Abstract
Background
The intestinal microbial community has major effects on human health, but optimal research methods are unsettled. To facilitate epidemiologic and clinical research, we sought to optimize conditions and to assess reproducibility of selected core functions of the distal gut microbiota, β-glucuronidase and β-glucosidase bioactivities.
Methods and results
A colorimetric kinetic method was optimized and used to quantify activities of β-glucuronidase and β-glucosidase in human feces. Enzyme detection was optimal with neutral pH, snap freezing in liquid nitrogen, and rapid thawing to 37°C before protein extraction. Enzymatic stability was assessed by delayed freezing for 2–48 hours to mimic field settings. Activities decayed approximately 20% within 2 hours and 40% within 4 hours at room temperature. To formally assess reproducibility, 51 volunteers (25 male; mean age 39) used two devices to self-collect and rapidly chill four replicates of a stool. Devices were compared for mean enzymatic activities and intraclass correlation coefficients (ICC) in paired replicates of the self-collected specimens. Reproducibility was excellent with both devices for β-glucuronidase (ICC 0.92). The larger collection device had significantly higher reproducibility for β-glucosidase (ICC 0.92 vs. 0.76, P<0.0001) and higher mean activities for both enzymes (P<0.0001).
Conclusions
Optimal measurement of these core activities of the microbiota required a sufficient quantity of rapidly chilled or frozen specimens collected in PBS at pH7.0. Application of these methods to clinical and epidemiologic research could provide insights on how the intestinal microbiota affects human health.
Keywords: β-glucuronidase activity, β-glucosidase activity, feces, reproducibility
Introduction
Studies of the gut microbiome have mainly focused on the analysis of DNA sequences, particularly the 16S rRNA gene, to describe the composition and relative abundance of the microbiota system [1]. In addition, metagenomic analysis has been used to identify the genetic capacity of the whole microbiome in a particular milieu [2]. These powerful techniques, however, do not measure specific bacterial functions, which may serve as better indicators of how microbes affect health or disease conditions.
Specific functional assays would help to clarify microbial-host interactions, but validation of the methods is required for epidemiological studies, especially if complex specimens such as feces are used. One particular function with application for understanding disease etiology and pathogenesis is microbial enzymatic activity. Freeman and colleagues reported the effect of dietary fiber on fecal bacteria enzymatic activity in a rat model of colon carcinogenesis [3]. Similarly, Goldin and Gorbach measured bacterial β-glucuronidase to determine the effect of diet and probiotic supplements on bacterial enzymatic activity [4]. These and other studies have established that deconjugation of glycosilated metabolites is a core function of the intestinal microbiota [5;6]. More recent studies have suggested that strains of the genus Bacteroides require deconjugation for nutrient acquisition and may be one of the main bacterial groups with this function [7–10]. Of interest is the association of Bacteroides and other members of the phylum Bacteroidetes in inflammatory bowel disease and Crohn’s disease [11;12]. It is possible that these conditions result, in part, from high levels of Bacteroidetes-related deconjugation leading to increased induction of pro-inflammatory compounds [13].
Prior to launching epidemiologic and clinical studies of bacterial functions, optimization of many parameters is needed to minimize variability of enzymatic measurements and facilitate comparison across studies. These parameters include specimen collection, specimen handling, and assay reproducibility. The current project aimed to evaluate the reproducibility of measurements of two microbial enzymes (β-glucuronidase and β-glucosidase) from fecal specimens that were self-collected by volunteers.
Methods
Assays to detect and quantify enzymatic activities were developed and optimized with fecal specimens from laboratory volunteers. Additional methods are provided in Supplemental Materials.
Protein extraction
Protein extraction and enzymatic activities were performed as described by Goldin and colleagues [4] with slight modifications to optimize detection of the enzymatic activities. Approximately 0.5gr of thawed feces in phosphate buffered saline (PBS) was transferred to a 10ml conical tube containing 5ml of extraction buffer (60mM Na2HPO4, 40mM NaH2PO4, 10mM KCl, 1mM MgSO4) and kept on ice. Fecal material was homogenized by heavy vortexing for 1 min, and bacterial cells were lysed by sonication using a Misonix XL2000 Ultrasonic Homogenizer (Fisher Scientific, Pittsburgh, PA) at maximum power for 90 sec (30 second intervals) on an ice bath. Lysates were centrifuged at 22Kg (20K rpm) for 30min at 4°C using an Eppendorf 5424 microcentrifuge, and supernatant containing extracted proteins was transferred to new tubes and used to measure protein concentration and enzymatic activities. Protein concentration in each lysate was estimated using the bicinchoninic acid method according to the manufacturer’s instructions (PIERCE, Rockford, IL) and used to normalize enzyme activity estimates.
Enzymatic activity assay
β-glucuronidase and β-glucosidase activities were measured in a 96-well microplate format using ~100mg of input protein from fecal lysates (in 100µl volume with PBS). The final reaction volume was 200µl/well, composed of 100µl sample and 100µl of either 10mM 4-Nitrophenyl-β-D-glucuronide pH7.0 (for β-glucuronidase) or 10mM 4-Nitrophenyl-β-D-glucopyranoside pH7.0 (for β-glucosidase) preincubated at 37°C, which was added immediately before starting the enzymatic reaction. Enzymatic activity was measured in triplicates by following real-time kinetics at 37°C of the product 4-nitrophenol. The increment of the product was monitored at 405nm on Spectramax M5 (Molecular Devices, Sunnyvale, CA) either for 60min for fecal extracts with sufficient protein concentration or 5Hr for diluted fecal samples. Enzymatic concentrations were determined from standard curves of pure enzymes [β-glucuronidase, SIGMA G7646 and β-glucosidase, SIGMA G4511, SIGMA-ALDRICH (St. Louis, MO)] as controls and normalized by protein input. Enzymatic activity was reported as the mean value of triplicate runs in IU/100mg protein.
Assay optimization and validation
A description of the protocols used for these experiments can be found in the Supplemental Materials. In short, several collection procedures and storage conditions were tested to assess the sensitivity and extraction yield of bacterial β-glucuronidase and β-glucosidase from fecal extracts. Among the parameters tested were collection buffers containing different media [RNAlater® (Ambion, Austin, TX) vs. PBS] with or without mild detergents (0.5% Triton X-100) and/or protease inhibitor cocktail (SIGMA, P8340). As described in Table 1 and the Supplemental Materials, specified freezing and thawing methods (no freeze; fast freeze/fast thaw; fast freeze/slow thaw; slow freeze/fast thaw; and slow freeze/slow thaw) and delayed freezing (0, 2, 4, 8, 12, 24 and 48 Hr incubation at room temperature prior to freeze) were tested to determine the best parameters for handling fecal specimens in epidemiological studies when self-collection is needed and immediate freezing is not an option. The effects on enzymatic activities of pH and dilution of protein content (from 1–20mg/reaction) were also assessed.
Table 1.
Protein yields and enzymatic activities in phosphate buffered saline without freezing and with each of four freeze/thaw regimes*
| No freeze | Any freeze- thaw |
Fast freeze Slow thaw |
Fast freeze Fast thaw |
Slow freeze Slow thaw |
Slow freeze Fast thaw |
|
|---|---|---|---|---|---|---|
| Analyte | Means (SEM), P-values compared to no freeze | |||||
| Total protein | 1.5 (0.04) | 2.38 (0.05) P=0.0001 |
2.45 (1.73) P= 0.01 |
2.02 (0.07) P= 0.13 |
2.54 (0.11) P= 0.01 |
2.53 (0.08) P= 0.01 |
| β-Glucuronidase activity† | 2.47 (0.05) | 2.61 (0.04) P=0.12 |
2.31 (0.12) P= 0.59 |
2.82 (0.01) P= 0.06 |
2.63 (0.05) P= 0.64 |
2.70 (0.03) P= 0.25 |
| β-Glucosidase activity† | 3.57 (0.02) | 6.16 (0.09) P=0.0001 |
6.06 (0.2) P= 0.0002 |
6.49 (0.02) P= 0.0001 |
6.48 (0.2) P= 0.0001 |
5.60 (0.01) P= 0.0005 |
Fast and slow freezing and thawing conditions are described in detail in Supplemental Materials. Means and standard error of the means (SEM) were calculated from triplicate measurements in the duplicate fecal subsamples. The P-values that compares each freezing condition with no freeze are from unpaired t-tests with 2 degrees of freedom, because there are n=2 fecal subsamples for each freeze/thaw condition.
Enzymatic activity expressed in IU/100mg input protein.
Research Subjects
To assess the reproducibility of microbial measures in self-collected fecal specimens, healthy volunteers (25 male, mean age 39; and 26 female, mean age 40) were recruited from the Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI). The study was approved by the NCI Special Studies Institutional Review Board. Following face-to-face instructions and signed informed consent, participants were provided a toilet-attached pouch (Protocult, Rochester, MN), from which they collected multiple aliquots from various parts of an early or mid-morning stool. As illustrated in Figure 1, four aliquots were collected with Polymedco OC-auto collection devices that were pre-loaded with 2ml sterile PBS. Polymedco is a leak-proof device with a snap-cap-attached probe that is widely used for colon cancer screening (FOBT-CHECK®). For comparison, four aliquots were collected with Sarstedt feces collection containers (SARSTEDT, Nümbrecht, Germany), a 10mL tube with a screw-cap-attached scoop, each of which was pre-loaded with 5ml PBS. The Sarstedt device holds approximately 20-fold more fecal matter (~1gm) than does the Polymedco device. All specimens were immediately chilled on frozen gel packs, frozen in liquid nitrogen within 1–3 hours, and stored at −80°C until thawing for protein extraction. Four aliquots from each participant (duplicates with each device) were used for the current study. The remaining aliquots were reserved for future studies.
Figure 1.
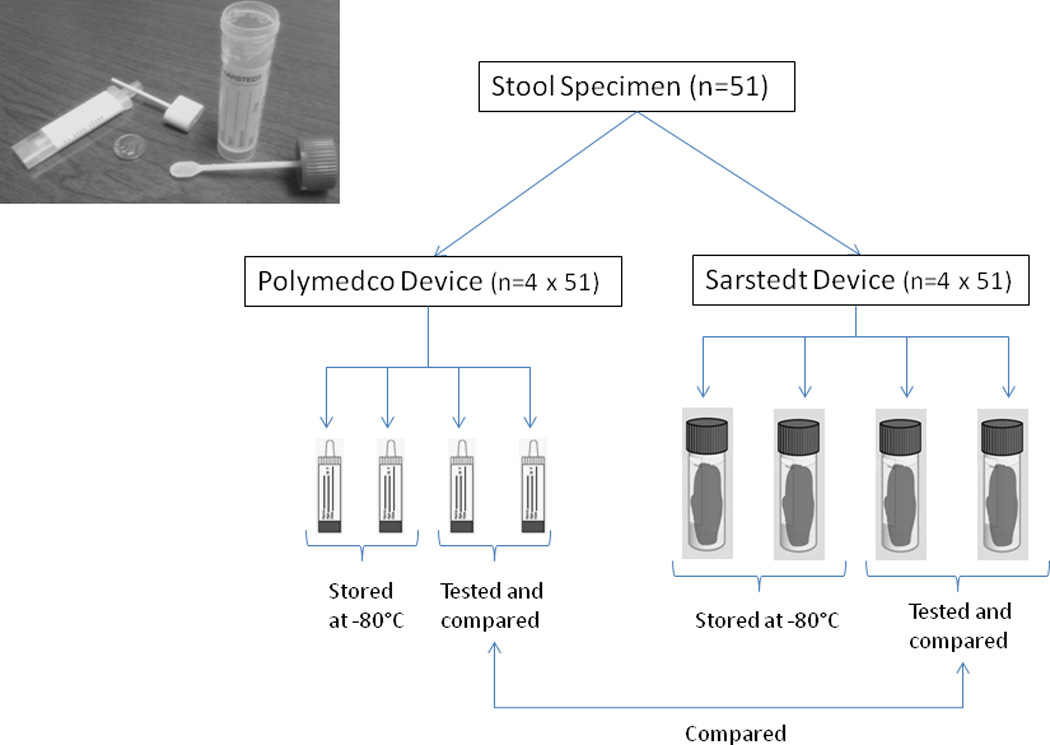
Schema for self-collection of replicate aliquots of stool specimens by 51 volunteers. Each volunteer collected eight aliquots from various parts of one stool using four Polymedco devices and four Sarstedt devices (shown in photographic insert). Pairs with each device were tested and compared for the current study.
Reproducibility and device comparison
To assess reproducibility of β-glucuronidase and β-glucosidase activity measurements, as well as to compare the two collection devices, extractions of independent duplicates of feces (one duplicate per device per participant [n=4]) were used (Figure 1). In short, fecal specimens were thawed on ice; proteins were extracted as described above; and activities of microbial β-glucuronidase and β-glucosidase were measured colorimetrically in triplicates, as described above. The mean of the triplicate values was used to determine the intra class correlation (ICC) between replicates; and the means of the replicates were used to assess correlation between devices.
Statistical analysis
Enzymatic activity was estimated as the mean [and standard error of the mean (SEM)] of the triplicate measures. Differences between the devices in mean enzyme activities were compared with Wilcoxon signed rank tests. ICC between each device’s duplicate aliquots was calculated for each enzyme. Difference in ICC between the devices was tested by bootstrapping with 1000 replicates. Significance was based on two-sided tests with α=0.05. Analyses were conducted using the statistical software SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
Optimization experiments with collection media
Bacterial β-glucuronidase and β-glucosidase from fecal extracts were sensitive to the collection media used. Both enzymes were inhibited by RNAlater, mild detergents, protease inhibitors and mild acidic pH (data not shown). Except as noted below, assessment of bacterial β-glucuronidase and β-glucosidase activities was restricted to specimens collected in PBS at pH7.0. With optimized conditions, including freezing and thawing (Table 1), β-glucosidase activity (mean±SEM, 6.16±0.09 IU/100 mg protein) was more than 2-fold higher than β-glucuronidase activity (mean±SEM, 2.61±0.04 IU/100 mg protein).
Freeze/thaw effects
As shown in Table 1, β-glucuronidase activity in specimens that had never been frozen did not differ significantly from activity in specimens that had been frozen and thawed under various conditions (P≥0.06 for all comparisons). There was, however, significant heterogeneity in β-glucuronidase activity among the freeze/thaw conditions (P=0.02), with the highest activity observed with the fast freeze/fast thaw condition and the lowest activity with the fast freeze/slow thaw condition. In contrast to β-glucuronidase, never frozen specimens had significantly lower β-glucosidase activity compared to all of the freeze/thaw conditions (by 26.8–38.7%, P≤0.0005 for all comparisons). As observed for β-glucuronidase, there was significant heterogeneity of β-glucosidase among the freeze/thaw conditions (P=0.03); activity was highest with the fast freeze/fast thaw condition and lowest with the slow freeze/fast thaw condition.
Delayed freezing and storage conditions
The effect of incubation at room temperature on fecal enzymatic activity for samples stored in different media is shown in Fig. 2. The highest activity of β-glucuronidase in the basal condition (at time 0) was observed in fecal specimens in PBS alone (P=0.02). This activity with PBS alone fell by 20% within 2 hours and by 40% during 4 hours of incubation at room temperature (Fig. 2a). Protease inhibitors and acidic pH both decreased the β-glucuronidase activity by more than 50% at time 0 and by lesser amounts with incubation at room temperature. The loss of β-glucosidase activity during 4 hours at room temperature was similar (Fig. 2b). Incubation at room temperature for more than 12 hours resulted in higher enzyme activity levels (Fig. 2a,b), presumably due to proliferation of bacteria.
Figure 2.
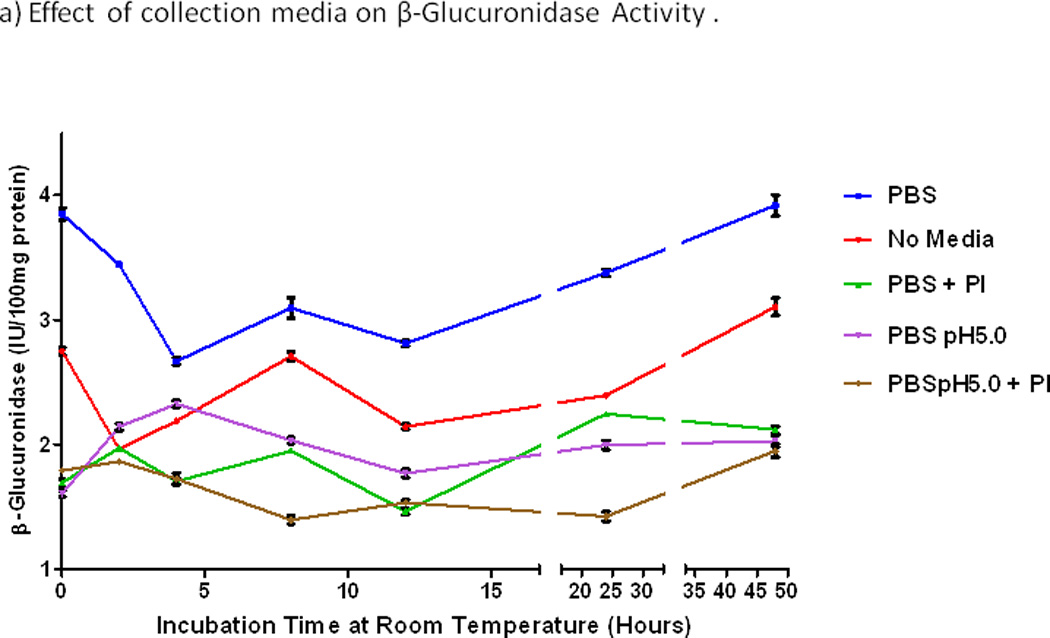
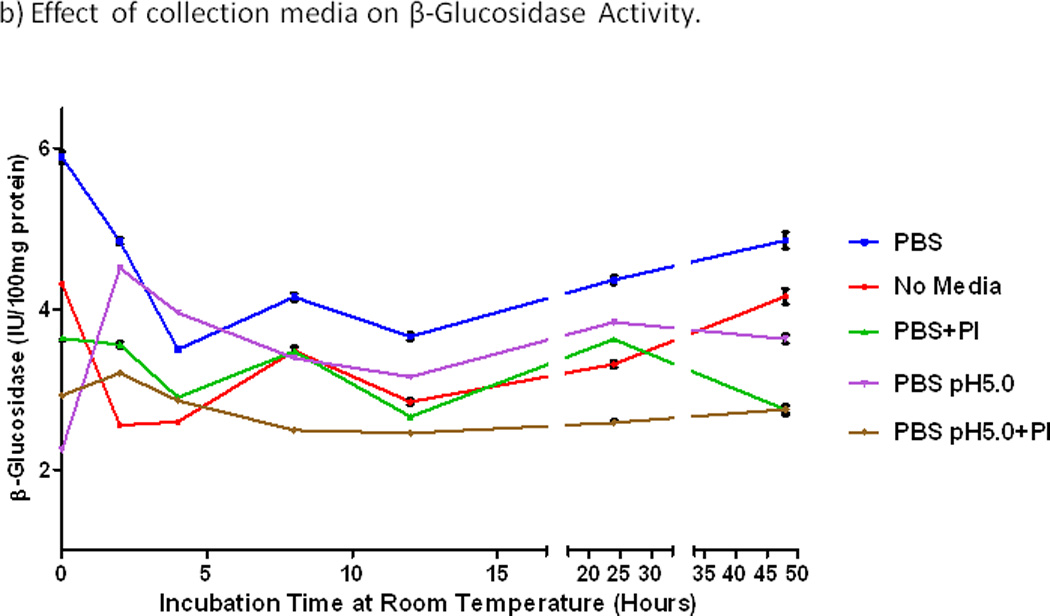
Effects of incubation time at room temperature and storage media on enzymatic activities. a) β-glucuronidase. b) β-glucosidase. PBS, phosphate buffered saline alone at pH7.0, else with protease inhibitor (+PI), or acid (pH5.0), or both. Activities of both enzymes were consistently highest with PBS alone at pH7.0. Activities of both enzymes fell steeply during 2–4 hours at room temperature, and they increased over 24–48 hours at room temperature, perhaps from bacterial proliferation.
Reproducibility of fecal microbial enzymatic activity
With specimens that were self-collected by 51 volunteers (Figure 1), reproducibility between duplicates in enzymatic activity levels was good to excellent with both devices (Fig.3a,b). With the larger (Sarstedt) device, β-glucuronidase had ICCSarstedt=0.921, and β-glucosidase had ICCSarstedt=0.920. With the smaller (Polymedco) device, the corresponding values were ICCPolymedco=0.92 for β-glucuronidase and ICCPolymedco=0.76 for β-glucosidase. ICC was significantly lower with Polymedco than Sarstedt for β-glucosidase (mean ICC difference 0.17, P<0.0001) but not for β-glucuronidase (mean ICC difference 0.003, P=0.91). Moreover, as shown in Table 2, mean (SEM) levels of activities were significantly lower with Polymedco than with Sarstedt [β-glucuronidase 0.74 (0.10) vs 1.20 (0.15), P=0.01; β-glucosidase 1.47 (0.08) vs 2.37 (0.23), P=0.003]. As also shown in Table 2, β-glucosidase activity was higher than β-glucuronidase activity with either device (P<0.0001 for each device).
Figure 3.
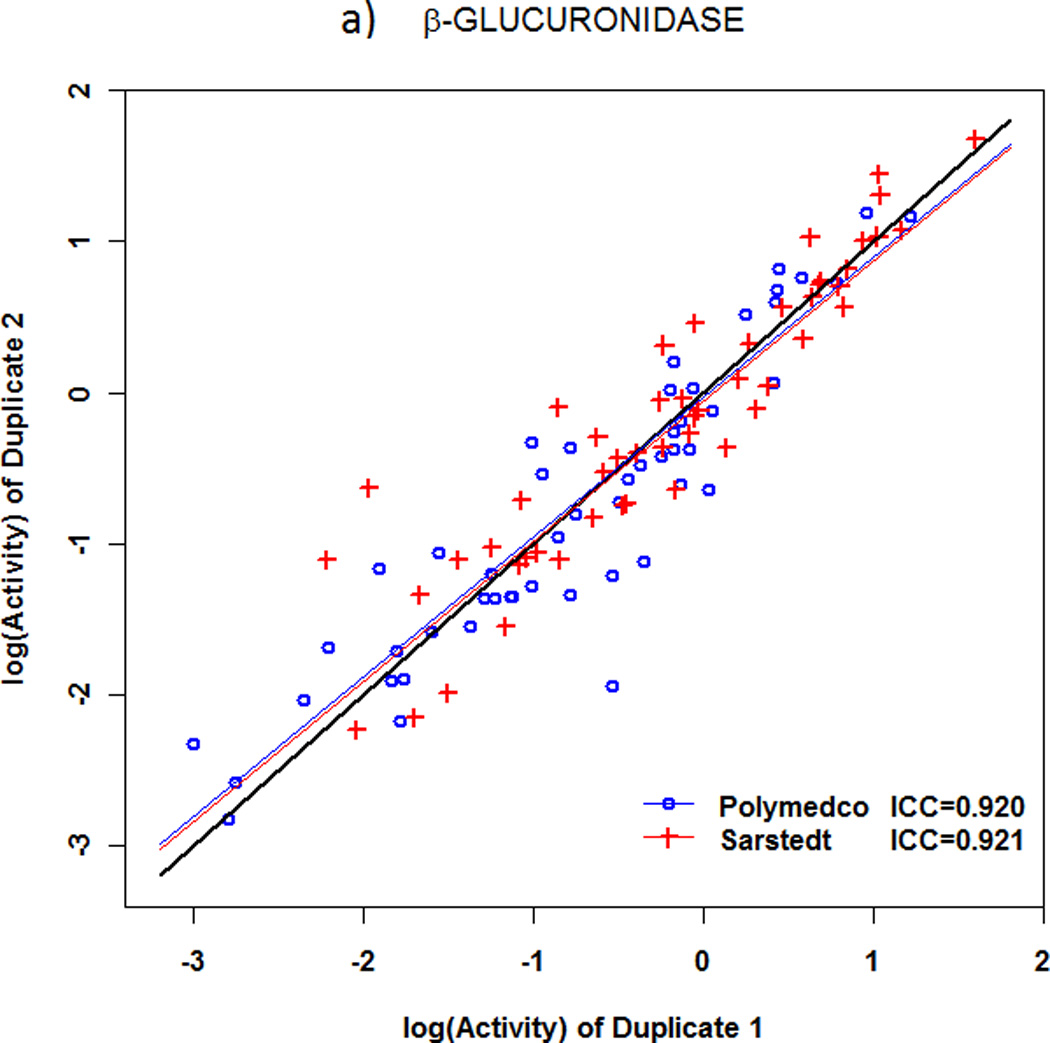
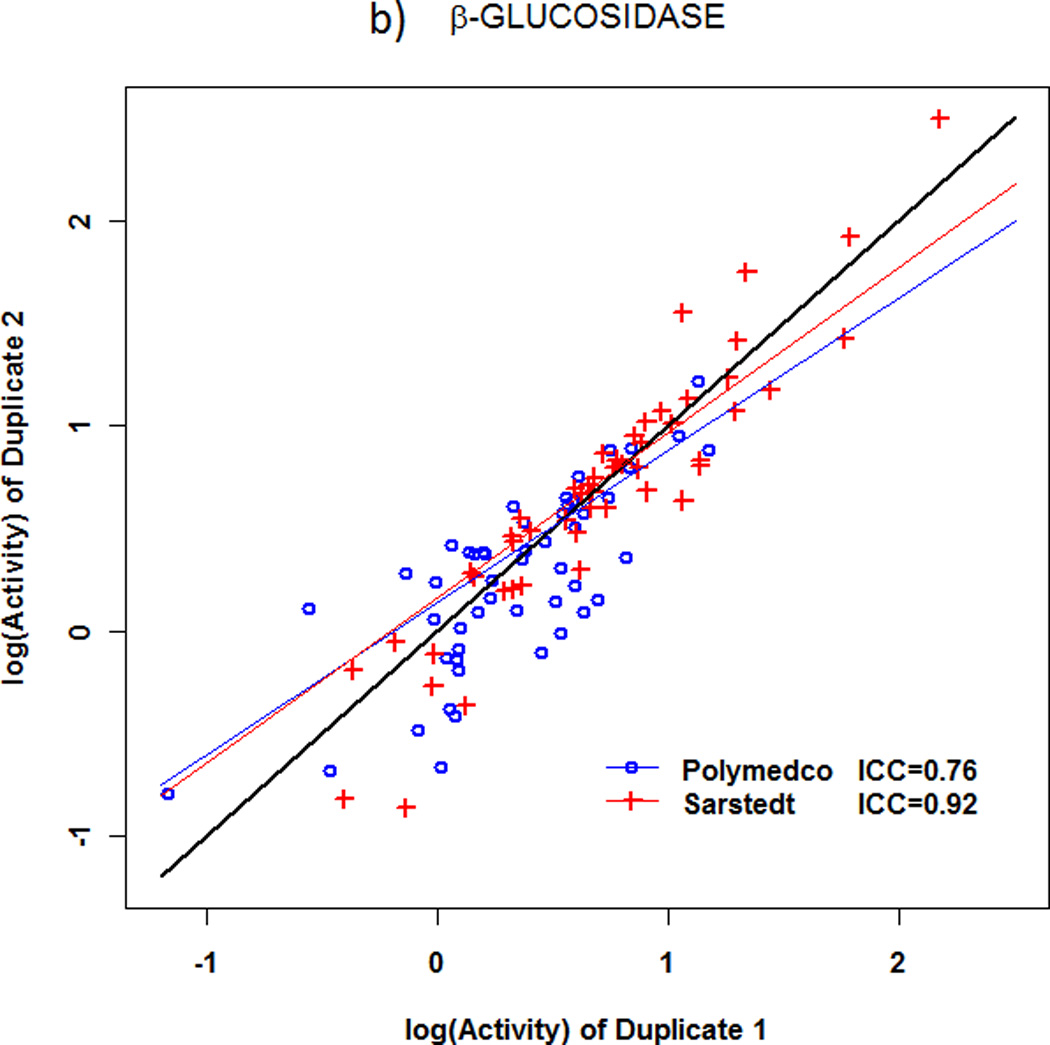
Reproducibility of microbial enzyme activities in fecal specimens obtained with two different collection devices. a) β-glucuronidase. b) β-glucosidase. For β-glucosidase activity, the intraclass correlation coefficient (ICC) was significantly lower with Polymedco (0.76) than with Sarstedt (0.92, P<0.0001), but the ICC was excellent with both devices for β-glucuronidase activity.
Table 2.
Inter- and intra-device comparison of enzymatic activity in the study population.
| Polymedco | Sarstedt | ||||||
|---|---|---|---|---|---|---|---|
| Enzyme | N | Mean† | SEM | N | Mean† | SEM | T-test* |
| β-glucuronidase | 51 | 0.74 | 0.1 | 51 | 1.2 | .15 | P=0.01 |
| β-glucosidase | 51 | 1.47 | 0.08 | 51 | 2.4 | .23 | P=0.003 |
| T-test* | P=0.0001 | P=0.0001 | |||||
Mean and standard error of the mean (SEM) enzymatic activity with each device, expressed in IU/100mg input protein.
Unpaired T-test with α=0.05
Discussion
To facilitate clinical and epidemiologic studies of the human microbiota, this project developed and assessed methods to accurately and reproducibly measure the functional activity of two microbial enzymes, β-glucuronidase and β-glucosidase, in self-collected fecal specimens. We found that immediately chilled specimens that were collected in sufficient quantity yielded robust and highly reproducible quantification of both enzymes. Results were less optimal with delay at room temperature or a smaller quantity of feces. In contrast to enzyme measures, delay before freezing and amount of feces appear to have less effect on characterization of the fecal microbiome based on 16S rRNA amplification and pyrosequencing [14;15].
Stool is a complex mixture of metabolites, indigestible and partially digested fibers, short-chain fatty acids, bile acids, mucus, viable and remnants of dead intestinal cells, and a dynamic and diverse microbial ecosystem of prokaryotes, eukaryotes, fungi and phage viruses. Even though bacterial diversity in the human gastrointestinal tract can surpass more than 1000 distinct bacterial species, the collective gut microbiome appears to have a relatively uniform core of metabolic functions [16;17]. When stool is removed from the gut microenvironment, however, some of these functions are affected by changes in oxygen tension, temperature, preservation media, and other conditions. Fluctuations in handling and processing of such specimens are likely to have marked effects on measures of bacterial functional activity. To understand these possible sources of variation, a series of experiments was performed on fresh human stool to define the effects of freezing, media, extraction techniques, and lag time on the activity of two bacterial enzymes, β-glucosidase and β-glucuronidase, involved in chemical cleavage of polysaccharides or deconjugation reactions.
Our experiments showed that both enzymes are sensitive to the collection media used. Previous reports of β-glucuronidase and β-glucosidase kinetics suggested an optimal acidic pH of 5.0 to maximize activity [18;19]. However, when an acidic pH of 5.0 was used during either extraction or enzyme measurement, the activity was reduced by 58% compared to a neutral pH of 7.0. Likewise, both enzymes were very susceptible to the presence of protease inhibitors, including mercaptoethanol, which have been reported in extraction buffers for proteins used in kinetic studies [20;21]. Consequently, enzymatic assay optimization and validation need to be established for specific biospecimens before launching any collection protocol used for clinical studies. Our experience suggests that neutral pH is preferred with human fecal samples.
Specimens collected in the field typically spend various amounts of time at room temperature, following which they are shipped to a central laboratory on frozen gel packs (4°C) or dry ice (−30°C). Once in the laboratory, samples are either processed immediately or stored in freezers. While this work flow is common in many studies, lag times from one step to another in specimen processing are often not determined. For specimens like human stool, harboring a concentration of bacteria on the order of 1014 cells/gr, lag time at room temperature or even on ice will most likely affect the metabolic rate and degradation of expressed proteins. To determine the effect of room temperature incubation on fecal bacterial enzymatic activity, we performed a time line experiment using different collection media. Irrespective of media used, the overall effect was a 20%–40% loss of enzymatic activity within 2–4 Hr at room temperature. These results illustrate that large bias may occur with delayed freezing. For our reproducibility assessment, we sought to minimize decay at room temperature by having our 51 volunteers immediately chill their self-collected specimens with gel packs, following which we froze the specimens in liquid nitrogen within 3 hours. We found that immediate chilling was sufficient for highly reproducible measurement of two enzymes.
In summary, technological advances in sequencing over the last 20 years have revolutionized the conceptual framework of microbiota/host interactions. Sequencing can be complemented by quantification of specific bacterial functions. As a first step in this functional-genomic continuum, herein we carefully evaluated the stability and effects of specimen storage, handling and media on bacterial enzymatic activity. We found that homogenization of stool in neutral pH, chilling and freezing soon after specimen collection, and fast thawing at 37°C before protein extraction preserved the activity of at least two bacterial enzymes. Because freezing soon after specimen collection is not always feasible in clinical and field settings, alternatives for stabilization of bacterial enzymatic activity are needed. With specimens that were self-collected in sufficient amount, then immediately chilled, we found that estimates of β-glucuronidase and β-glucosidase activities were highly reproducible. Application of these methods to clinical and epidemiologic research could provide insights on how the intestinal microbiota affects human physiology and health.
Supplementary Material
Acknowledgements
We thank Mr. Andrew Para for help with collection of the specimens and data, Dr. Charles Rabkin for helpful comments on the manuscript. We are especially grateful to the study participants.
Financial support: This project (Z01-CP010214) was funded by the Intramural Research Program of the National Cancer Institute, National Institutes of Health
Footnotes
Conflict of Interest: The authors have no conflicts of interest
Reference List
- 1.Hamady M, Knight R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res. 2009;19(7):1141–1152. doi: 10.1101/gr.085464.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman HJ. Effects of differing purified cellulose, pectin, and hemicellulose fiber diets on fecal enzymes in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Cancer Res. 1986;46(11):5529–5532. [PubMed] [Google Scholar]
- 4.Goldin BR, Gorbach SL. Effect of Lactobacillus acidophilus dietary supplements on 1,2-dimethylhydrazine dihydrochloride-induced intestinal cancer in rats. J Natl Cancer Inst. 1980;64(2):263–265. doi: 10.1093/jnci/64.2.263. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura J, Kubota Y, Miyaoka M, Saitoh T, Mizuno F, Benno Y. Comparison of four microbial enzymes in Clostridia and Bacteroides isolated from human feces. Microbiol Immunol. 2002;46(7):487–490. doi: 10.1111/j.1348-0421.2002.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 6.Renwick AG, Tarka SM. Microbial hydrolysis of steviol glycosides. Food Chem Toxicol. 2008;46(Suppl 7):S70–S74. doi: 10.1016/j.fct.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, et al. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299(5615):2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 8.Narushima S, Itoha K, Miyamoto Y, Park SH, Nagata K, Kuruma K, et al. Deoxycholic acid formation in gnotobiotic mice associated with human intestinal bacteria. Lipids. 2006;41(9):835–843. doi: 10.1007/s11745-006-5038-1. [DOI] [PubMed] [Google Scholar]
- 9.Van der Meulen R, Camu N, Van VT, Heymans C, De VL. In vitro kinetic analysis of carbohydrate and aromatic amino acid metabolism of different members of the human colon. Int J Food Microbiol. 2008;124(1):27–33. doi: 10.1016/j.ijfoodmicro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6(2):121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 11.Cucchiara S, Iebba V, Conte MP, Schippa S. The microbiota in inflammatory bowel disease in different age groups. Dig Dis. 2009;27(3):252–258. doi: 10.1159/000228558. [DOI] [PubMed] [Google Scholar]
- 12.Lee B, Lee JH, Lee HS, Bae EA, Huh CS, Ahn YT, et al. Glycosaminoglycan degradation-inhibitory lactic acid bacteria ameliorate 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice. J Microbiol Biotechnol. 2009;19(6):616–621. doi: 10.4014/jmb.0808.479. [DOI] [PubMed] [Google Scholar]
- 13.Kasper DL. A paradigm for commensalism: the role of a specific microbial polysaccharide in health and disease. Nestle Nutr Workshop Ser Pediatr Program. 2009;64:1–8. doi: 10.1159/000235779. [DOI] [PubMed] [Google Scholar]
- 14.Flores R, Shi J, Gail MH, Gajer P, Ravel J, Goedert JJ. Assessment of the human fecal microbiota: II. Reproducibility and associations of 16S rRNA pyrosequences. 2011 doi: 10.1111/j.1365-2362.2012.02659.x. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu GD, Lewis JD, Hoffmann C, Chen YY, Knight R, Bittinger K, et al. Sampling and pyrosequencing methods for characterizing bacterial communities in the human gut using 16S sequence tags. BMC Microbiol. 2010;10:206. doi: 10.1186/1471-2180-10-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu B, Wang X, Li L. Human gut microbiome: the second genome of human body. Protein Cell. 2010;1(8):718–725. doi: 10.1007/s13238-010-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turnbaugh PJ, Henrissat B, Gordon JI. Viewing the human microbiome through three-dimensional glasses: integrating structural and functional studies to better define the properties of myriad carbohydrate-active enzymes. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66(Pt 10):1261–1264. doi: 10.1107/S1744309110029088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowland IR. Factors affecting metabolic activity of the intestinal microflora. Drug Metab Rev. 1988;19(3–4):243–261. doi: 10.3109/03602538808994135. [DOI] [PubMed] [Google Scholar]
- 19.Ball AL, Chambers KA, Hewinson M, Navaratnarajah S, Samrin L, Thomas N, et al. A microtitre plate assay for measuring glycosidase activity. J Enzyme Inhib Med Chem. 2008;23(1):131–135. doi: 10.1080/14756360701384252. [DOI] [PubMed] [Google Scholar]
- 20.Breyne PDLM, Dedonder A, Van Montagu M, Depicker A. Quantitative kinetic analysis of B-gluronidase activities using a computer-directed microtiter plate reader. Plant Molecular Biology Reporter. 1993;11:21–31. [Google Scholar]
- 21.Honda H, Kataoka F, Nagaoka S, Kawai Y, Kitazawa H, Itoh H, et al. Beta-galactosidase, phospho-beta-galactosidase and phospho-beta-glucosidase activities in lactobacilli strains isolated from human faeces. Lett Appl Microbiol. 2007;45(5):461–466. doi: 10.1111/j.1472-765X.2007.02176.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


