Abstract
The frequency or trajectory of vital sign abnormalities in children with pneumonia has not been described. In a cohort of 2,714 patients with severe pneumonia identified and treated as per the World Health Organization definition and recommendations, tachypnea, fever and hypoxia were found in 68.9%, 23.6% and 15.5% of children, respectively. Median oxygen saturation returned to a normal range by 10 hours following initiation of treatment, followed by temperature at 12 hours and respiratory rate at 22 hours for subjects less than 12 months and at 48 hours for those greater than or equal to 12 months of age.
Keywords: pneumonia, vital signs, tachypnea, hypoxia, trajectory
INTRODUCTION
Acute lower respiratory tract infections are a common cause of morbidity and the principal cause of mortality in children less than 5 years of age in developing countries1,2. Vital signs including respiratory rate, temperature and oxygen saturation are often measured routinely in acute care settings and help to guide the management of lower respiratory tract infections. Respiratory rates are additionally used in World Health Organization (WHO) case management guidelines for management decisions3. Until recently little evidence supported the established normal ranges for such vital signs. Meta-analyses of observational studies of children have now led to age-specific percentiles for normal respiratory rate and heart rate4,5. Data on normal ranges for oxygen saturation measured by pulse-oximetry and temperature, however, remain limited despite widely accepted definitions of hypoxia and fever in the pediatric population6,7.
The frequency of abnormalities in respiratory rate, temperature and oxygen saturation among children with acute lower respiratory tract infection has not been described. Furthermore, the resolution of vital sign abnormalities with appropriate treatment has never been demonstrated despite the common use of persistent fever and tachypnea as markers of treatment failure8–11. In this study we set out to demonstrate the ranges of abnormalities seen in respiratory rate, temperature, and oxygen saturation in a population of children with severe pneumonia. In these patients, severe pneumonia was identified as per the Integrated Management of Childhood Illness (IMCI) algorithm using the standard World Health Organization case definition of cough or difficulty breathing and lower chest indrawing that persists despite a bronchodilator trial12.
MATERIALS AND METHODS
Study Design and Population
This study is an observational cohort study using a pooled, individual level dataset from 2,714 subjects enrolled in two randomized clinical trials of therapeutic interventions for severe pneumonia13,14. Subjects were recruited from sixteen sites within eight countries: Colombia, Ghana, India, Mexico, Pakistan, South Africa, Vietnam and Zambia. Recruitment spanned from May 1999 to May 2002 for the first study and February 2005 to August 2006 for the second study. Both trials recruited patients aged 2–59 months with WHO-defined severe pneumonia from pediatric referral hospitals and used standardized clinical methods which were intentionally similar13,14. Those with non-severe pneumonia or very severe pneumonia were excluded from both studies. To minimize the impact of reactive airway disease, both studies uniformly excluded patients with a known history of asthma and those whose symptoms resolved after up to three bronchodilator treatments at screening.
Children were exposed to one of three therapeutic regimens with demonstrated equivalence given at or within 2 hours of baseline evaluation: parenteral ampicillin, parenteral penicillin, or oral amoxicillin. In all sites, subjects were hospitalized for the first 48 hours of treatment and reevaluated in the community 6 and 14 days following enrollment. Standardized measurements of respiratory rate by timer and axillary temperature by digital thermometer were performed every 6 hours in the hospital and once at each follow-up visit.
For the purpose of analysis we define tachypnea as values above the 99th percentile as established by Fleming and colleagues approximately equating to greater than 60 breaths per minute in those less than 12 months of age and greater than 40 breaths per minute in those greater than or equal to 12 months of age4. We define fever (abnormally high temperature) as greater than 38 degrees centigrade regardless of age15.
Data on oxygen saturation were collected in only one of the two cohorts contributing to this pooled analysis14. Oxygen saturation was measured in a non-crying child on room air (Nellcor N-20E, N-25 sensor, Pleasanton, CA, USA). Measurements were only made during hospitalization and are thus not available for day 6 and 14 community visits. Oxygen saturation measurements in two sites at high elevation (> 2000 m) were excluded from the pooled analysis. Hypoxia (abnormally low arterial oxygen saturation) was defined as a pulse oximeter measurement less than 92% regardless of age.
Statistical Methods
We calculated median estimates of respiratory rate, axillary temperature and oxygen saturation over time and present them with a distribution plot identifying the 5th, 25th, 75th, and 95th percentiles. Analyses were limited to children with the condition at baseline (e.g. temperature measurements limited to those with fever at baseline). Children were stratified by whether or not they had the outcome of interest (e.g. tachypnea, fever, low oxygen saturation) to assess when median results fell within normal ranges.
RESULTS
Of 2,714 included subjects, 61.1% (1,659) were male and 62.7% (1,703) were between 2 and 11 months of age.
Respiratory Rate
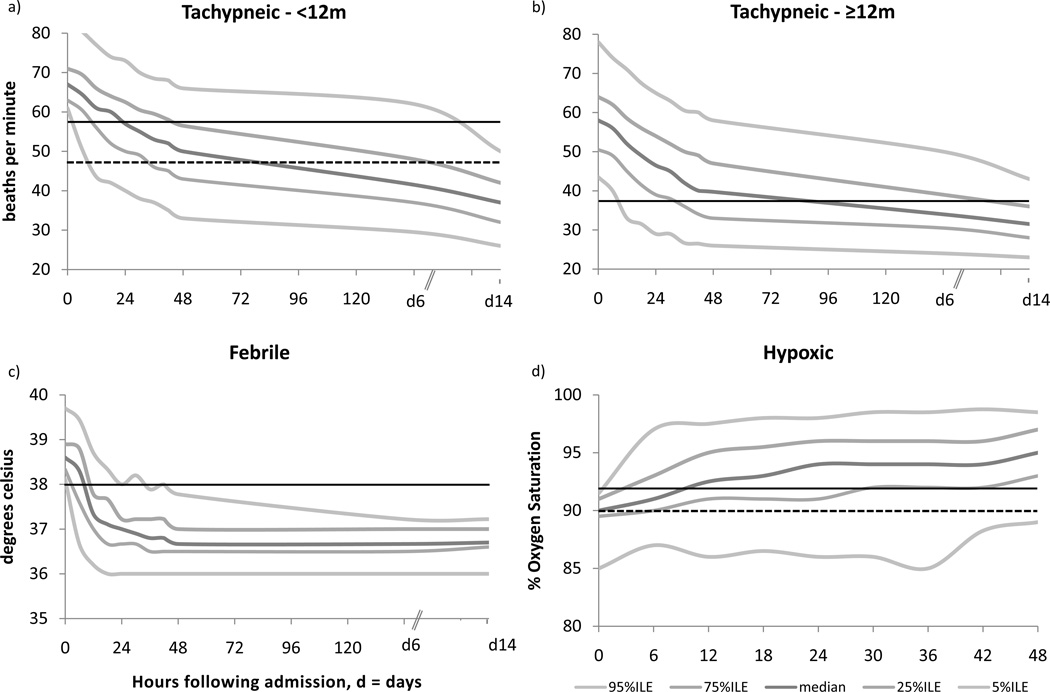
Approximately 70% of subjects (n=1,861) were tachypneic at baseline by our age-adjusted definition. Among these, the median (50th percentile) respiratory rate had returned to normal at approximately 22 hours for children <12 months and at approximately 48 hours for children ≥12 months. In both age groups, median respiratory rates trended downwards through hospitalization and discharge (Figure 1a and 1b). At time of discharge from the hospital (48 hours), 14.5% of children <12 months (n=135) and 43% of children ≥12 months (n=381) had persistent tachypnea. By the final follow-up visit (day 14), these values were 1.3% (n=11) for children < 12 months and 8.2% (n=70) for children ≥12 months.
Figure 1.
Percentiles of respiratory rate (a and b), temperature (c) and oxygen saturation (d) in children with severe pneumonia and the respective vital sign abnormality, presented with solid lines representing evidence-based norms and dotted lines representing World Health Organization standard for ‘fast breathing’ in (a) and for ‘hypoxia’ in (d)
Temperature
A total of 23.6% of subjects (n=644) were febrile at baseline by our definition. Among these, median temperature had returned to the normal range at approximately 12 hours of treatment. Temperature measurements plateaued at a median value of 36.7° (day 6 and day 14) following discharge from the hospital (Figure 1c). Among these children 2.4% (n=15) remained febrile at time of discharge from the hospital.
Oxygen Saturation
Oxygen Saturation was recorded on 1,439 subjects, of which 223 (15.5%) were hypoxic at baseline by our definition. Among these, median oxygen saturation had returned to greater than 92% at approximately 10 hours of treatment. At 48 hours following study enrollment, the median oxygen saturation was found to be 95% and 13.6% (31/223) had persistent hypoxia (Figure 1d).
DISCUSSION
Although the World Health Organization definition of severe pneumonia does not rely on the presence of tachypnea, we found that the majority of subjects (68.9%) were tachypneic on enrollment. Significantly smaller proportions, however, presented with fever (23.6%) or hypoxia (15.5%), two vital signs that commonly increase the clinical suspicion of pneumonia and have been shown to contribute to specificity of the diagnosis when a radiographic infiltrate is taken as the gold standard16,17.
Among the three investigated vital sign abnormalities, oxygen saturation was the first to correct from the abnormal range, despite median values not returning to expected norms of 97–100% within the 48 hour follow-up. Oxygen saturation has been previously shown to be an accurate estimate of functional arterial hemoglobin saturation and in the setting of pneumonia is known to be associated with disease severity and greater ventilation/perfusion (V/Q) mismatch18. The relatively rapid resolution of low oxygen saturation noted in our analysis may be related to this being an initial sign of convalescence. However, the absence of a longer follow-up period for this measurement (48 hours vs. 14 days for respiratory rate and temperature) precludes our ability to demonstrate a true plateau indicating a return to baseline values.
Despite its relatively low prevalence among subjects, the persistence of low oxygen saturations at 48 hours underscores the importance of supplemental oxygen in the treatment of patients with pneumonia. This is consistent with recent published guidelines for management of Community Acquired Pneumonia by the Infectious Diseases Society of America and The British Thoracic Society which integrate oxygen saturation measurement and supplemental oxygen treatment into decisions regarding patient care and disposition19,20.
Our study demonstrated similar trajectories in resolution of tachypnea regardless of age. The specific timing for correction from an abnormal range however was different between the two age groups. This finding may be due to the crude nature of identifying a single cut-off for an age range over which there is significant difference in baseline respiratory rate. A systematic review of observational studies measuring normal respiratory rate proposed rates as high as 64 and as low as 29 serving as the 99th percentile for ages 2 and 59 months, respectively4. In general however, the subjects aged greater than 12 months took longer to return to reported norms as their degree of tachypnea was relatively higher than age-adjusted norms on enrollment. Among the vital signs studied, temperature had the most reliable stabilization and plateau at 36.7°C. The relatively rapid decline of temperature in response to antibiotic treatment among febrile subjects supports the use of persistent fever as a sign of treatment failure among similar patients.
Overall, our analysis demonstrated low rates of vital sign abnormalities. Even among children with vital signs defined as abnormal, there was a relatively rapid return to normal ranges with the initiation of hospitalization and treatment. Furthermore, the rate of return to normal values was found to be relatively parallel within a narrow timeframe. This is not surprising as it is well known that vital signs are closely inter-related and often have similar trajectories in convalescence6,7. Specifically, fever and hypoxia in response to pneumonia can both increase the respiratory drive and lead to tachypnea. Conversely, resolution of tachypnea may require the initial resolution of hypoxia and fever as demonstrated in the attached figure.
The relatively low baseline prevalence of vital sign abnormalities in our study likely reflects the poor specificity of WHO-defined severe pneumonia and may have been improved if children with WHO-defined very severe pneumonia were also included3. Additionally, the relatively rapid return to normal values within our study may reflect the high efficacy of beta lactam antibiotics in the treatment of acute lower respiratory tract infection assuming that subjects with fever, hypoxia and tachypnea were more likely to be true cases of bacterial pneumonia. Unfortunately, our study did not investigate the etiology of pneumonia in each patient and we are thus unable to comment on vital sign trajectories in patients with bacterial causes as compared with those with viral or mixed causes of pneumonia. Further studies of vital sign abnormalities particularly among children with a more specific and microbiological diagnosis of pneumonia are needed in order to understand the true trajectory of disease and convalescence.
ACKNOWLEDGMENTS
Matthew Fox was supported by the National Institute of Allergy and Infectious Diseases (NIAID) under Award Number K01AI083097. The NIAID and USAID had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of WHO, the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or other parties.
SOURCES OF SUPPORT: World Health Organization, USAID and NIAID
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT: S A Qazi is a medical officer in the Department of Child and Adolescent Health and Development, WHO. The authors and contributors have declared no conflict of interest.
REFERENCES
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86(5):408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Acute respiratory infections in children: case management in small hospitals in developing countries. Geneva: World Health Organization; 1990. WHO Programme for the Control of Acute Respiratory Infections. Report Number 5. [Google Scholar]
- 4.Fleming S, Thompson M, Stevens R, Heneghan C, Plüddemann A, Maconochie I, Tarassenko L, Mant D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377:1011–1018. doi: 10.1016/S0140-6736(10)62226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson MJ, Harnden A, Perera R. Deriving temperature and age appropriate heart rate centiles for children with acute infections. Arch Dis Child. 2009;94:361–365. doi: 10.1136/adc.2008.145011. [DOI] [PubMed] [Google Scholar]
- 6.Fouzas S, Prifitis KN, Anthracopouos MB. Pulse Oximetry in Pediatric Practice. Pediatrics. 2011;128(4):740–752. doi: 10.1542/peds.2011-0271. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein JA, Christiansen CL, Platt R. Fever in pediatric primary care: occurrence, management, and outcomes. Pediatrics. 2000;105:260. [PubMed] [Google Scholar]
- 8.Straus WL, Qazi SA, Kundi Z, Nomani NK, Schwartz B. Antimicrobial resistance and clinical effectiveness of co-trimoxazole versus amoxycillin for pneumonia among children in Pakistan: randomised controlled trial. Lancet. 1998;352:270–274. doi: 10.1016/s0140-6736(97)10294-x. [DOI] [PubMed] [Google Scholar]
- 9.Catchup Study Group. Clinical efficacy of co-trimoxazole versus amoxicillin twice daily for treatment of pneumonia: a randomised controlled clinical trial in Pakistan. Archives of Diseases in Childhood. 2002;86:113–89. doi: 10.1136/adc.86.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qazi SA, Rehman GN, Khan MA. Standard management of acute respiratory infections in a children's hospital in Pakistan: impact on antibiotic use and case fatality. Bulletin of the World Health Organization. 1996;74:501–507. [PMC free article] [PubMed] [Google Scholar]
- 11.Haider BA, Saeed MA, Bhutta ZA. Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Systematic Review. 2008;2:CD005976. doi: 10.1002/14651858.CD005976.pub2. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Technical updates of the guidelines on the Integrated Management of Childhood Illness. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 13.Hazir T, Fox LM, Bin Nisar Y, Fox MP, Ashraf YP, MacLeod WB, et al. Ambulatory short-course high-dose oral amoxicillin for treatment of severe pneumonia in children: a randomised equivalency trial. Lancet. 2008;371:49–56. doi: 10.1016/S0140-6736(08)60071-9. [DOI] [PubMed] [Google Scholar]
- 14.Addo-Yoba E, Chisaka N, Hassan M, Hibberd P, Lozano JM, et al. Oral amoxicillin versus injectable penicillin for severe pneumonia in children aged 3 to 59 months: a randomized multicenter equivalency study. Lancet. 2004;364:1141–1148. doi: 10.1016/S0140-6736(04)17100-6. [DOI] [PubMed] [Google Scholar]
- 15.Alpern ER, Henretig FM. Fever. In: Fleisher GR, Ludwg S, Henretig FM, editors. Textbook of Pediatric Emergency Medicine. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 295–306. [Google Scholar]
- 16.Mathews B, Shah S, Cleveland RH, Lee EY, Bachur RG, Neuman MI. Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124(1):e29–e36. doi: 10.1542/peds.2008-2062. [DOI] [PubMed] [Google Scholar]
- 17.Neuman MI, Monuteaux MC, Scully KJ, Bachur RG. Prediction of pneumonia in a pediatric emergency department. Pediatrics. 2011;128(2):246–253. doi: 10.1542/peds.2010-3367. [DOI] [PubMed] [Google Scholar]
- 18.Salyer JW. Neonatal and pediatric pulse oximetry. Respiratory Care. 2003;48(4):386–396. [PubMed] [Google Scholar]
- 19.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH, Jr, Moore MR, St Peter SD, Stockwell JA, Swanson JT. Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Executive summary: the management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clinical Infectious Diseases. 2011;53(7):617–630. doi: 10.1093/cid/cir625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, Thomson A. British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66:s2, 1–3. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]