical twins with discordant outcomes. J Pediatr Hematol Oncol. 2003;25:491–494. doi: 10.1097/00043426-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez C, Bouvier C, Sevenet N, et al. Congenital disseminated malignant rhabdoid tumor and cerebellar tumor mimicking medulloblastoma in monozygotic twins: pathologic and molecular diagnosis. Am J Surg Pathol. 2002;26:266–270. doi: 10.1097/00000478-200202000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Howlett DC, King AP, Jarosz JM, et al. Imaging and pathological features of primary malignant rhabdoid tumours of the brain and spine. Neuroradiology. 1997;39:719–723. doi: 10.1007/s002340050494. [DOI] [PubMed] [Google Scholar]
- 27.Nicolaides T, Tihan T, Horn B, et al. High-dose chemotherapy and autologous stem cell rescue for atypical teratoid/rhabdoid tumor of the central nervous system. J Neurooncol. 2010;98:117–123. doi: 10.1007/s11060-009-0071-6. 10.1007/s11060-009-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puri DR, Meyers PA, Kraus DH, et al. Radiotherapy in the multimodal treatment of extrarenal extracranial malignant rhabdoid tumors. Pediatr Blood Cancer. 2008;50:167–169. doi: 10.1002/pbc.20947. 10.1002/pbc.20947. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez E, Castellote A, Mayolas N, et al. Congenital tumours involving the head, neck and central nervous system. Pediatr Radiol. 2009;39:1158–1172. doi: 10.1007/s00247-009-1369-4. 10.1007/s00247-009-1369-4. [DOI] [PubMed] [Google Scholar]
- 30.Cecen E, Gunes D, Uysal KM, et al. Atypical teratoid/rhabdoid tumor in an infant conceived by in vitro fertilization. Childs Nerv Syst. 2010;26:263–266. doi: 10.1007/s00381-009-1005-5. 10.1007/s00381-009-1005-5. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Macaluso M, Cohen B, et al. Accuracy of assisted reproductive technology information on the Massachusetts birth certificate, 1997-2000. Fertil Steril. 2010;94:1657–1661. doi: 10.1016/j.fertnstert.2009.10.059. 10.1016/j.fertnstert.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 32.Inskip PD, Harvey EB, Boice JD, Jr., et al. Incidence of childhood cancer in twins. Cancer Causes Control. 1991;2:315–324. doi: 10.1007/BF00051671. [DOI] [PubMed] [Google Scholar]
- 33.Rodvall Y, Hrubec Z, Pershagen G, et al. Childhood cancer among Swedish twins. Cancer Causes Control. 1992;3:527–532. doi: 10.1007/BF00052749. [DOI] [PubMed] [Google Scholar]
- 34.Yip BH, Pawitan Y, Czene K. Parental age and risk of childhood cancers: a population-based cohort study from Sweden. Int J Epidemiol. 2006;35:1495–1503. doi: 10.1093/ije/dyl177. 10.1093/ije/dyl177. [DOI] [PubMed] [Google Scholar]
- 35.Dockerty JD, Draper G, Vincent T, et al. Case-control study of parental age, parity and socioeconomic level in relation to childhood cancers. Int J Epidemiol. 2001;30:1428–1437. doi: 10.1093/ije/30.6.1428. [DOI] [PubMed] [Google Scholar]
- 36.Pellestor F, Anahory T, Hamamah S. Effect of maternal age on the frequency of cytogenetic abnormalities in human oocytes. Cytogenet Genome Res. 2005;111:206–212. doi: 10.1159/000086891. 10.1159/000086891. [DOI] [PubMed] [Google Scholar]
- 37.Collier AC, Tingle MD, Paxton JW, et al. Metabolizing enzyme localization and activities in the first trimester human placenta: the effect of maternal and gestational age, smoking and alcohol consumption. Hum Reprod. 2002;17:2564–2572. doi: 10.1093/humrep/17.10.2564. [DOI] [PubMed] [Google Scholar]
- 38.Yamada Z, Kitagawa M, Takemura T, et al. Effect of maternal age on incidences of apoptotic and proliferative cells in trophoblasts of full-term human placenta. Mol Hum Reprod. 2001;7:1179–1185. doi: 10.1093/molehr/7.12.1179. [DOI] [PubMed] [Google Scholar]
- 39.Gosden RG. Maternal age: a major factor affecting the prospects and outcome of pregnancy. Ann N Y Acad Sci. 1985;442:45–57. doi: 10.1111/j.1749-6632.1985.tb37504.x. [DOI] [PubMed] [Google Scholar]
- 40.Bourdeaut F, Freneaux P, Thuille B, et al. Extra-renal non-cerebral rhabdoid tumours. Pediatr Blood Cancer. 2008;51:363–368. doi: 10.1002/pbc.21632. 10.1002/pbc.21632. [DOI] [PubMed] [Google Scholar]
- 41.Morgenstern DA, Gibson S, Brown T, et al. Clinical and pathological features of paediatric malignant rhabdoid tumours. Pediatr Blood Cancer. 2010;54:29–34. doi: 10.1002/pbc.22231. 10.1002/pbc.22231. [DOI] [PubMed] [Google Scholar]
- 42.Bishop AJ, McDonald MW, Chang AL, et al. Infant Brain Tumors: Incidence, Survival, and the Role of Radiation Based on Surveillance, Epidemiology, and End Results (SEER) Data. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2010.08.020. 10.1016/j.ijrobp.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Athale UH, Duckworth J, Odame I, et al. Childhood atypical teratoid rhabdoid tumor of the central nervous system: a meta-analysis of observational studies. J Pediatr Hematol Oncol. 2009;31:651–663. doi: 10.1097/MPH.0b013e3181b258a9. 10.1097/MPH.0b013e3181b258a9. [DOI] [PubMed] [Google Scholar]
- 44.Northam S, Knapp TR. The reliability and validity of birth certificates. J Obstet Gynecol Neonatal Nurs. 2006;35:3–12. doi: 10.1111/j.1552-6909.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- 45.Roohan PJ, Josberger RE, Acar J, et al. Validation of birth certificate data in New York State. J Community Health. 2003;28:335–346. doi: 10.1023/a:1025492512915. [DOI] [PubMed] [Google Scholar]
- 46.Piper JM, Mitchel EF, Jr., Snowden M, et al. Validation of 1989 Tennessee birth certificates using maternal and newborn hospital records. Am J Epidemiol. 1993;137:758–768. doi: 10.1093/oxfordjournals.aje.a116736. [DOI] [PubMed] [Google Scholar]