Abstract
Objective
To investigate oral cancer in pregnant women, a rare but therapeutically challenging patient subset.
Methods
After IRB approval, an EMERSE search was used to identify all women treated at the University of Michigan from 1998–2010 with head and neck squamous cell carcinoma (HNSCC) during pregnancy. This identified four patients with tongue cancer. Biomarkers and HPV were assessed by immunohistochemistry and multiplex PCR/Mass spectrometry, respectively.
Results
Two patients responded well to therapy and are alive more than 10 years after diagnosis; two died of disease. All tumors overexpressed EGFR and Bcl-xL, three of four overexpressed c-Met, both tumors that progressed overexpressed p53. All tumors were negative for HPV, p16, ER, PR, and HER-2.
Conclusions
Biomarkers of aggressive tumors (high EGFR, Bcl-xL, c-Met; low p53) did not correlate with outcome. Additional studies are needed to determine whether perineural invasion, delay in diagnosis, and p53 overexpression are factors related to poorer survival.
Keywords: Oral cancer, Biomarkers, HPV, pregnancy, risk factors
Introduction
Head and neck squamous cell carcinoma (HNSCC) is typically considered to be a disease that predominantly affects older males, with a male: female ratio of approximately 4:1(1). Long term alcohol and tobacco use have been identified as the traditional risk factors for this disease. Interestingly, recent trends have shown an increase in the incidence of HNSCC in younger patients without these risk factors, and there is controversy over whether these represent a more aggressive form of cancer(2). Recently, an increased incidence of HNSCC of the tongue in female patients has been described, which is partially responsible for a noted fall in the male: female ratio of patients affected by this disease(3). Therefore, while it is still rare for a young woman of reproductive age to be diagnosed with HNSCC, recent developments suggest an increasing risk for this population.
When HNSCC is diagnosed in a pregnant patient, clinicians and patients are faced with the challenge of balancing maternal and fetal health. While early detection and intervention is key, it is also important to weigh the risks of diagnostic and treatment modalities to the fetus. The patient is faced with difficult ethical decisions, and the clinician is often tasked with providing both optimal treatment for the cancer and protection of the fetus.
There is a paucity of data regarding the etiology of cancers of the head and neck in pregnant women. The studies that do exist are primarily case reports discussing the challenges that clinicians face in administering treatment that is of maximal benefit to the patient and minimal risk to the fetus(4–7). While the hypothesis that these tumors are hormonally induced during pregnancy seems logical, it has not yet been determined whether or not a biological predisposition to HNSCC during pregnancy exists. It seems plausible that these patients may suffer from a latent human papillomavirus (HPV) that becomes active and oncogenic during pregnancy. However, none of the cases reported in the literature have been assessed for HPV status. We report a series of cases in which pregnant women presented with HNSCC to the University of Michigan Department of Otolaryngology - Head & Neck Surgery between 1998 and 2010. These patients’ tumors were analyzed for biomarker expression and HPV status.
Materials and Methods
Patient population
Permission from the Institutional Review Board for Human studies was granted to identify patients that presented to the Department of Otolaryngology between 1998 and 2010 with head and neck squamous cell carcinoma during pregnancy using the University of Michigan’s Electronic Medical Record Search Engine (EMERSE). This query identified four patients. IRB approval was also granted for use of existing patient specimens and data.
DNA Isolation
DNA was extracted from a core of formalin-fixed, paraffin-embedded tissue taken from each pretreatment biopsy or surgical resection specimen for HPV analysis. The core of tissue was deparaffinized, then DNA was isolated according to the manufacturer’s protocol (QIAmp DNA Mini Kit; Qiagen).
Immunostaining
Slides containing tissue sections from the resected tumors were deparaffinized, rehydrated, and peroxidase-quenched (DAKO Cytomation) as described previously(8). All slides were incubated in Antigen Retrieval Solution (Dako Cytomation, Glostrup Denmark) for 40 minutes in 92C water bath with a buffer change midway and allowed to cool to room temperature for 20 minutes. For EGFR, an additional antigen retrieval step was performed with pepsin incubation for 10 minutes at 37C. Horse serum was used for blocking (30 minutes at room temperature). Primary antibodies, prediluted EGFR/31G7 and 1:300 dilution of c-Met/3D4 (Zymed Laboratories, South San Francisco, CA), 1:100 dilution of p53/DO1 and 1:100 dilution of Bcl-xL/7D9 (Lab-Vision, Fremont, CA), prediluted p16/E6H4 (CINtec Histology, Westborough, MA), 1:200 dilution of ERa/SP1 (BioCare Medical, Concord, CA), 1:200 dilution of ERb/88 (Biogenex, San Ramon, CA), 1:50 dilution of PR/636 and 1:100 dilution of cErbB2 (HER2) (Dako, Carpinteria, CA), were allowed to incubate overnight at 4C. Slides were washed and secondary antibodies linked to avidin/biotin peroxidase (ABC Kit; Vector Laboratories, Burlingame CA) were used to detect primary antibody binding. All stained slides were reviewed and scored by an experienced pathologist using an intensity scale from 1 to 4 (1= no staining, 2= weak, 3= moderately strong, 4= intense signal) and a four-point proportion-positive scale where 1= <10%, 2= 11–25%, 3= 26–50%, 4= 51–100%. The intensity and proportion were multiplied together to give an overall IHC score from 0–16.
HPV analysis
HPV analysis from the extracted DNA from pretreatment biopsies or surgical specimens was accomplished using a sensitive and quantitative real-time PCR with primers that are specific to HPV-E6 region of each of the 15 most common high-risk HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73). HPV analysis was carried out using the Attosense multiplex PCR–mass spectrometry method developed at the University of Michigan and licensed to Sequenom as previously described(9).
Results
Case reports
Patient 1
A 26-year-old Caucasian woman with no significant past medical history presented to our clinic with pain on the right side of her tongue, mild dysphagia, and left-sided otalgia. She had recently found out she was pregnant. The patient had no history of alcohol use but had a 6-pack-year tobacco use history. She was no longer smoking at the time of presentation. She had no family history of cancer. Her exam was notable for a large, ulcerated, tender lesion of the right lateral tongue with palpable induration extending across the midline. Biopsy of her right lateral tongue revealed well-differentiated invasive squamous cell carcinoma (SCC). Pre-operative CT scan revealed an irregular 3.5 cm by 2.5 cm mass in the right posterior tongue that extended inferiorly. Multiple scattered, enlarged lymph nodes were noted bilaterally.
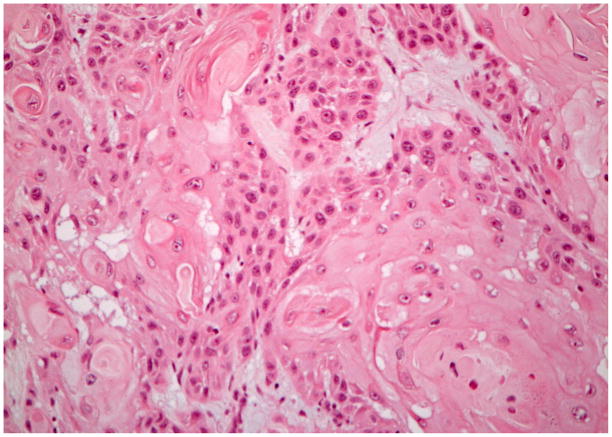
Following elective termination of her pregnancy, the patient underwent bilateral selective neck dissections of levels I through IV, subtotal glossectomy, and an anterolateral thigh fasciocutaneous free flap transfer. Tumor pathology revealed a 5 cm well-differentiated, ulcerative SCC with a depth of invasion of 2 cm. There was extensive perineural invasion and several unilateral lymph nodes positive for malignancy, some of which had extra-capsular extension. The final tumor margins were negative, and the tumor was staged as T4aN2bM0 (Figure 1A), with a type 3 pattern of invasion(10).
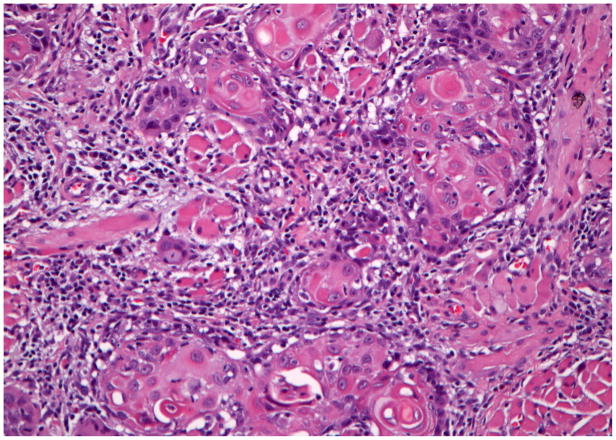
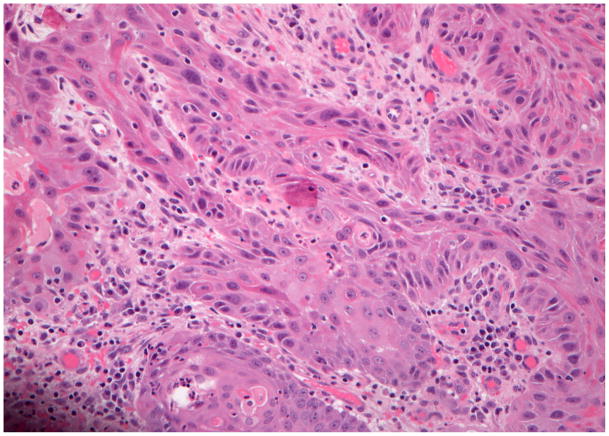
Figure 1.
Hematoxylin and eosin staining of the invasive squamous cell carcinoma tissue from each of the cases. Case #1 (A), case #2 (B), case #3 (C), case #4 (D). (A–D ×200 original magnification).
The patient’s post-operative recovery was complicated by an oral-cutaneous fistula. Adjuvant platinum/taxol chemotherapy and radiation to a dose of 60 Gray were recommended. After receiving three doses of chemotherapy and 5.4 Gray of radiation, she was unable to continue making her scheduled appointments.
Approximately 6 months after her surgery, the patient presented to the emergency department with neck swelling and was admitted for a large neck abscess. Biopsy revealed recurrent invasive well- to moderately-differentiated SCC. PET scan demonstrated FDG-avidity in the right neck but was negative for distant lesions. Her recurrence was deemed unresectable, and she was started on a palliative chemoradiation regimen. The patient subsequently developed lung metastases and passed away approximately one year after diagnosis.
Patient 2
A 33-year-old Caucasian female presented with soreness of the left tongue and a one-month history of a neck mass. She first noted these symptoms during her last month of pregnancy. After delivery, she continued to notice increasing soreness and fullness on the left side of her tongue as well as fullness in the left upper neck. Notably, she had no history of tobacco or alcohol use.
Physical exam revealed a 1.7 cm by 3.0 cm area of ulceration and erythroplakia on the left lateral edge, surrounded by a 0.5 cm rim of submucosal firmness. There was no involvement of her floor of mouth or base of tongue. Her left neck revealed a 4.0 cm by 3.0 cm mass in the submandibular region, levels I and II. Biopsy confirmed SCC of the tongue.
She underwent a partial glossectomy and left selective lymph node dissection. The primary tumor was a moderately differentiated, 2.3 cm mass with pushing borders. There was no vascular invasion. She had several positive level II lymph nodes, some with extracapsular spread. Her tumor was staged as T2N2bM0 (Figure 1B) with a type 3 pattern of invasion(10). She received post-operative radical external beam radiation therapy. She has returned for follow up every 6–12 months and was free of disease at her most recent appointment 12 years after diagnosis.
Patient 3
A 30-year-old Caucasian woman presented with a history of intermittent soreness on her left lateral tongue which over several months had worsened until her pain became persistent, and interfered with her ability to eat. She had recently found out she was pregnant. She had no other specific complaints related to the head and neck. She had no history of tobacco or alcohol use, and no family history of cancer. Her exam revealed a 2 cm mucosal lesion on the lateral border of her left tongue. No apparent mass or induration was present upon palpation of the tongue and no other masses were noted. A biopsy confirmed SCC of her left lateral tongue with a depth of invasion of 2 mm. MRI showed a 3–3.5 cm lesion of the tongue with underlying skeletal muscle invasion without evidence of cervical adenopathy.
She underwent a left hemiglossectomy, a left selective neck dissection of levels I–III, and a tracheostomy. Pathology revealed a 0.7 cm moderately-differentiated SCC with infiltrative borders and a depth of invasion of 0.9 cm. Extensive perineural invasion was present. All cervical lymph nodes were negative. There were positive margins, requiring reoperation after which clear margins were obtained. The tumor was staged as T2N0M0 (Figure 1C) with a type 4 pattern of invasion(10). Post-operative radiation was considered but not performed.
Approximately four months later, the patient noted intermittent pain that became progressively worse. Imaging was postponed until after delivery. At that time, a PET scan showed FDG-avidity in the posterolateral tongue extending into the base of tongue and floor of mouth. Options for therapy included a total glossectomy and possible laryngectomy versus radiation therapy with chemotherapy. After discussion the latter treatment option was chosen.
She began a platinum/taxol chemotherapy regimen followed by 70 Gray of radiation. Three months after completing this therapy, a PET scan revealed findings consistent with local recurrence, a new active lesion in the left thyroid, and multiple metabolically active lesions in both lungs. After receiving chemotherapy with cetuximab/rapamycin, a follow-up PET scan showed a solitary primary lung lesion that was biopsied to confirm metastatic disease. There was no residual activity in the primary tumor site. She underwent resection of the lung lesion, in addition to cetuximab chemotherapy. She subsequently developed hoarseness and biopsies revealed squamous cell cancer of the anterior larynx. This likely represented a new primary as it developed remotely from her original primary but could have been a recurrence of her original tumor. This was treated with a laryngectomy. At the time of surgery the hypermetabolic lesion in the thyroid was found to be a papillary thyroid cancer. She developed distant metastasis, was treated with palliative chemotherapy and expired several months later.
Patient 4
A 37-year old Caucasian female with a history of premalignant changes of the right lateral tongue, including numerous biopsies for dysplasia as well as CO2 laser ablation for possible carcinoma in situ and leukoplakia. She became pregnant and noted that her tumor “took off” after the birth of her child. Shortly after she completed her pregnancy, she noticed a mucosal tear of the glossotonsillar sulcus following an episode of vomiting. One month later, she noticed an enlarging lump in the area of the right posterior tongue and a loose molar that was extracted. She had no history of tobacco or alcohol use, but her family history was positive for head and neck cancer in her grandfather. Pathology revealed SCC on the right lateral tongue and right retromolar trigone.
She underwent a right-sided composite resection of the lateral tongue, floor of mouth, and lateral mandible with a right selective neck dissection of levels I–IV. The primary tumor was a moderately differentiated, infiltrative 5.5 cm by 4.4 cm mass. Several lymph nodes were positive, but none exhibited extracapsular extension. This tumor was staged as T4aN2bM0 (Figure 1D) with a type 2 pattern of invasion(10). She completed radiation therapy approximately three months later. As of her last follow-up visit, she has been free of disease 12 years from diagnosis.
Biomarker analysis
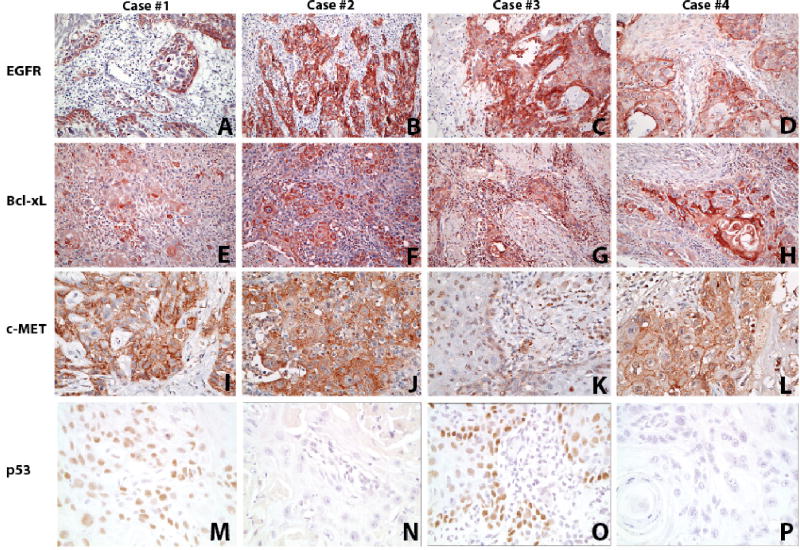
The patients’ tumors had similar biomarker expression patterns. We used biomarkers that have been previously implicated in tumor behavior and response to therapy. All four tumors overexpressed epidermal growth factor receptor (EGFR), all with IHC score of 16 (Figure 2A–D), and Bcl-xL, with IHC score of 12 (3, 4) for patient 1 and 16 for patients 2–4 (Figure 2E–H). C-Met overexpression was observed in tumors from patients 1, 2, and 4, with IHC scores of 16 for patients 1 and 2 and 12 (3, 4) for patient 4 (Figures 2I, 2J, and 2L) but not patient 3, with IHC score of 2 (2, 1) (Figure 2K). p53 was overexpressed in patients 1 and 3 only (Figure 2M and 2O). All four of the tumors were negative for p16, ER, PR, and HER-2 staining (not shown). Table 1 summarizes these biomarker results.
Figure 2.
Staining for EGFR (A–D), Bcl-xL (E–H), c-MET (I–L), and p53 (M–P) of the squamous cell carcinoma primary tumor from each patient. Fig 2A,E,I,M from patient 1; Fig 2B,F,J,N from Patient 2; Fig 2C,G,K,O Patient 3; Fig 2D,H,L,P Patient 4. (A–H ×200, I–L ×400 and M–P ×800 original magnification).
Table 1.
Summary of tumor characteristics and biomarker status in the four patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| TNM classification | T4aN2bM0 | T2N2bM0 | T2N0M0 | T4aN2bM0 |
| Tobacco use (pk/yr) | Yes (6pk/yr) | No | No | No |
| Pattern of invasion | 3 | 3 | 4 | 3 |
| Perineural invasion | Yes | No | Yes | No |
| Tumor differentiation | Well | Moderate | Moderate | Moderate |
| Extracapsular spread | Yes | Yes | N/A | No |
| Delay in diagnosis (months) | 7 | 0 | 10 | Unknown |
| Breaks in treatment | Yes | No | No | No |
| EGFR overall IHC score* | 16 | 16 | 16 | 12 (3, 4) |
| Bcl-xL overall IHC score* | 12 (3, 4) | 16 | 16 | 16 |
| c-MET overall IHC score* | 16 | 16 | 2 (2, 1) | 12 (3, 4) |
| ER overall IHC score* | 0 | 0 | 0 | 0 |
| PR overall IHC score* | 0 | 0 | 0 | 0 |
| HER-2 overall IHC score* | 0 | 0 | 0 | 0 |
| p53 overall IHC score* | 12 (3, 4) | 0 | 16 | 0 |
| p16 overall IHC score* | 2 (2, 1) | 0 | 2 (2, 1) | 2 (2, 1) |
| HPV status | Negative | Negative | Negative | Negative |
| Survival outcome | Dead from disease | Alive, no evidence of disease | Dead from disease | Alive, no evidence of disease |
Immunohistochemistry (IHC) score represents a product of a four-point intensity scale (1= no staining, 2= weak, 3= moderately strong, 4= intense signal) and a four-point proportion-positive scale where 1= <10%, 2= 11–25%, 3= 26–50%, 4= 51–100%. For scores less than 16, numbers in parentheses are (intensity score, proportion score)
Etiology
Smoking and alcohol use were not a consistent factor in the etiology of these tumors. Only one patient admitted to a tobacco use history of 6 pack-years and none admitted to alcohol abuse. Only one patient had a family history of head and neck cancer in a grandparent. All four tumors were also negative for high-risk HPV as assessed by a sensitive and accurate PCR mass spectroscopy assay(9) as well as by p16INK4a expression, a sensitive surrogate marker for transcriptionally active high risk HPV involvement (11). Except for the common factor of pregnancy, the etiology of these tumors remains a mystery.
Discussion
Although HNSCC in pregnant patients is rare, it is important to understand the biology of these tumors to treat the cancer appropriately while minimizing risk to the fetus. Unfortunately, the increasing incidence of HNSCC in young patients means that likely there will be an increasing incidence of this disease in young pregnant women. The etiology of oral squamous cancer in young women in general and particularly pregnant women is perplexing. HNSCCs are typically the result of many years of tobacco and alcohol abuse, and have been predominantly a disease of men in their 7th and 8th decades of life who exhibit these social behaviors(1, 2). HNSCCs are rare in women, although with the increase in social acceptance of cigarette smoking during the 1940s and 1950s and active tobacco marketing campaigns for women in the 1960s and 1970s, the proportion of women with these cancers has been slowly rising such that instead of 4:1 male to female ratio the current ratio is closer to 3:1 or less(3). Nevertheless, most women that develop head and neck cancers have long histories of exposure to tobacco smoke and are in the later decades of life when diagnosed. The four pregnant patients ranged in age from 26–37. Interestingly, a review of oral cavity cancer cases in our database revealed a total of 13 patients age 30 and under. Remarkably, there was an inverted gender ratio, with a 11:2 ratio of women: men, including two of the pregnant women, both of whom died of their disease. In contrast, the remaining 8 of 11 women are currently alive with no evidence of disease. On the other hand, both men under 30 died from disease within 16 months of diagnosis. Although the number of patients age 30 and under is small, the predominance of women in this age group is striking, as is the better survival among the non-pregnant women in this age category. Thus, it is important to determine the factors associated with the development of these tumors in young women, and especially during pregnancy.
There were few clues to the differences in outcome among the pregnant patients. One factor we considered was whether any of these women experienced a delay in diagnosis. In fact, patient 1 initially presented with a sore on her tongue that was thought to be a chancre sore and was subsequently treated with antibiotics six months prior to diagnosis of malignancy. Patient 3 noted an intermittent soreness that waxed and waned for ten months before the pain became severe enough for her to seek medical opinion. Patient 4 had a long history of a dysplastic lesion that was being closely monitored and thus was diagnosed when it seemed to accelerate growth after pregnancy. It is unclear whether diagnosis was delayed in patient 2, as she was initially seen and diagnosed at a different institution and complete records were not available.
Pregnancy may induce physiologic changes that can promote neoplastic growth, such as a high metabolic state, increased circulating growth factors and amplified hormonal responses mediated through the estrogen and progesterone receptors(4, 12, 13).
Increased expression of estrogen receptor (ER) and progesterone receptor (PR) have been linked to several types of cancers. The binding of the respective ligands to ER and PR stimulates the proliferation of certain cell types, which may enable DNA mutations to persist as a result of increased cell division(14). Because these patients were pregnant, it seemed plausible that the high levels of estrogen and progesterone circulating during pregnancy would contribute to oncogenesis if the malignant cells expressed ER and/or PR. Some studies have shown an absence of both estrogen and progesterone receptors in SCC cells(15). However, others have reported that the majority of cases of HNSCC in males overexpress these receptors, and the highest tumor-free survival in that study was in the ER(+)/PR(−) group(16). Our results in the present study fail to implicate expression of either ER- α or PR in these tumors.
HER-2 is a cell membrane-bound tyrosine kinase that is involved in a signal transduction pathway that leads to cell proliferation. HER-2 is an important biomarker in breast cancer, and targeting HER-2 with herceptin is an important component of breast cancer therapy. Recently, some studies have explored HER-2 expression in HNSCC(17, 18), but were inconclusive with respect to its effect on prognosis. Because of the altered hormonal milieu during pregnancy, we assessed HER-2 expression in these oral cancers as a possible biomarker. However, all of the tumors were HER-2 negative. In the case of breast cancers, HER-2 in combination with ER-α and PR is used as an indicator of prognosis(19). Triple-negative breast cancers (negative for ER-α, PR, and HER-2) are known to have a low response to drug therapy and a worse prognosis(20). Although our patients’ tumors were all triple negative for these markers, in the absence of a population of oral tumors that express these markers, it is not possible to ascribe any significance to this negative finding.
The TNM classification system is used for assessing the tumor stage of HNSCC. This system is mostly based on primary tumor size, regional lymph node involvement, and distant metastases as variables for staging. While tumor staging can help to determine prognosis, recent studies have demonstrated that more specific histopathological markers play a significant role in assessing prognosis. Among these biomarkers are perineural invasion and pattern of invasion. Perineural invasion has been associated with poor prognosis in many cancers, including HNSCC(21). Because of its association with tumor aggressiveness, it may be one of the most significant tumor characteristics influencing survival(21, 22), and local recurrence(23). Patients 1 and 3 had tumors that exhibited perineural invasion. Both of these patients experienced aggressive disease progression with multiple recurrences and death from their disease compared to patients 2 and 4, whose tumors lacked perineural invasion. Our results are consistent with perineural invasion as an indicator poor prognosis.
Another histopathologic biomarker that has been shown to influence prognosis is pattern of invasion. Pattern of invasion (POI) is defined on a scale from type 1 to 4. A POI type 1 is characterized by broad, pushing borders at the invasive front, type 2 indicates a tumor with broad, invasive fingers (separate large tumor islands), type 3 is characterized by smaller invasive islands with more than 15 cells per island, and a POI type 4 is the most invasive type, defined as containing invasive tumor islands smaller than 15 cells per island(10). It has been shown that using this histopathologic assessment over margin status is more predictive of local disease-free and overall survival(24). Patient 4 with POI 2 is a long-term survivor, while only 1 of the 3 patients with more invasive POI has survived long term without disease recurrence. Patient 3 with the most invasive POI, type 4, experienced significant disease progression including multiple distant metastases.
EGFR is a receptor tyrosine kinase that plays a role in several physiologic and pathologic processes, including apoptosis, cell cycle progression, and metastasis. It is commonly upregulated in many cancers, especially HNSCC(29, 30). Overexpression of EGFR in oropharyngeal cancer is inversely associated with response to induction chemotherapy and chemoradiation therapy, and thus it is directly associated with a poor prognosis(8). In HPV(−) oropharyngeal tumors, we found that the tumors with EGFR overexpression had a poor prognosis, and all HPV-negative patients in a recent organ sparing trial with the EGFR-high phenotype died of their disease within two years of diagnosis(8). In the HPV(+) cases in the same trial, high expression of EGFR was associated with significantly reduced disease-specific and overall survival when compared to HPV(+)/EGFR(−) cases. In spite of the high EGFR/HPV(−) phenotype of all four tumors, two of these four patients have enjoyed long disease-free survival.
The tumor suppressor p53 is a key component of cell cycle regulation. In the setting of DNA damage, p53 induces cell cycle arrest and DNA repair mechanisms. Mutations in the p53 gene have been implicated in numerous cancers(8, 31) and are particularly common in smoking-induced head and neck cancers. Wild-type p53 is only expressed transiently and, therefore, it typically cannot be detected by immunohistology in normal cells. Tumors that stain positive for p53 typically have a mutation that allows p53 to accumulate within the cell(32). Although p53 is often mutated in oral cancers, in tumors induced by HPV, the HPV E6 protein causes degradation of p53, inactivating it. Thus, p53 is typically wild-type in HPV-induced cancers, but it is uncommon in HPV-negative HNSCC.
We found low or no expression of p53 in two of the tumor specimens, consistent with wild-type p53 in these patients; however, both patients whose tumors progressed overexpressed p53, consistent with mutant p53. We previously had established a cell line from patient 1 and in the course of characterizing the line we sequenced the full-length cDNA for p53. This revealed a point mutation at codon 157 that substitutes alanine for valine (V157A). This is consistent with the high expression of p53 in the patient’s tumor. Although we have not sequenced p53 in the tumor from patient 3, the high expression indicates a mutation in that tumor as well.
High-risk human papillomaviruses (HPV), especially HPV16, are now clearly implicated as etiologic factors in many oropharyngeal and some oral cavity cancers(25, 26). HPV-positive oropharyngeal cancers are also associated with younger age at diagnosis and may arise in people without a history of tobacco use. Furthermore, HPV-positive oropharyngeal cancers are more likely to respond to induction chemotherapy and chemoradiation therapy(8, 9). p16INK4a is a cyclin-dependent kinase inhibitor that blocks cell cycle progression by inhibiting pRb phosphorylation(27). While silencing of p16 INK4a is common in many smoking related head and neck cancers(28), it is nearly always overexpressed in HPV-positive tumors secondary to the effect of HPV E7 oncoprotein sequestering of Rb resulting in release of E2F, and upregulation p16 expression. Thus, immunohistology for p16 is often used as a marker of HPV expression(11). Three tumors expressed only low intensity staining of p16 in a minority of cells, which is in contrast to the high p16 intensity and 100% tumor cell positivity that is typically observed in HPV-positive tumors. This finding was confirmed by quantitative real-time PCR and MALDI-TOF mass spectroscopy(9), which failed to identify any high risk HPV in the tumor specimens. Thus, we can eliminate HPV as a likely etiologic factor to explain the development of these tumors at such an early age. However, the absence of HPV in all four of these tongue cancers arising in non-smokers is perplexing. It suggests that there is an unusual mechanism of carcinogenesis, or perhaps that even an inherited predisposition to cancer development is at work in these young pregnant women. High throughput assessment of the full cancer genome in each of these tumors may produce insight into the carcinogenic mechanism.
BcL-xL, an anti-apoptotic protein, is frequently overexpressed in HNSCCs(8, 32, 33). Bcl-xL contributes to tumorigenesis and treatment resistance by enabling cells that have been damaged by carcinogens, chemotherapeutics, or radiation to evade apoptosis. In laryngeal cancer, Bcl-xL overexpression in combination with low expression of wild-type p53 is associated with a decrease in response to chemotherapy and a 16-fold increased risk of laryngectomy(34). The combination of low p53 and high Bcl-xL levels is also associated with decreased overall survival in oropharyngeal cancer(8). Bcl-xL was overexpressed, and p53 was low or undetectable in the pregnant patients suggesting that chemotherapy would be unlikely to add significant benefit. Interestingly, both patients who received chemotherapy failed to exhibit a strong benefit.
C-Met overexpression is highly correlated with tumor invasiveness and poor overall survival in multiple cancer types(35–37). C-Met inhibitors are being developed that decrease metastatic growth and invasiveness in several types of cancer(38–40). While the data that implicates c-Met specifically in HNSCCs is sparse, the data that does exist indicates that it is an important biomarker of poor prognosis and outcome in this disease. Interestingly, patient 3 had a tumor with a pattern of invasion type 4, the most invasive type. This patient was the only of the four that had an aberrant pattern of c-Met expression and did not floridly overexpress c-Met. Clearly more work on the association between c-Met and pattern of invasion as well as the basis for aberrant c-Met expression in oral cancer requires more study. Similarly, the presence of c-Met overexpression in three of the four tumors from pregnant patients suggests that the use of c-Met inhibitors may have a therapeutic role in these cancers.
While the biomarker expression pattern is similar among all four patients in this study, disease progression and survival vary greatly. Two patients, 2 and 4, had completed their pregnancy by the time their cancer was diagnosed and treated. It is possible that critical hormonal influences had diminished in these patients after they completed their pregnancy, allowing for their better outcomes. The lack of ER and PR expression does not exclude the possibility of other hormonal influences impacting the growth and spread of these cancers, as estrogen and progesterone are not the only hormonal variables that change dramatically during pregnancy. For example, growth factors such as IGF-1 and others that are elevated during pregnancy could have a potent influence on epithelial cell proliferation and tumor progression. Further study is required to determine this possibility.
Patients 2 and 4 were able to undergo surgery followed by radiation, as they had both delivered their babies. These two patients are alive and well more than 12 years after diagnosis. Patients 1 and 3 both exhibited extensive perineural invasion, a known poor prognostic factor in head and neck cancer, and patient 3 exhibited the most aggressive POI. These findings prompted consideration for adjuvant therapy.
Patient 1 had interrupted post-operative radiation and chemotherapy and returned to complete her treatment months after her initial therapy and after her recurrent cancer was inoperable. It is known that prolonged treatment breaks can affect ultimate local and regional control rates and prognosis. Although this patient chose to terminate her pregnancy, her inability to complete the recommended course of therapy may have contributed to her poor outcome.
Patient 3 continued her pregnancy and did not receive radiation or chemotherapy postoperatively, as she had no detectable nodal spread, and her only concerning risk factor was perineural invasion. It is unclear whether radiation with or without chemotherapy improves local and regional control rates or overall survival in patients whose tumors exhibit perineural invasion. The NCCN guidelines suggest considering radiation or radiation with chemotherapy for patients whose tumors exhibit perineural invasion, but so far there is no study that indicates that this added therapy improves prognosis. Given the uncertainty that additional treatment would prove to be beneficial, the patient chose to continue her pregnancy. This patient’s cancer behaved in a very aggressive manner and the influence of aberrant c-Met expression on her tumor biology warrants further investigation.
Pregnancy complicates the management of patients. Pregnancy is a state of relative immunosuppression and increased metabolic and hormonal activity that may affect the outcome of treatment for a wide variety of diseases. Any treatment that is chosen will affect both the mother and the fetus. The physician and mother must try to balance the need for treatment and the risks to the fetus. Whenever possible, if equivalent therapies are available, the one with the least risk to the patient and fetus should be selected. Treatment decisions are much more difficult when the efficacy of the treatment is unclear and the risks may be significant, as is the case with perineural invasion in head and neck cancer. The decision to terminate a pregnancy is generally accepted to be a personal one. The treating physician has an obligation to advise the mother regarding treatment options, expected effects, and risks to the fetus. The mother ultimately decides with the help of this advice and that from other individuals; then she may choose to continue the pregnancy or not.
Physicians and expectant mothers may have a tendency to avoid therapy or postpone full treatment until after the completion of pregnancy for fear of causing harm to the fetus. Ideally, treatments that are highly effective and have little or no risk to the fetus would be available. New treatments for HNSCC have begun to focus on the significance of molecular inhibitors that silence one or more molecular pathways that may decrease the severity of side effects while increasing the response to therapy. Administration of a more specific treatment regimen that is tailored to the specific tumor biology would be more effective and decrease the toxicity to the patient. This concept is particularly important in pregnant women to minimize harm to the fetus while treating the cancer appropriately. Further investigation is necessary to help determine the exact biology of these tumors to facilitate a more specific treatment regimen that will balance both maternal and fetal health.
Acknowledgments
This work was supported by NIH NIDCR 1 R01-DE019126 (TEC), NCI P50 CA97248 (Head and Neck SPORE), NCI P30 CA46592 (Cancer Center Core Grant), NIH NIDCD T32 DC05356 (AT), NIDCD P30 DC05188 (Research Center Core Grant), MICHR UL 1RR024986.
Footnotes
A poster presentation of this manuscript was shown at the Triological Society Combined Sections Meeting in Scottsdale, AZ on January 26-29, 2011.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103(9):1843–9. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 3.Callery CD, Spiro RH, Strong EW. Changing trends in the management of squamous carcinoma of the tongue. Am J Surg. 1984;148(4):449–54. doi: 10.1016/0002-9610(84)90368-4. [DOI] [PubMed] [Google Scholar]
- 4.Layton SA, Rintoul M, Avery BS. Oral carcinoma in pregnancy. Br J Oral Maxillofac Surg. 1992;30(3):161–4. doi: 10.1016/0266-4356(92)90148-c. [DOI] [PubMed] [Google Scholar]
- 5.Cheung EJ, Wagner H, Jr, Botti JJ, Fedok F, Goldenberg D. Advanced oral tongue cancer in a 22-year-old pregnant woman. Ann Otol Rhinol Laryngol. 2009;118(1):21–6. doi: 10.1177/000348940911800104. [DOI] [PubMed] [Google Scholar]
- 6.Chow VL, Chan JY, Ng RW, Wei WI. Management of head and neck tumours during pregnancy: case report and literature review. Asian J Surg. 2008;31(4):199–203. doi: 10.1016/s1015-9584(08)60086-x. [DOI] [PubMed] [Google Scholar]
- 7.Succo G, Crosetti E, Torta R, et al. Oropharyngeal carcinoma during pregnancy: clinical and psycho-oncological aspects. Acta Otorhinolaryngol Ital. 2003;23(6):440–5. [PubMed] [Google Scholar]
- 8.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26(19):3138–46. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989;18(8):432–7. doi: 10.1111/j.1600-0714.1989.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 11.Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162(3):747–53. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibuya H, Saiot M, Horiuchi JI, Suzuki S. Treatment of malignant head and neck tumors during pregnancy--a report of 3 cases. Acta Oncol. 1987;26(3):237–8. doi: 10.3109/02841868709091439. [DOI] [PubMed] [Google Scholar]
- 13.Lasaridis N, Tilaveridis I, Karakasis D. Management of a carcinoma of the tongue during pregnancy: report of case. J Oral Maxillofac Surg. 1996;54(2):221–4. doi: 10.1016/s0278-2391(96)90453-x. [DOI] [PubMed] [Google Scholar]
- 14.Ostrander JH, McMahon CM, Lem S, et al. Optical redox ratio differentiates breast cancer cell lines based on estrogen receptor status. Cancer Res. 2010;70(11):4759–66. doi: 10.1158/0008-5472.CAN-09-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuller DE, Abou-Issa H, Parrish R. Estrogen and progesterone receptors in head and neck cancer. Arch Otolaryngol. 1984;110(11):725–7. doi: 10.1001/archotol.1984.00800370027006. [DOI] [PubMed] [Google Scholar]
- 16.Budai B, Remenar E, Orosz Z, Szamel I, Kralovanszky J, Kasler M. [Steroid hormone receptors in squamous cell carcinoma of the head and neck] Orv Hetil. 1997;138(12):723–7. [PubMed] [Google Scholar]
- 17.Williams MD. Integration of biomarkers including molecular targeted therapies in head and neck cancer. Head Neck Pathol. 2010;4(1):62–9. doi: 10.1007/s12105-010-0166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali MA, Gunduz M, Gunduz E, et al. Expression and mutation analysis of her2 in head and neck squamous cell carcinoma. Cancer Invest. 2010;28(5):495–500. doi: 10.3109/07357900903476778. [DOI] [PubMed] [Google Scholar]
- 19.Filipovic S, Kocic B, Petrovic B, Milovanovic L, Poultsidi AA. Hormone sensitivity of primary breast carcinoma. J BUON. 2010;15(2):255–62. [PubMed] [Google Scholar]
- 20.Kan N, Kuwata K, Mise K, Kodama H. [Effective therapeutic regimens for patients with triple-negative (ER/PgR/HER2-negative) metastatic breast cancer] Gan To Kagaku Ryoho. 2010;37(7):1259–64. [PubMed] [Google Scholar]
- 21.Goepfert H, Dichtel WJ, Medina JE, Lindberg RD, Luna MD. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148(4):542–7. doi: 10.1016/0002-9610(84)90385-4. [DOI] [PubMed] [Google Scholar]
- 22.Brown B, Barnes L, Mazariegos J, Taylor F, Johnson J, Wagner RL. Prognostic factors in mobile tongue and floor of mouth carcinoma. Cancer. 1989;64(6):1195–202. doi: 10.1002/1097-0142(19890915)64:6<1195::aid-cncr2820640606>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Hosal AS, Unal OF, Ayhan A. Possible prognostic value of histopathologic parameters in patients with carcinoma of the oral tongue. Eur Arch Otorhinolaryngol. 1998;255(4):216–9. doi: 10.1007/s004050050046. [DOI] [PubMed] [Google Scholar]
- 24.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167–78. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 25.Strome SE, Savva A, Brissett AE, et al. Squamous cell carcinoma of the tonsils: a molecular analysis of HPV associations. Clin Cancer Res. 2002;8(4):1093–100. [PubMed] [Google Scholar]
- 26.Li W, Thompson CH, Cossart YE, et al. The expression of key cell cycle markers and presence of human papillomavirus in squamous cell carcinoma of the tonsil. Head Neck. 2004;26(1):1–9. doi: 10.1002/hed.10335. [DOI] [PubMed] [Google Scholar]
- 27.Zhang HS, Postigo AA, Dean DC. Active transcriptional repression by the Rb-E2F complex mediates G1 arrest triggered by p16INK4a, TGFbeta, and contact inhibition. Cell. 1999;97(1):53–61. doi: 10.1016/s0092-8674(00)80714-x. [DOI] [PubMed] [Google Scholar]
- 28.Wong TS, Man MW, Lam AK, Wei WI, Kwong YL, Yuen AP. The study of p16 and p15 gene methylation in head and neck squamous cell carcinoma and their quantitative evaluation in plasma by real-time PCR. Eur J Cancer. 2003;39(13):1881–7. doi: 10.1016/s0959-8049(03)00428-3. [DOI] [PubMed] [Google Scholar]
- 29.Takes RP, Baatenburg de Jong RJ, Schuuring E, Litvinov SV, Hermans J, Van Krieken JH. Differences in expression of oncogenes and tumor suppressor genes in different sites of head and neck squamous cell. Anticancer Res. 1998;18(6B):4793–800. [PubMed] [Google Scholar]
- 30.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24(17):2666–72. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 31.Menendez D, Inga A, Resnick MA. Potentiating the p53 network. Discov Med. 2010;10(50):94–100. [PubMed] [Google Scholar]
- 32.Kumar B, Cordell KG, D’Silva N, et al. Expression of p53 and Bcl-xL as predictive markers for larynx preservation in advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2008;134(4):363–9. doi: 10.1001/archotol.134.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma H, Sen S, Mathur M, Bahadur S, Singh N. Combined evaluation of expression of telomerase, survivin, and anti-apoptotic Bcl-2 family members in relation to loss of differentiation and apoptosis in human head and neck cancers. Head Neck. 2004;26(8):733–40. doi: 10.1002/hed.20059. [DOI] [PubMed] [Google Scholar]
- 34.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 35.Lo Muzio L, Farina A, Rubini C, et al. Effect of c-Met expression on survival in head and neck squamous cell carcinoma. Tumour Biol. 2006;27(3):115–21. doi: 10.1159/000092716. [DOI] [PubMed] [Google Scholar]
- 36.De Herdt MJ, Baatenburg de Jong RJ. HGF and c-MET as potential orchestrators of invasive growth in head and neck squamous cell carcinoma. Front Biosci. 2008;13:2516–26. doi: 10.2741/2863. [DOI] [PubMed] [Google Scholar]
- 37.Grugan KD, Miller CG, Yao Y, et al. Fibroblast-secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc Natl Acad Sci U S A. 2010;107(24):11026–31. doi: 10.1073/pnas.0914295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holgren C, Dougherty U, Edwin F, et al. Sprouty-2 controls c-Met expression and metastatic potential of colon cancer cells: sprouty/c-Met upregulation in human colonic adenocarcinomas. Oncogene. 2010 doi: 10.1038/onc.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uddin S, Hussain AR, Ahmed M, et al. Inhibition of c-MET is a potential therapeutic strategy for treatment of diffuse large B-cell lymphoma. Lab Invest. 2010 doi: 10.1038/labinvest.2010.108. [DOI] [PubMed] [Google Scholar]
- 40.Canadas I, Rojo F, Arumi-Uria M, Rovira A, Albanell J, Arriola E. C-MET as a new therapeutic target for the development of novel anticancer drugs. Clin Transl Oncol. 2010;12(4):253–60. doi: 10.1007/s12094-010-0501-0. [DOI] [PubMed] [Google Scholar]