Abstract
Manganese (Mn) has been implicated in the impairment of the glutamate-glutamine cycling (GGC) by deregulation of Glu and glutamine (Gln) turnover in astrocytes. Here we have examined possible mechanisms involved in the Mn(II)-mediated disruption of Glu turnover, including those related to protein degradation, such as the proteasomal and lysosomal machinery. Our study revealed that lysosome, but not proteasomal inhibition is responsible for downregulation of the Glu transporter after Mn(II) treatment. Because protein kinase C (PKC) activation leads to the downregulation of Glu carriers, and Mn(II) increases PKC activity, we hypothesized that the PKC signalling contributes to the Mn(II)-mediated disruption of Glu turnover. Our results show that PKC activation causes a decrease in Glu uptake and that inhibition of PKC reverses Mn(II)–dependent downregulation of Glu influx as well as glutamate transporter 1 (GLT1) and glutamate–aspartate transporter (GLAST) protein level. Co-immunoprecipitation studies show association of GLT1 with the PKCδ and PKCα isoforms and Mn(II)–induced specific increase in PKCδ-GLT1 interaction. Additionally, astrocytes transfected with shRNA against PKCδ show decreased sensitivity to Mn(II) compared to those transfected with control shRNA or shRNA targeted against PKCα. Taken together, these findings demonstrate that PKCδ signalling is involved in the Mn(II)-induced deregulation of Glu turnover in astrocytes.
Keywords: manganese, GGC, GLT1, GLAST, PKC signalling
INTRODUCTION
Manganese (Mn) is a naturally occurring trace metal, which is commonly found in the environment and has well-defined physiological functions within all tissues, including the brain (Prohaska, 1987; Takeda 2003). However, excessive exposure to Mn(II) causes neurodegeneration and/or neuronal dysfunction (Erikson and Aschner, 2003; Roth and Garrick, 2003; Weiss, 2006). Excessive accumulation of Mn(II) in specific brain areas, such as the substantia nigra, the globus pallidus and the striatum, causes a neurological brain disorder, referred to as manganism, which is characterized by early psychotic symptoms and frequently followed by chronic symptoms analogous to those in idiopathic Parkinson’s disease (Racette et al. 2001). Although the underlying mechanisms of manganism are not completely understood, disturbance in glutamine(Gln)/glutamate(Glu) cycling (GGC) between neurons and astrocytes have been posited as a major executor in its etiology (Brouillet et al. 1993; Lee et al. 2009; Sidoryk-Wegrzynowicz et al. 2009).
GGC is critical for optimal CNS function. Given the inability of neurons to perform de novo synthesis of Glu and gamma-amino butyric acid (GABA) from glucose, they are metabolically dependent upon astrocytes (Berl and Clarke, 1983). Under optimal conditions, a major proportion of astroglia-derived Gln is shuttled to neurons, where it is degraded and gives rise to the excitatory neurotransmitter Glu and indirectly GABA (Sonnewald et al. 1993). Our earlier study has demonstrated that Mn(II) exposure leads to altered expression and function of astrocytic transporters involved in neuronal Gln supply (Sidoryk-Wegrzynowicz et al. 2009).
The importance of GGC cycling is also dramatically illustrated in the deregulation of the astrocytic Glu transporters, glutamate–aspartate transporter (GLAST) and GLT1 in various neuropathological conditions (Danbolt, 2001). In optimal physiological conditions, astrocytes are responsible for neuronal activity regulation by removal of neurotransmitters from synaptic cleft (80% of Glu released from neurons is taken up by astrocytes) (Choi et al. 1987; Rothstein et al. 1996). Glu transporters are active during the first week of postnatal life, suggesting that astrocytes are primed for regulating neurotransmission at early life stages (Regan et al., 2007). Both in vivo and in vitro studies have shown that knockdown of glial GLAST or GLT1 produces elevated extracellular Glu levels and excitotoxic neurodegeneration (Rothstein et al. 1996).
Astrocytes possess a greater ability to accumulate Mn(II) than neurons, and an excess of brain Mn(II) primarily affects the functioning of glial cells (Aschner et al., 1992). Several studies have shown than excessive exposure to Mn(II) leads to the downregulation of Glu transporters and to impairment in glutamatergic neurotransmission, representing one of the most prominent neuropathologic effects of Mn(II) ( Erikson et al. 2002; Erikson et al. 2008; Lee et al. 2009).
Here we sought to examine possible pathways involved in Mn(II)-induced disruption of Glu turnover and extend our previous investigations in defining the effect of Mn(II) on GGC and astroglial dysfunction. Since protein kinase C (PKC) activation leads to the downregulation of Glu transporters (Conradt and Stoffel, 1997; Ramamoorthy 1998; Loder et al. 2003; Gonzalez et al. 2005), and Mn(II) increases PKC activity (Sidoryk-Wegrzynowicz et al. 2011), we hypothesized that the PKC signaling pathway contributes to the Mn(II)-induced disruption of Glu turnover. Our previous study showed that Mn(II) exposure increases phosphorylation of PKCδ and the interaction of this isoform with the Glu transporter, ASCT2 (Sidoryk-Wegrzynowicz et al., 2011). Based on this finding, as well as evidence on the important role of PKCδ in Mn(II) neurotoxicity, we hypotesized that this isoform may be specifically involved in Mn(II) – dependent disruption of GGC (Anantharam et al. 2002; Kitazawa et al. 2005, Latchoumycandane et al. 2005).
Previous studies showed that both PKC activation and treatment with Mn(II) led to protein poliubiquitination (Sidoryk-Wegrzynowicz et al. 2011). Specifically, Mn(II) was shown to be associated with the ubiquitin proteolytic system where it increased the expression of ubiquitin ligases Nedd4-2 (neuronal precursor cell expressed, developmentally down-regulated 4-2 activity), which promotes protein/substrate targeting to ubiquitin (Sidoryk-Wegrzynowicz et al. 2010). Ubiquitination of protein leads to its degradation by the 26 S proteasome or may initiate endocytosis signal targeting of proteins for lysosomal degradation (Hicke 1997; Miranda et al. 2007). Accordingly, both pathways, namely the proteasomal and lysosomal pathway, as well as PKC signalling were investigated in the current study due their sensitivity to Mn(II).
MATERIAL AND METHODS
Chemicals, reagents, antibodies and cell culture supplies
Manganese chloride(MnCl2); the proteasome inhibitor, ALLN (N-acetyl-leu-leu-norleucinal); the lysosomal inhibitors: bafilomycine A1 (BAF), chloroquine diphosphate (CHLORO); PKCs inhibitors: bisindolylmaleimide II (BIS II), Go6976, Rottlerin (ROT); caspase inhibitor Z-VAD-FMK (Z-Ala-Glu(OMe)-Val-Asp(OMe)-fluoromethyl ketone); PKC activator α-phorbol 12-myristate 13-acetate (PMA), glutamate (Gln) were purchased from Sigma Chemical Co. (St Louis, MO, USA). Minimal essential medium (MEM) with Earle’s salts, heat-inactivated horse serum, penicillin and streptomycin were purchased from Invitrogen (Carlsbad, CA, USA). Primary antibodies against GLAST, GLT1, PKCα, PKCδ were obtained from Abcam, (Cambridge, MA, USA); beta-actin was obtained from Sigma Chemical Co. Secondary, HRP-conjugated antibodies against mouse and rabbit IgGs and anti-Lamin B were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Primary cultures of astrocytes
Primary cultures of astrocytes were prepared according to previously established protocols (Aschner et al. 1992). One-day-old Sprague-Dawley rats were decapitated under halothane anesthesia, and the cerebral cortices were dissected out and digested with bacterial neutral protease (Dispase, Invitrogen, Eugene, OR, USA). All experiments were performed in accordance with the guiding principles of the Institutional Animal Care and Use Committee at Vanderbilt University Medical School. Astrocytes were then recovered by the repeated removal of dissociated cells and plated at a density of 1 × 105 cells/ml. Twenty-four hours after the initial plating, the medium was changed to preserve the adhering astrocytes and to remove neurons and oligodendrocytes. The cultures were maintained at 37°C in a 95% incubator for 3 weeks in MEM with Earle’s salts supplemented with 10% air/5% CO2 fetal bovine serum, 100 U/mL of penicillin and 100 μg/mL of streptomycin. The medium was replaced twice per week. The surface-adhering monolayer cultures were > 95% positive for the astrocytic marker, glial fibrillary acidic protein (GFAP). All experiments were performed 3 weeks post-isolation.
Treatment Protocols
Where indicated, MnCl2 was added to the culture medium. The chosen MnCl2 concentrations were based on our previous findings. The physiological range (no symptoms) was 75–100 μM, and clinical signs increased in frequency and severity above this level. Cells were treated with 100 μM (physiological range) or 500 μM MnCl2, representing a range of Mn(II) concentrations considered to be toxic in cultured astrocytes (Milatovic et al. 2007), as well as in the in vivo brain (Suzuki et al. 1975). For assessment on the role of the proteasome and lysosome in Mn(II)-induced downregulation of Glu turnover, cells were treated with 10 μM ALLN (N-acetyl-leu-leu-norleucinal) and 500 nM BAF (bafilomycine A1) or 100 μM CHLORO (chloroquine diphosphate), respectively. The role of PKC signaling or caspase 3 in Mn(II)-induced downregulation of Glu transport was evaluated in the presence of 100 nM PMA (α-phorbol 12-myristate 13-acetate), 10 μM BIS II (bisindolylmaleimide II), 1μM Gö6976, 5 μM Rot (Rottlerin). Caspase-3 involvement in Mn(II)-dependent disruption of Glu turnover were performed in the presence of caspase-3 inhibitor - 50 μM Z-VAD-FMK (Z-Ala-Glu(OMe)-Val-Asp(OMe)-fluoromethyl ketone.
PKCδ and PKCα Knockdown by Short Hairpin RNA (shRNA)
At 50–60% confluence, primary astrocytes were infected for 24 h with 15 μl lentiviral particles containing 1 × 106 infectious units of virus (IFU) prior to 1, 4, 8 and 24 h 500 μm Mn(II) treatment. Knockdown efficiency was assessed by western blot analysis. Lentiviral particles containing expression shRNA constructs against PKCδ and PKCα (Santa Cruz Biotechnology, Santa Cruz, CA) were used to knock down PKCδ. Control shRNA lentiviral particles containing a sequence with no known gene target (Santa Cruz Biotechnology, Santa Cruz, CA) was used as the negative control.
Astrocyte glutamate uptake assay
Astrocytes (24-well plates) were treated with 10 μM ALLN, 100 μM CHLORO, 10 μM BIS II, 1μM Gö6976, 5 μM Rot or 50 μM Z-VAD-FMK, for 1, 4, 8, 24 h or with 100 nM PMA for 0.5, 1, 2, 4, 6, 8, or 24 h. Next, the culture media were washed and replaced with the uptake buffer, pre-warmed HEPES (4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid) -buffered solution containing 122 mM NaCl, 3.3 mM KCl, 0.4 mM MgSO4, 1.3 mM CaCl2, 1.2 mM KH2PO4, 25 mM HEPES, and 10 mM D-(+)-glucose, pH of 7.4. Five min later, pre-warmed uptake buffer containing 0.25 μCi/ml L-[3H]-glutamate (specific activity: 49.0 Ci/mmol, Amersham Pharmacia Biotech, Piscataway, NJ, USA) and unlabelled Glu at a final concentration of 100 nM (Lee et al. 2009) was added. Uptake was terminated after 10 min of incubation at 37°C with ice-cold sodium-HEPES buffer, and cells were lysed by incubation with 1 M NaOH for 30 min at 37°C. An aliquot of the lysate was transferred to a scintillation vial, LSC cocktail (Fisher Scientific) was added, and radioactivity was measured in a scintillation counter (LS 6500 Beckman, Fullerton, CA, USA). The remainder of the lysate was used for protein determination by the bicinchoninic acid protein assay (Pierce, Rockford, IL, USA). Radioactivity counts were corrected for protein levels and calculated as Glu nmol/mg protein/min. Experiments were performed in quadruplicates in three-five independent cultures.
Nuclear and cytosol fractionation
Cells were exposed to 100 μM or 500 μM Mn for 1, 4, 8 or 24 h, and after each treatment the nuclear and cytoplasmic were fractionated with the Nuclear/cytosol fractionation kit, following the manufacturer’s protocol (BioVison, Mountain View, CA, USA).
Western blot analysis
After treatment, astrocytes were washed twice with phosphate-buffered solution (PBS) and then lysed with RIPA buffer (radio immunoprecipitation assay buffer: 150 mM NaCl, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate and 50 mM Tris, pH 8.0), containing a protease inhibitor and phosphatase inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA). Protein concentrations in the astrocytic lysates were determined with the bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). An equal amount of protein (30 μg) was loaded and run on a 12% SDS-PAGE and then transferred to a polyvinylidenefluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked for 1h at room temperature in 5% non-fat dry milk in Tris Buffered Saline with 0.1% Tween 20 (TBS-T). Incubations with primary antibodies were carried out at 4°C for 24 h in 5% milk in TBS-T buffer at the following concentrations: anti-GLAST (1:1,000), anti-GLT1 (1:1,000), anti-Lamin B (1:500), anti-PKCδ (1: 2,000), anti-PKCα (1:1,000), anti-beta actin (1:5,000). Secondary antibody incubations were performed at room temperature for 1.5 h using anti-rabbit IgG or anti-mouse IgG (1:2,000). Western blots were visualized with Thermo Scientific Pierce Supersignal West Dura Extended Duration Chemiluminescent Substrate (Pierce, Rockford, IL, USA). Band intensities were quantified with the AlphaEaseFC Imaging System software (Alpha Innotech, San Leandro, CA, USA).
Co-immunoprecipitation
Cells were washed twice with PBS and then lysed with RIPA buffer containing protease and phosphatase inhibitors. Lysates containing 1000 μg of protein were pre-cleared by shaking at 4°C for 1 h with 40 μl of Pansorbin cells (Calbiochem, San Diego, CA, USA) followed by centrifugation at 3,000 × g for 5 min at 4°C. The supernatant was incubated overnight at 4°C with GLAST and GLT1 or control rabbit IgG antibodies. Proteins were precipitated by incubation for 2 h at 4°C with 25 μl of protein-G agarose beads (Sigma-Aldrich, St Louis, MO, USA) and subsequent centrifugation at 1,000 x g for 1 min at 4°C. Immunocomplexes were washed three times with RIPA buffer and once with wash buffer (100 mM NaCl, 50 mM Tris-HCl, pH 7.5, 0.5% Nonidet P-40) solubilized at 95°C for 5 min in 40 μl of SDS-PAGE loading buffer with 0.1M DTT, and then subjected to western blot analysis.
Statistical analysis
Results are expressed as the mean ± S.D. from a minimum of three independent astrocytic cultures. Statistical analysis was carried out with two-way and/or one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. All analyses were performed with GraphPad Prism 4.02 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Glu uptake in astrocytes exposed to Mn(II) and inhibitors of the lysosomal/endosomal or proteasome machinery of protein degradation
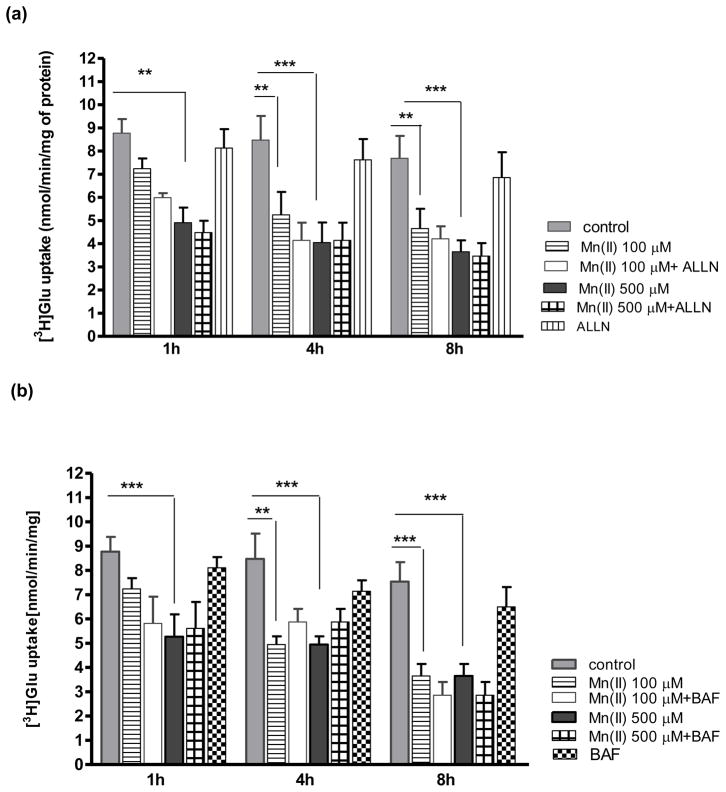
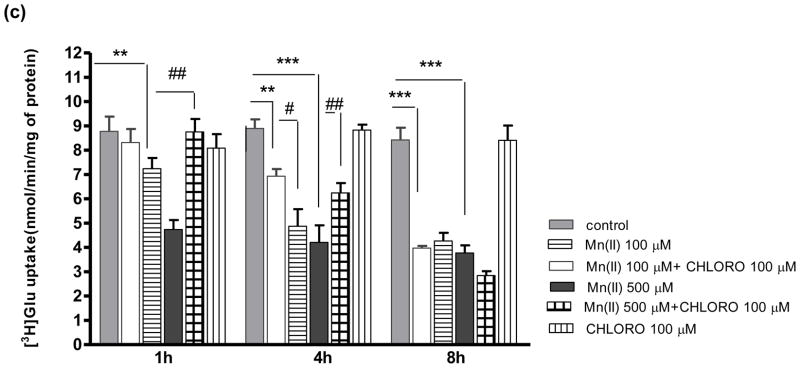
To investigate whether Mn(II) targets the Glu transporter to the lysosomal/endosomal or proteasome machinery of protein degradation we performed a series of experiments. Cultured primary astrocytes were incubated for various time points with the lysosomal inhibitors chloroquine (CHLORO) and bafiliomycin (BAF), or the proteasome inhibitor ALLN. BAF (500 nM) and ALLN (10 μM) failed to reverse the Mn(II)-induced decrease in astrocytic Glu uptake at 1, 4, 8 h (Fig. 1a, b). However, co- treatment with 500 μM Mn(II) for 1 h, or with 100 μM and 500 μM Mn(II) for 4 h and CHLORO reversed the Mn(II)–induced decrease in Glu transport (Fig. 1c).
Fig. 1. Glu uptake in astrocytes exposed to Mn(II) and inhibitors of the lysosomal/endosomal or proteasome machinery of protein degradation.
Total L-(G-3H)-Gln (0.25 μCi, specific activity: 49.0 Ci/mmol) uptake was measured after 1, 4 or 8 h exposure to 100 or 500 μM Mn alone or in the presence of the proteasome inhibitor ALLN, two -way ANOVA found main effect of Mn(II) exposure ((F(1,101)=34.12, p<0.0001) and Mn(II) x time interaction ((F(1, 101)=45.43, p<0.001) on Glu uptake (a); the lysosomal inhibitors: bafiliomycin (BAF), two -way ANOVA found main effect of Mn(II) exposure ((F(1,98)=78.56, p<0.0001) and Mn(II) x time interaction ((F(1, 98)=45.46, p<0.001) on Glu uptake (b); chloroquine (CHLORO), two -way ANOVA found main effect of Mn(II) exposure ((F(1,102)=66.42, p<0.0001), Mn(II) x time interaction ((F(1, 102)=65.23, p<0.001) and Mn(II) x CHLORO interaction ((F(1, 102)=5.133, p<0.025) on Glu uptake (c). Data represent the mean ± S.D. from 4–5 independent sets of cultures, each performed in triplicate; **p < 0.01, ***p < 0.001 vs. control; #p < 0.05, ##p < 0.01 vs. Mn(II) exposed cells (one-way ANOVA followed by post hoc Tukey’s test).
Glu uptake in astrocytes exposed to Mn (II) and inhibitors or activators of the PKC pathway
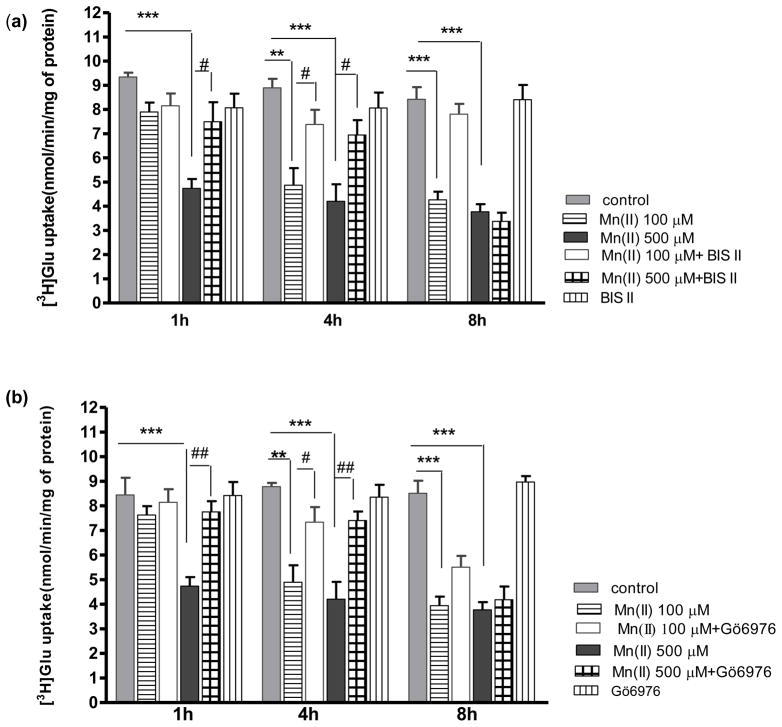
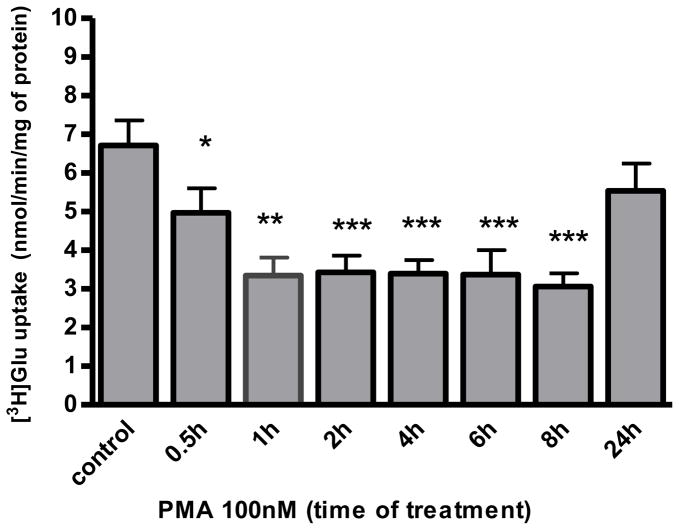
Our previous study established that Mn(II) increases PKC activity (Sidoryk-Wegrzynowicz et al. 2011). Given that PKC activation leads to the downregulation of a number of neurotransmitter transporters, including Glu transporters (for review see Gonzalez and Robinson, 2004), we hypothesized that the PKC pathway contributes to the Mn(II)-induced disruption of Glu turnover. To address whether modulation of PKC activity may affect Glu turnover, an uptake assay was performed in the presence of the PKC activator - phorbol 12-myristate 13-acetate (PMA) and the PKC inhibitor - bisindolylmaleimide II (BIS II). As shown in Fig. (2), PKC stimulation with PMA from 0.5 - 8 h significantly decreased astrocytic Glu uptake with a maximal effect at 8 h (43 % ± 4% of control); however, no effect on Glu uptake was observed after 24 h of PKC stimulation (data not shown). Co-treatment with Bis II reversed Mn(II)-induced Glu uptake inhibition after 1 h and 4 h (Fig. 3a). These results strongly support the hypothesis that PKC signaling is involved in Mn (II)–mediated disruption of Glu transport.
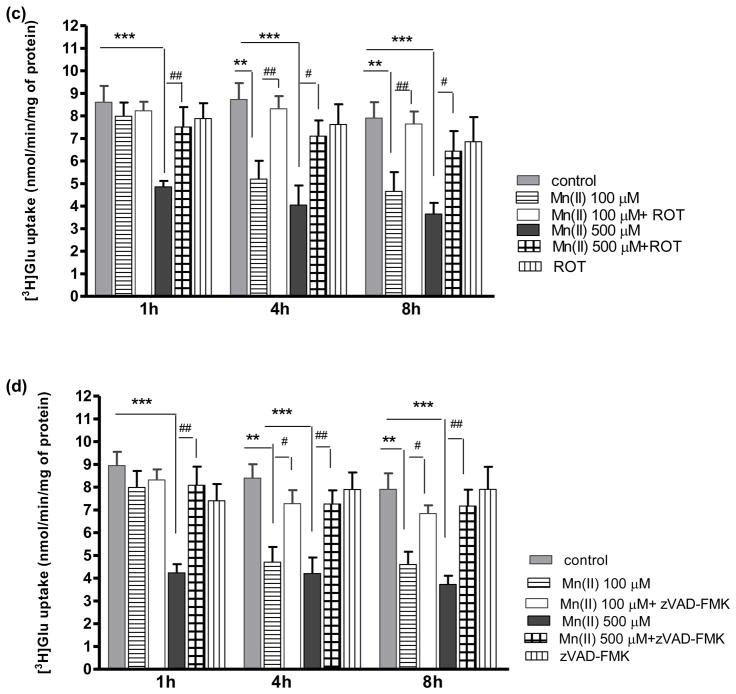
Fig. 3. Effect of PKC inhibition on Mn(II)-induced downregulation of astrocytic Glu uptake.
Total L-(G-3H)-Gln (0.25 μCi, specific activity: 49.0 Ci/mmol) uptake was measured after 1, 4 or 8 h exposure to 100 μM or 500 μM of Mn(II) alone or in presence of the general PKC inhibitor bisindolylmaleimide II (BIS II), two -way ANOVA found main effect of Mn(II) exposure ((F(1,103)=54.62, p<0.0001), Mn(II) x time interaction ((F(1, 103)=22.33, p<0.05) and Mn(II) x BIS II interaction ((F(1, 103)=15.099, p<0.0002) on Glu uptake (a); the selective PKCα inhibitor Gö6976, two -way ANOVA found main effect of Mn(II) exposure ((F(1,104)=73.60, p<0.0001), Mn(II) x time interaction ((F(1, 73)=62.13, p<0.05) and Mn(II) x Gö6976 interaction ((F(1, 103)=8.153, p<0.0052) on Glu uptake (b); the selective PKCδ inhibitor Rottlerin (ROT), two- way ANOVA found main effect of Mn(II) exposure ((F(1,87)=14.18, p<0.0002), Mn(II) x time interaction ((F(1, 93)=42.23, p<0.05) and Mn(II) x ROT interaction ((F(1, 87)=17.18, p<0.0001) on Glu uptake (c); the caspase inhibitor Z-VAD-FMK (Z-Ala-Glu(OMe)-Val-Asp(OMe)-fluoromethyl ketone), two -way ANOVA found main effect of Mn(II) exposure ((F(1,101)=20.69, p<0.0001), Mn(II) x time interaction ((F(1, 77)=46.24, p<0.05) and Mn(II) x zVAD-FMK interaction ((F(1, 101)=26.80, p<0.0001) on Glu uptake (d). Data represent the mean ± S.D. from 4–5 independent sets of cultures, each performed in triplicate; **p < 0.01, ***p < 0.001 vs. control; #p < 0.05, ##p < 0.01 vs. Mn(II) exposed cells (one-way ANOVA followed by post hoc Tukey’s test).
Our previous study demonstrated that Mn(II) treatment increases the phosphorylation of both the PKCα and PKCδ isoforms (Sidoryk-Wegrzynowicz et al. 2011). Therefore, we sought to determine if these isoforms mediate the Mn(II)-induced decrease in Glu transport. As shown in Fig. 3(b) the inhibition of astrocytic Glu uptake after Mn(II) treatment for 1 h (500 μM Mn(II)) or 4 h (100 μM and 500 μM Mn(II)) was abrogated by the specific inhibitor of PKCα, 1 μM Go6976. When present in the medium for >4 h there appeared to be a trend towards restoration of the Mn(II)-induced decrease in Glu uptake; however, this effect was statistically indistinguishable from the controls. Inhibition of PKCδ by 5 μM rottlerin (ROT) reversed the Mn(II)-induced downregulation in Glu uptake both in response to 500 μM Mn(II) treatment for 1 h as well as to 100 μM and 500 μM Mn(II) for 4 or 8 h (Fig. 3c).
Glu uptake in astrocytes exposed to Mn(II) and the caspase inhibitor Z-VAD-FMK
Our previous study showed that PKCδ, among all investigated isoforms, was the most affected by Mn(II) (Sidoryk-Wegrzynowicz et al. 2011). We and others postulated that Mn(II) leads to activation of PKCδ by caspase-3-dependent proteolytic cleavage (Latchoumycandane et al. 2005; Sidoryk-Wegrzynowicz et al. 2011). Herein, we demonstrate that Mn(II)–induced downregulation of astrocytic Glu uptake is abrogated by the caspase inhibitor Z-VAD-FMK in analogous way to ROT treatment (Fig. 3d).
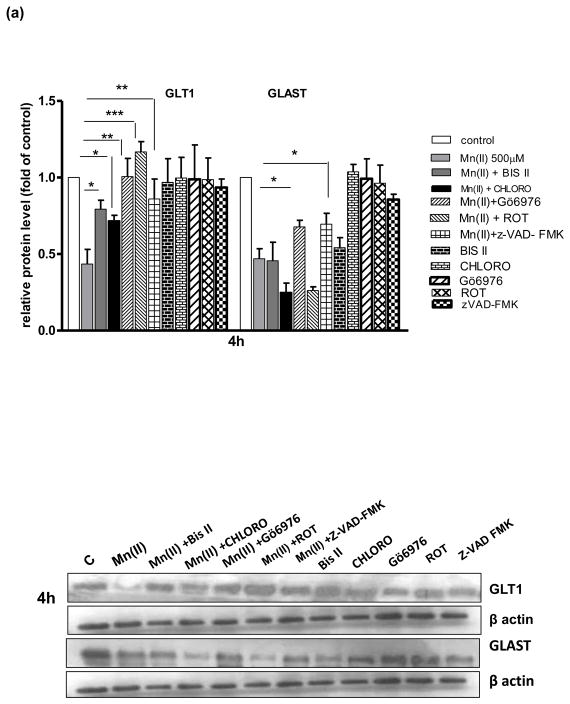
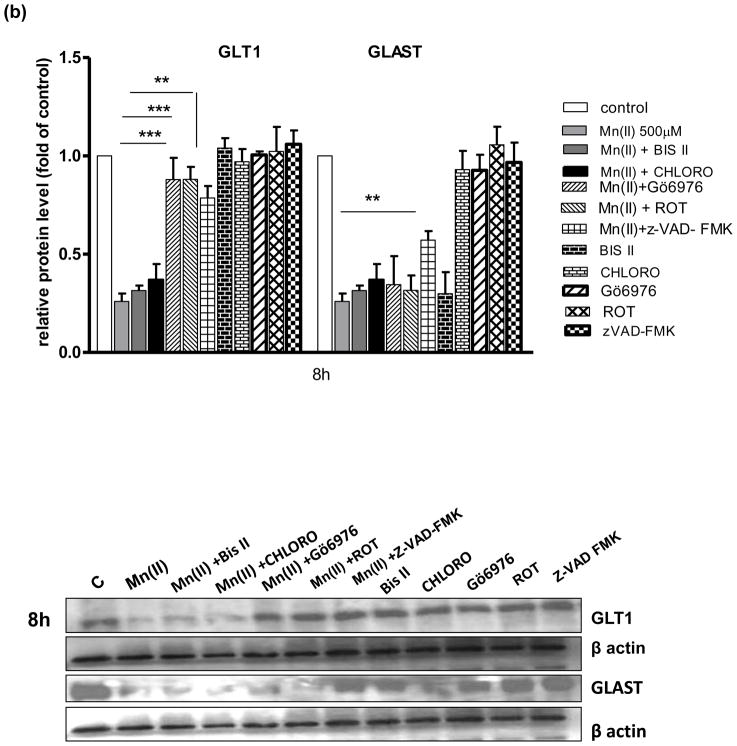
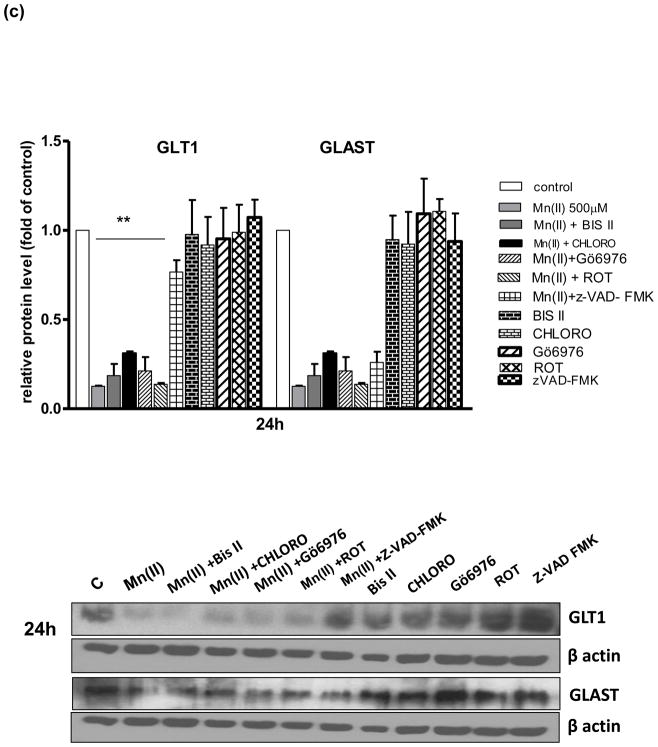
Astrocytic GLAST and GLT1 protein levels after treatment with Mn(II) and/or PKC pathway and caspase inhibitors
Next, we performed a series of experiments to determine GLAST and GLT1 protein expression levels in astrocytes treated with Mn and/or inhibitors of pathways associated with Mn(II)-induced downregulation of Glu uptake. As shown on Fig. 4–500 μm Mn(II) treatment decreased GLAST and GLT1 protein expression levels at all time points (4, 8 or 24 h). Co-treatment with Bis II, CHLORO, Gö6976, ROT or Z-VAD-FMK after 4 h, and Gö6976, ROT or Z-VAD-FMK after 8 h reversed the Mn(II)-induced GLT1 protein downregulation. After 24 h treatment, only Z-VAD-FMK fully restored the Mn(II)-induced decrease in GLT1 protein level (Fig. 4). As shown on Fig. 4(a, b) 4 h co-treatment with Gö6976, as well as 4 h or 8 h with Z-VAD-FMK, reversed the Mn(II)-induced downregulation of astrocytic GLAST protein expression.
Fig. 4. Glutamate transporter protein levels after Mn exposure and/or inhibition of PKC signalling, caspase 3 and lysosomal pathways.
GLT1 and GLAST protein levels were determined by western blotting after 4, 8 or 24 h exposure to 500 μM Mn(II) alone or in presence of the general PKC inhibitor bisindolylmaleimide II BIS, the selective PKCα inhibitor Gö6976, the selective PKCδ inhibitor Rottlerin (ROT), the caspase inhibitor Z-VAD-FMK (Z-Ala-Glu(OMe)-Val-Asp(OMe)-fluoromethyl ketone), and the lysosomal inhibitor chloroquine (CHLORO). Data represent the mean ± S.D. from 4 independent sets of cultures, each performed in triplicate; two -way ANOVA found main effect of Mn(II) exposure ((F(1,42)=45.62, p<0.0001), Mn(II) x time interaction ((F(1, 42)=22.4 p<0.005), Mn(II) x Gö6976 interaction ((F(1, 42)=10.9 p<0.0052), and Mn(II) x zVAD-FMK interaction ((F(1,39)=12.89, p<0.0034) on GLAST protein level; two- way ANOVA found main effect of Mn(II) exposure ((F(1,38)=10.19, p<0.005; Mn(II) x time interaction ((F(1, 38)=31.3 p<0.005), Mn(II) x BIS II interaction ((F(1, 38)=7.848, p<0.031); Mn(II) x CHLORO interaction ((F(1, 38)=5.49, p<0.042), Mn(II) x Gö6976 interaction ((F(1, 40)=4.42, p<0.049), Mn(II) x ROT interaction ((F(1, 42)=3.19, p<0.0228); Mn(II) x zVAD-FMK interaction ((F(1, 42)=4.22, p<0.0252) on GLT1 protein level; *p < 0.05, **p < 0.01 vs. control (one-way ANOVA followed by post hoc Tukey’s test).
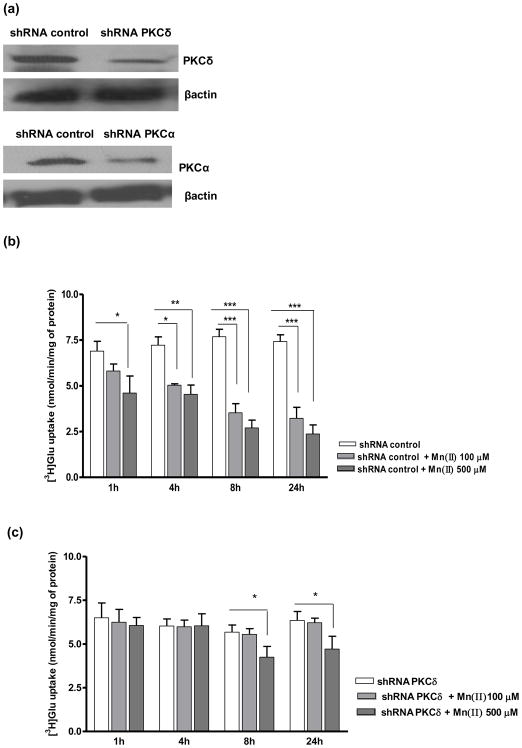
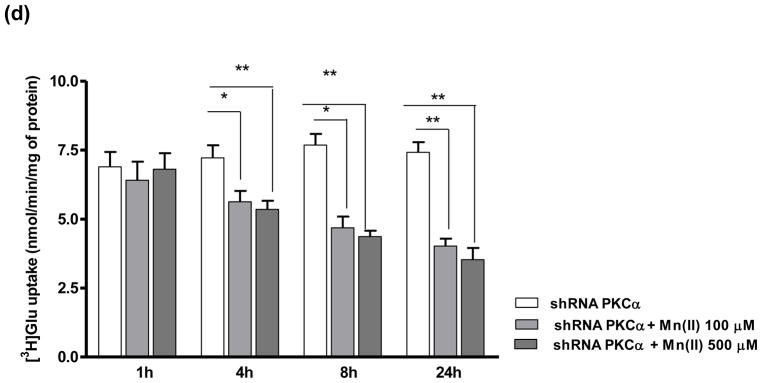
Glu uptake in PKCδ and PKCα knockdown astrocytes
Since our results established a critical role for the PKC pathway in mediating Glu uptake in the presence of Mn(II), additional uptake experiments were performed after knockdown of the PKCδ and PKCα isoforms. Western blot analysis from 3 inedpendent transfection showed significant difference in the gene knockdown efficiency (47 % ± 9% of control for PKCδ and 51 % ± 8% of control for PKCα) (Fig. 5a). As shown in Fig. 5b, cells transfected with control shRNA were sensitive to Mn(II) and showed decreased Glu uptake after 1 h treatment with 500 μM of Mn(II), and after 4, 8 or 24 h treatment with 100 μM or 500 μM of Mn(II). Cells transfected with shRNA against PKCδ were unaffected by treatment with 500 μM of Mn(II) for 1 h, or by 100 μM and 500 μM Mn(II) treatment for 4 h, as well by 100 μM Mn(II) treatment for 8 h or 24 h. Transfected cells remained sensitive to 500 μM Mn(II) after 8 h or 24 h of treatment (Fig. 5c). Knockdown of PKCα led to insensitivity in response to 1 h treatment with 500 μM Mn(II) (Fig. 5c). PKCα shRNA transfected cells exhibited higher sensitivity at longer Mn(II) treatments (4, 8 or 24 h), but lesser than those transfected with control shRNA (Fig. 5c, d).
Fig. 5. Astrocytic glutamate uptake upon PKCα and PKCδ knockdown.
Cells were transfected with lentivirus for 24 h (a). Next cells were exposed for 1, 4, 8 or 24 h to 100 μM or 500 μM of Mn(II) followed by Glu uptake analysis (b–d). Data represent the mean ± S.D. from 4 independent sets of cultures, each performed in triplicate; *p < 0.05, **p < 0.01, ***p < 0.001 control vs. Mn(II)-exposed cells (one-way ANOVA followed by post hoc Tukey’s test).
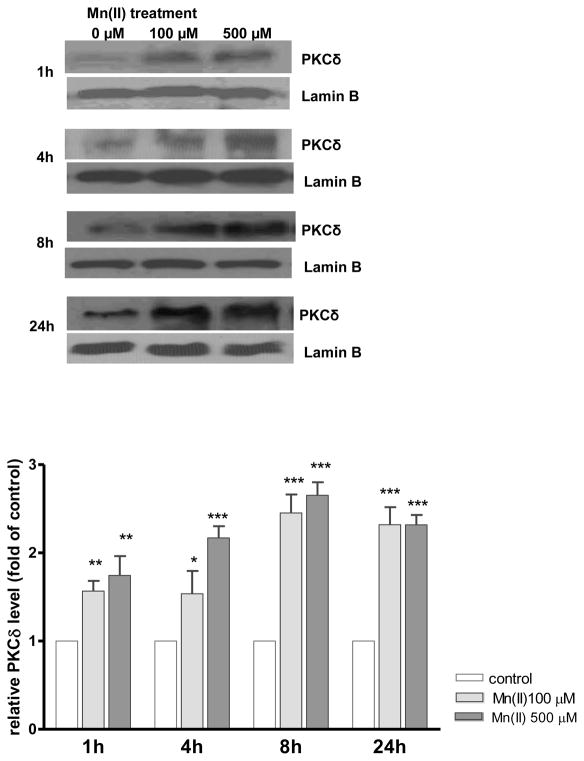
Mn(II)-induced PKCδ nuclear translocation in astrocytes
Our previous findings established that Mn(II) promotes PKCδ activation by its phosphorylation or caspase-3-dependent proteolytic cleavage (Sidoryk-Wegrzynowicz et al. 2011). Herein, we have further demonstrated that Mn(II) treatment increases astrocytic nuclear PKCδ levels after 1 h (1.64 ± 0.8-fold; 100 μM Mn(II) and 1.57± 0.9-fold; 500 μM Mn(II)), 4 h (1.49 ± 0.6-fold; 100 μM Mn(II) and 2.21 ± 0.7-fold; 500 μM Mn(II)), 8 h (2.34 ± 0.8-fold; 100 μM Mn(II) and 2.52 ± 0.7-fold; 500 μM Mn(II)) and 24 h (2.24 ± 0.8-fold; 100 μM Mn(II) and 2.22 ± 0.9-fold; 500 μM Mn(II) (Fig. 6).
Fig. 6. Mn(II)-induced astrocytic PKCδ nuclear translocation.
Cells were treated with 100 μM or 500 μM of Mn(II) for 1, 4, 8 or 24 h. Next, the nuclear-rich fraction was isolated with the Nuclear/cytosol fractionation kit following the manufacturer’s protocol (BioVison, Mountain View, CA, USA) and analyzed for PKCδ and the nuclear marker Lamin B by western blotting. Data represent the mean ± S.D. from 3 independent sets of cultures, each performed in triplicate; *p < 0.05, **p < 0.01, ***p < 0.001 control vs. Mn(II)-exposed cells (one-way ANOVA followed by post hoc Tukey’s test).
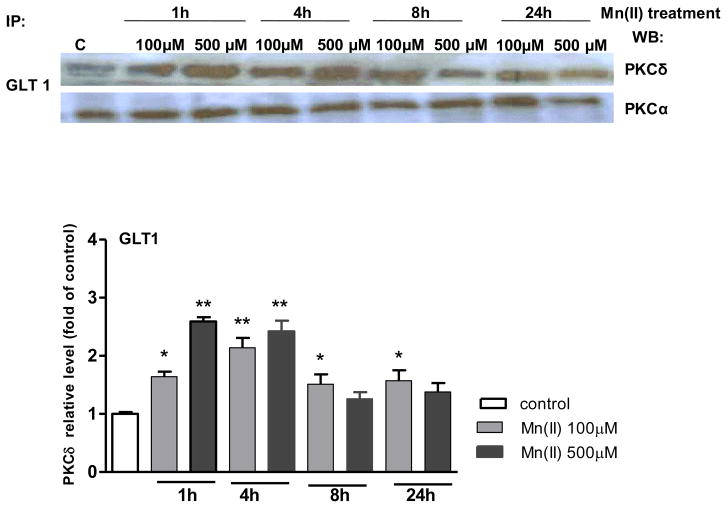
PKCδ and PKCα associate with glutamate transporters in astrocytes
We performed a series of experiments to examine the possible complexation of Glu transporters with the PKCα and PKCδ isoforms. Specifically, we sought to determine whether Mn(II) could affect this interaction. Cell lysates were subjected to immunoprecipitation using antibodies against the Glu transporters, GLAST or GLT1. As shown in Fig. 7, Mn(II) significantly increased the association of PKCδ with GLT1 after 1 h (2.62 ± 0.5-fold 500 μM Mn(II) relative to the control), 4 h (2.04 ± 0.4-fold 100 μM Mn(II) relative to the control; 2.42 ± 0.5-fold 500 μM Mn(II) relative to the control), 8 h (100 μM Mn(II) relative to the control) and 24 h (100 μM Mn(II) relative to the control). PKCα immunoreactivity was evident in GLT1 immunoprecipitates, however Mn(II) treatment had no effect on the association of GLT1 with PKCα (Fig. 7). Furthermore, no interaction was observed between PKCα or PKCδ and GLAST.
Fig. 7. PKCδ and PKCα associate with glutamate transporters in primary astrocyte cultures.
Cells were exposed to 100 or 500 μM Mn(II) for 1, 4, 8 or 24 h, then lysed and subjected to immunoprecipitation with antibodies against GLT1, and probed for PKCδ or PKCα. In each experiment, an antibody against control IgG was additionally used for immunoprecipitation controlling for nonspecific binding. Similar results were obtained in three independent sets of cultures; *P < 0.05 and **P < 0.01 control (0 h) vs. Mn(II)-exposed (one-way ANOVA followed by post hoc Tukey’s test).
DISCUSSION
Our previous studies have shown that impairment in GGC, resulting from deregulation of Gln transporters may contribute to Mn(II)-induced neurotoxicity (Sidoryk-Wegrzynowicz et al. 2009). We hypothesized that Mn(II) deregulates Gln turnover via PKC signalling and the ubiquitin-dependent proteolytic system (Sidoryk-Wegrzynowicz et al. 2010, Sidoryk-Wegrzynowicz et al. 2011). Several additional studies have established the propensity of Mn(II) to disrupt other components of the GGC, leading to both a reduction in Glu uptake and elevation in extracellular Glu level (Erikson et al. 2002; Erikson et al. 2008; Lee et al. 2009). The objective of the present study was to extend these early observations and to specifically address mechanism(s) associated with the Mn(II)-induced disruption of Glu turnover.
It was previously reported that polyubiquitination targets proteins for proteasomal degradation, while monoubiquitination serves as a signal to trigger the internalization of membrane-bound proteins and to regulate the activity of components of the endocytotic/lysosomal machinery (Sheldon et al. 2008; Yang et al. 2008). Our study demonstrated that the activation of the lysosomal, rather than the proteasomal pathway, might be responsible for downregulation of Glu transporter activity after Mn(II) exposure (Fig. 1). Similarly, studies by Susarla and Robinson (2007) and González-González et al. (2008) revealed that lysosomal proteolysis is responsible for Glu transporter (GLT1) degradation. Specifically, it was suggested that precisely controlled internalization followed by lysosomal targeting represents a significant mechanism of transmembrane transporters activity regulation (Miranda et al. 2005).
The PKC pathway is responsible for neurotransmitter transporters internalization and degradation by promoting their ubiquitiniation. This pathway was previously shown to be involved in the degradation of the dopamine transporter (DAT) (Miranda et al. 2007; Foster et al. 2008) as well as GLT1 (Gonzalez-Gonzales et al. 2008). PKC-dependent ubiquitination is mediated either by direct or indirect interaction of the target protein with E3 ubiquitin ligase(s) (Vina-Vilaseca et al. 2011). It was shown that knockdown of ubiquitin ligase, Nedd4-2, results in pronounced reduction in the PKC-dependent ubiquitination of DAT (Sorkina et al. 2006). Our previous study demonstrated that Mn(II) exposure increased expression of Nedd4-2 as well as the global polyubiquitination of proteins, and that these events were preceded by the activation of PKC signalling (Sidoryk-Wegrzynowicz et al. 2011). We also showed that PKC signalling was involved in Mn(II)-induced downregulation of Gln turnover (Sidoryk-Wegrzynowicz et al. 2011). Based on the current results, it appears that similar mechanisms are involved in the regulation of Glu turnover. PKC stimulation by PMA significantly decreased astrocytic Glu uptake, while treatment with the general PKC inhibitor, BIS II, protected astrocytes from the Mn(II)-induced downregulation of Glu transport (Figs 2, 3a). We have previously shown that Mn(II) treatment promotes the specific phosphorylation of PKCα and PKCδ isozymes (Sidoryk-Wegrzynowicz et al. 2011). Accordingly, in the present study we performed a series of Glu uptake studies in the presence of Gö6976 and ROT, specific inhibitors of the PKCα and PKCδ isoforms, respectively. Mn(II)-induced downregulation of astrocytic Glu uptake was reversed by ROT and this effect lasted longer than with Gö6976 (Fig. 3b, c). Interestingly, inhibition of caspase 3 by Z-VAD-FMK caused an analogous effect on astrocytic Glu uptake as observed for ROT (Fig. 3d). This is consistent with PKCδ representing a target of caspase 3 proteolytic activity (Hanrott et al. 2008). PKCδ was also shown to be activated by Mn(II) via proteolytic cleavage both in dopaminergic neurons and in cultured primary astrocytes (Latchoumycandane et al. 2005; Sidoryk-Wegrzynowicz et al. 2011). Thus, caspase-3-dependent proteolytic cleavage of PKCδ may contribute to Mn(II)–induced impairment in Glu transport. Notably, previous studies have shown that caspase-3-dependent PKCδ activation not only contributes to neuronal apoptosis, but also has a significant feedback regulatory role in amplification of the apoptotic cascade during neurotoxic stress associated with Mn treatment (Anantharam et al., 2002 Latchoumycandane et al., 2005).
Fig. 2. Astrocytic Glu uptake upon stimulation of PKC signalling.
Total L-(G-3H)-Gln (0.25 μCi, specific activity: 49.0 Ci/mmol) uptake was measured in control and 100 nM PKC-activating phorbol 12-myristate 13-acetate (PMA) treated primary cultures of astrocytes for 0.5, 1, 2, 4, 6, 8 or 24 h. Data represent the mean ± S.D. from 3 independent sets of cultures, each performed in triplicate; *p < 0.05, **p < 0.01, ***p < 0.001 control vs. PMA-exposed cells (one-way ANOVA followed by post hoc Tukey’s test).
Glu uptake upon PKC isoform knockdown established the involvement of the PKC pathway in Mn(II)–induced deregulation of Glu turnover. Astrocytes transfected with shRNA against PKCδ were unaffected by Mn(II) after 1 or 4 h treatment as well as by lower Mn(II) concentrations for longer duration, while cells transfected with shRNA against PKCα exhibited higher sensitivity to Mn(II) (Fig. 5). Additionally, co-immunoprecipitation revealed co-localization of PKCδ and PKCα with GLT1 (Fig. 7). Corroborating these findings, an earlier study has shown that GLT1 and PKCα co-expression is responsible for transporter internalization in C6 glioma cells (Gonzalez et al. 2005). Furthermore, we showed that Mn(II) increased the interaction between PKCδ and GLT1, but did not affect the interaction between PKCα and GLT1 (Fig. 7). These observations suggest that PKCδ rather than PKCα is involved in Mn(II)-induced disruption of Glu turnover. As shown previously in astrocytes, PKCδ co-localized with the Gln transporters SNAT3 and ASCT2. In addition, ASCT2 was found to be a major PKCδ target among Gln transporters after Mn(II) treatment (Sidoryk-Wegrzynowicz et al. 2011). Here, we have demonstrated that Mn(II) treatment increases the nuclear expression levels of this isoforms (Fig. 6). These events toghether with the observed increase in PKCδ and GLT1 interaction may play prominent role in modulating Mn(II)-induced glutamate dyshomeostasis. Proteolytically activated PKCδ in the nucleus was found to mediate DNA fragmentation as well as gene transcription (Kanthasamy et al. 2003). Additionally, Su et al. demonstrated that during proteasome dysfunction in dopaminergic neuronal cells mitochondrial translocation/accumulation of the proteolytically cleaved PKCδ triggers mitochondria-mediated apoptotic cascade (Su et al., 2008). Combined, our findings suggest that Mn(II) can regulate multiple cellular processes, including GGC in a PKCδ–dependent pattern.
In the present study we also show that Mn(II) decreases the expression of GLAST and GLT1. Co-treatment with Bis II, CHLORO, Gö6976, ROT or Z-VAD-FMK after 4 h, and with Gö6976, ROT or Z-VAD-FMK for 8 h reversed the Mn(II)-induced GLT1 protein downregulation. Attenuation of the Mn(II) effect on GLT1 remained persistent in the presence of Z-VAD-FMK (Fig. 4). These observations are consistent with the results of the uptake study, especially at shorter incubation time points (Figs 1c, 3, 4). Longer (>4h) treatments with the specific PKCα inhibitor, Gö6976 were ineffective in blocking the Mn(II)–induced downregulation of Glu uptake, while an opposite effect was observed for GLT1 protein levels (Figs 3b, 4). These results suggest that PKCδ is involved in GLT1 regulation in specific cellular compartments. This could include short-term GLT1 regulation by alteration of transporter trafficking and thereby its cell surface expression. We have demonstrated that the general inhibitor of PKC-BIS II was less effective in blocking Mn(II)-induced Glu uptake and protein expression downregulation than the specific inhibitors of the PKCs isoforms (Figs 3, 4). A possible explanation for this observation may be that the general PKC inhibitor BIS II is more specific for the different PKC isoforms investigated in the present study.
GLAST was regulated by Mn(II) in a pattern distinct from GLT1 (Fig. 4B). We found that PKCα inhibition reverses Mn(II)-induced downregulation of GLAST protein expression (Fig. 4b). However, this effect was not observed after longer Mn(II) treatments, suggesting short-term PKCα regulation of GLAST expression. Several groups have studied the effects of PKC on GLAST-mediated transport with contradictory results, showing that activation of PKC increases, decreases or has no effect on GLAST expression and activity (Casado et al. 1991; Conradt and Stoffel, 1997; González and Ortega 1997; Daniels and Vickroy, 1999). Other studies showed that activation of PKC rapidly decreases GLT1 cell surface expression in C6 glioma stably transfected with GLT1 and in primary cultures that endogenously express GLT1 (Gonzalez et al. 2005; González-González 2008). In our study we found that both GLAST and GLT1 may be regulated via PKC-dependent pathway, however contribution of α and δ PKC isoforms is different for either transporter (Figs 3, 4).
Our results demonstrate that PKC signalling is involved in Mn(II)–dependent impairment of glutamate uptake by neurons, leading to excessive extracellular neurotransmitter accumulation. Furthermore, the PKC pathway may also contribiute to elevation in extracellular Glu levels by increasing its release from neurons. PKC has been previously shown to regulate of extracellular glutamate levels in cerebral cortical synaptosomes, playing an important role in synaptic plasticity (Coffey et al., 1994). Another study showed the involvement of PKC in the temperature-dependent elevation of extracellular glutamate levels in the striatum during ischemia, suggesting that inhibition of PKC activation may be protective against ischemic brain damage (Boris-Möller and Wieloch, 1998).
Caspase-3 inhibition appeared to have the most potent effect in reversing the Mn(II)-induced inhibition of Glu transport (Figs 3d, 4). Notably, inhibition of caspase-3 was more effective in reversing Mn(II)–dependent deregulation of GLT1 and GLAST expression than inhibition of PKCδ (Fig. 4). These findings suggest that other PKCδ-independent mechanism(s) may be involved in disruption of Glu turnover upon Mn(II) treatment. Notably, Mn(II) was found to be associated with caspase- 3 activation in a number of studies (Choi et al. 2007; Yin et al. 2008); yet, it was also shown that caspase-3 also directly cleaves and inactivates GLT1 transporter, while GLAST was lacks conventional cleavage sites for caspase-3 (Boston-Howes et al. 2006). Accordingly, this selective cleavage of GLT1 may explain the more pronounced effect of caspase-3 inhibition on GLT1 than on GLAST transporter expression (Fig. 4a, b). However, at the same time point (24 h), Mn(II)-dependent deregulation of GLT1 expression was reversed by caspase -3 inhibition, but had no effect on Mn(II)–induced disruption in Glu uptake (data not shown). This suggests that other mechanisms, e.g. postranslational modifications, may be involved in Mn(II)-induced dysfunction in GLT1.
In summary, our studies show disruption of GGC in Mn(II) treated astrocytes, leading to the accumulation of extrasynaptic Glu. Notably, Mn(II) is implicated in the impairment of GGC at two key regulatory steps, namely both Glu and Gln turnover. Herein, we identified mechanisms responsible for Mn(II)-induced deregulation of Glu turnover that are mediated by GLT1 and GLAST. Our findings along with previous observations establish that Mn(II) affects both Glu and Gln transport via similar PKC signalling events. Future studies should be directed at uncovering therapeutic modalities that reverse these effects and restore optimal GGC in astrocytes.
Acknowledgments
This manuscript was supported in part by Grants from the National Institute of Environmental Health Sciences ES R01-10563, R01-07331, and the Molecular Toxicology Center ES P30 000267.
Abbreviations used
- ALLN
N-acetyl-leu-leu-norleucinal
- BAF
bafilomycine A1
- BIS II
bisindolylmaleimide II
- CHLORO
chloroquine diphosphate
- DAT
dopamine transporter
- GGC
the glutamate-glutamine cycling
- GLAST
glutamate–aspartate transporter
- GLT1
glutamate transporter
- IFU
infectious units of virus
- PMA
activator α-phorbol 12-myristate 13-acetate, PKC, protein kinase C
- ROT
rottlerin
- Nedd4-2
neuronal precursor cell expressed, developmentally down-regulated 4-2 activity
- Z-VAD-FMK
Z-Ala-Glu(OMe)-Val-Asp(OMe)-fluoromethyl ketone
References
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22:1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem. 1992;58:730–735. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- Berl S, Clarke DD. The metabolic compartmentation concept. In: Hertz L, Kvamme E, McGeer EG, Schousboe A, editors. Glutamine, Glutamate and GABA in the Central Nervous System. Liss; New York: 1983. pp. 205–217. [Google Scholar]
- Boris-Möller F, Wieloch T. The effect of 4 beta-phorbol-12,13-dibutyrate and staurosporine on the extracellular glutamate levels during ischemia in the rat striatum. Mol Chem Neuropathol. 1998;35:133–147. doi: 10.1007/BF02815120. [DOI] [PubMed] [Google Scholar]
- Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Jr, Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281:14076–1484. doi: 10.1074/jbc.M600653200. [DOI] [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Casado M, Zafra F, Aragón C, Giménez C. Activation of high-affinity uptake of glutamate by phorbol esters in primary glial cell cultures. J Neurochem. 1991;57:1185–1190. doi: 10.1111/j.1471-4159.1991.tb08278.x. [DOI] [PubMed] [Google Scholar]
- Choi CJ, Anantharam V, Saetveit NJ, Houk RS, Kanthasamy A, Kanthasamy AG. Normal cellular prion protein protects against manganese-induced oxidative stress and apoptotic cell death. Toxicol Sci. 2007;98:495–509. doi: 10.1093/toxsci/kfm099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical c ell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey ET, Herrero I, Sihra TS, Sánchez-Prieto J, Nicholls DG. Glutamate exocytosis and MARCKS phosphorylation are enhanced by a metabotropic glutamate receptor coupled to a protein kinase C synergistically activated by diacylglycerol and arachidonic acid. J Neurochem. 1994;63:1303–1310. doi: 10.1046/j.1471-4159.1994.63041303.x. [DOI] [PubMed] [Google Scholar]
- Conradt M, Stoffel W. Inhibition of the high-affinity brain glutamate transporter GLAST-1 via direct phosphorylation. J Neurochem. 1997;68:1244–125. doi: 10.1046/j.1471-4159.1997.68031244.x. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Daniels KK, Vickroy TW. Reversible activation of glutamate transport in rat brain glia by protein kinase C and an okadaic acid-sensitive phosphoprotein phosphatase. Neurochem Res. 1999;24:1017–1025. doi: 10.1023/a:1021004809991. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Aschner M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int. 2003;43:475–48. doi: 10.1016/s0197-0186(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dorman DC, Lash LH, Aschner M. Duration of airborne-manganese exposure in rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Neurotoxicology. 2008;29:377–385. doi: 10.1016/j.neuro.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Suber RL, Aschner M. Glutamate/aspartate transporter (GLAST), taurine transporter and metallothionein mRNA levels are differentially altered in astrocytes exposed to manganese chloride, manganese phosphate or manganese sulfate. Neurotoxicology. 2002;23:281–288. doi: 10.1016/s0161-813x(02)00041-4. [DOI] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-González IM, García-Tardón N, Giménez C, Zafra F. PKC-dependent endocytosis of the GLT1 glutamate transporter depends on ubiquitylation of lysines located in a C-terminal cluster. Glia. 2008;56:963–974. doi: 10.1002/glia.20670. [DOI] [PubMed] [Google Scholar]
- González MI, Ortega A. Regulation of the Na+-dependent high affinity glutamate/aspartate transporter in cultured Bergmann glia by phorbol esters. J Neurosci Res. 1997;50:585–590. doi: 10.1002/(SICI)1097-4547(19971115)50:4<585::AID-JNR9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Susarla BT, Robinson MB. Evidence that protein kinase C alpha interacts with and regulates the glial glutamate transporter GLT1. J Neurochem. 2005;94:1180–1188. doi: 10.1111/j.1471-4159.2005.03330.x. [DOI] [PubMed] [Google Scholar]
- Hanrott K, Murray TK, Orfali Z, Ward M, Finlay C, O’Neill MJ, Wonnacott S. Differential activation of PKC delta in the substantia nigra of rats following striatal or nigral 6-hydroxydopamine lesions. Eur J Neurosci. 2008;27:1086–1096. doi: 10.1111/j.1460-9568.2008.06097.x. [DOI] [PubMed] [Google Scholar]
- Hicke L. Ubiquitin-dependent internalization and down-regulation of plasma membrane proteins. FASEB J. 1997;11:1215–1226. doi: 10.1096/fasebj.11.14.9409540. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Role of proteolytic activation of protein kinase Cdelta in oxidative stress-induced apoptosis. Antioxid Redox Signal. 2003;5:609–620. doi: 10.1089/152308603770310275. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Yang Y, Hirata Y, Kanthasamy A, Kanthasamy AG. Activation of protein kinase C delta by proteolytic cleavage contributes to manganese-induced apoptosis in dopaminergic cells: protective role of Bcl-2. Biochem Pharmacol. 2005;69:133–146. doi: 10.1016/j.bcp.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Ther. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J Neurochem. 2009;110:530–544. doi: 10.1111/j.1471-4159.2009.06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003;278:22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol Sci. 2007;98:198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- Miranda M, Dionne KR, Sorkina T, Sorkin A. Three ubiquitin conjugation sites in the amino terminus of the dopamine transporter mediate protein kinase C-dependent endocytosis of the transporter. Mol Biol Cell. 2007;18:313–323. doi: 10.1091/mbc.E06-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Wu CC, Sorkina T, Korstjens DR, Sorkin A. Enhanced ubiquitylation and accelerated degradation of the dopamine transporter mediated by protein kinase C. J Biol Chem. 2005;280:35617–35624. doi: 10.1074/jbc.M506618200. [DOI] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Function of trace elements in brain metabolism. Physiol Rev. 1987;67:858–901. doi: 10.1152/physrev.1987.67.3.858. [DOI] [PubMed] [Google Scholar]
- Racette L, McGee-Minnich SM, Moerlein JW, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: Clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J Biol Chem. 1998;273:2458–2466. doi: 10.1074/jbc.273.4.2458. [DOI] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JA, Garrick MD. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol. 2003;66:1–13. doi: 10.1016/s0006-2952(03)00145-x. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, González MI, Krizman-Genda EN, Susarla BT, Robinson MB. Ubiquitination-mediated internalization and degradation of the astroglial glutamate transporter, GLT1. Neurochem Int. 2008;53:296–308. doi: 10.1016/j.neuint.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Lee E, Albrecht J, Aschner M. Manganese disrupts astrocyte glutamine transporter expression and function. J Neurochem. 2009;110:822–830. doi: 10.1111/j.1471-4159.2009.06172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Lee ES, Ni M, Aschner M. Manganese-induced downregulation of astroglial glutamine transporter SNAT3 involves ubiquitin-mediated proteolytic system. Glia. 2010;58:1905–19012. doi: 10.1002/glia.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Lee E, Mingwei N, Aschner M. Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway. Glia. 2011;59:1732–1743. doi: 10.1002/glia.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Schousboe A, Svendes JS, Unsgard G, Petersen SB. Direct demonstration by [13C] NMR spectroscopy that glutamine from astrocytes is a precursor for GABA synthesis in neurons. Neurochem Int. 1993;22:19–29. doi: 10.1016/0197-0186(93)90064-c. [DOI] [PubMed] [Google Scholar]
- Sorkina T, Miranda M, Dionne KR, Hoover BR, Zahniser NR, Sorkin A. RNA interference screen reveals an essential role of Nedd4–2 in dopamine transporter ubiquitination and endocytosis. J Neurosci. 2006;26:8195–8205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na-dependent glutamate/aspartatetransporter from rat brain. Proc Natl Acad Sci USA. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Kanthasamy A, Song C, Yang Y, Anantharam V, Kanthasamy AG. Proteasome inhibitor-induced apoptosis is mediated by positive feedback amplification of PKCdelta proteolytic activation and mitochondrial translocation. J Cell Mol Med. 2008;16:2467–2481. doi: 10.1111/j.1582-4934.2008.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Mouri T, Nishiyama K, Fujii N. Study of subacute toxicity of manganese dioxide in monkeys. Tokushima J Exp Med. 1975;22:5–10. [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Vina-Vilaseca A, Bender-Sigel J, Sorkina T, Closs EI, Sorkin A. Protein kinase C-dependent ubiquitination and clathrin-mediated endocytosis of the cationic amino acid transporter CAT-1. J Biol Chem. 2011;286:8697–8706. doi: 10.1074/jbc.M110.186858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Economic implications of manganese neurotoxicity. Neurotoxicology. 2006;27:362–368. doi: 10.1016/j.neuro.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang S, Sung B, Lim G, Mao J. Morphine induces ubiquitin-proteasome activity and glutamate transporter degradation. J Biol Chem. 2008;283:21703–21713. doi: 10.1074/jbc.M800809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Aschner JL, dos Santos AP, Aschner M. Mitochondrial-dependent manganese neurotoxicity in rat primary astrocyte cultures. Brain Res. 2008;1203:1–11. doi: 10.1016/j.brainres.2008.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]