Abstract
The regulation of bone and fat homeostasis and its relationship to energy expenditure has recently been the focus of increased attention due to its potential relevance to osteoporosis, obesity and diabetes. Although central effectors within the hypothalamus have been shown to contribute to the regulation of both energy balance and bone homeostasis, little is known of the underlying mechanisms, including the possible involvement of transcriptional factors within the hypothalamus. Transgenic mice overexpressing ΔFosB, a splice variant of the AP1 transcription factor FosB with mixed agonist-antagonistic properties, have increased energy expenditure and bone mass. Since these mice express ΔFosB in bone, fat and hypothalamus, we sought to determine 1) whether overexpression of ΔFosB within the hypothalamus was sufficient to regulate energy expenditure and whether it would also regulate bone mass, and 2) whether these effects were due to antagonism to AP1. Our results show that stereotactic injection of an adeno-associated virus vector to restrict overexpression of ΔFosB to the ventral hypothalamus of wildtype mice induced a profound increase in both energy expenditure and bone formation and bone mass. This effect was phenocopied, at an even stronger level, by overexpressiong of a dominant-negative DNJunD, a pure AP1 antagonist. Taken together these results suggest that downregulation of AP1 activity in the hypothalamus profoundly increases energy expenditure and bone formation, leading to both a decrease in adipose mass and an increase in bone mass. These findings may have physiological implications since ΔFosB is expressed and regulated in the hypothalamus.
Keywords: AP-1, hypothalamus, energy expenditure, bone formation
INTRODUCTION
Osteoporosis and obesity, two widespread diseases with major social and economic impact, have long been considered as independent clinical entities. In recent years, a large body of work has however indicated not only that there is abundant cross-talk between bone and fat cells, but also that they share common precursors and a link to the regulation of energy and metabolism by the central nervous system (1,2). Whole body adiposity is affected predominantly by changes in the size of individual adipocytes, which is influenced by the balance between food intake and energy requirements. Skeletal homeostasis is maintained by the balance between the resorptive osteoclasts and the bone forming osteoblasts, which is influenced by many factors, including Ca2+ and phosphate metabolism and insulin. An increasing body of evidence suggests that both of these tissues can also be regulated by neuronal influences arising from the hypothalamus, a potent regulator of food intake and energy expenditure, via the sympathetic nervous system (SNS) (3–5) (6–10).
While this work has clearly established that the regulation of on the one hand bone and on the other energy metabolism and fat can be linked, the exact nature of this link and the relative roles of peripheral vs central pathways remain elusive. Insight into these pathways may also be gleaned from observations made in mouse models exhibiting altered homeostasis of both bone and fat. One such model, the ENO2-ΔFosB mice, in which the AP-1 transcription factorΔFosB is overexpressed in several tissues including adipose, bone and brain, exhibit a striking decrease in fat mass and increase in bone formation rates and bone mass (11,12).
ΔFosB is a naturally occurring spliced variant of FosB, and it acts as a mixed agonist-antagonist of AP1 in vitro and in vivo (13,14). We have previously shown that targeted expression of ΔFosB to the osteoblast was able to cell-autonomously increase bone formation and bone mass, albeit less than in the ENO2-ΔFosB model, but had no effect on adipose mass, indicating that the decrease in fat is not osteoblast-dependent (15). In contrast, targeted expression of ΔFosB to the adipocyte failed to recapitulate either the bone or the adipose phenotype (16), demonstrating that neither the decreased adipose mass nor the increased bone mass is the result of a cell-autonomous effect of ΔFosB within adipocytes. Detailed metabolic analysis of the ENO2-ΔFosB mice revealed that these mice display an increase in energy expenditure due to increased fatty-acid oxidation within skeletal muscle (16). Taken together, these observations suggested that the two phenotypes might not be linked at the peripheral level and that the changes in energy expenditure, which led to the decrease in adipose mass, were the result of changes in another organ, independent of bone and adipose tissue. Whether the changes in bone and/or fat were the result of AP1 activity in the brain and whether they were mechanistically linked remained to be determined.
In the present study we sought to determine if the expression of ΔFosB or a pure AP1 antagonist (an N-terminal Δ1–149aa truncation of JunD termed DNJunD) within the hypothalamus was able to affect energy expenditure, fat mass, or bone formation and thereby reproduce the changes we observed in the ENO2-ΔFosB mice. Our results reveal that targeted expression of either ΔFosB or DNJunD to the ventral hypothalamus was sufficient to give rise to an increase in energy expenditure. In addition, we observed a profound increase in bone formation and bone mass in these mice. These results demonstrate that repression of AP1 transcriptional activity within the hypothalamus centrally regulates both energy expenditure and bone formation, opening new avenues for the treatment of both obesity and osteoporosis.
METHODS AND MATERIALS
Animals
All animals were maintained on normal chow (60% carbohydrate/10% fat/30% protein calories). 8 to 12 week old male C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). The ENO2-ΔFosB mice were generated as previously described (11). All animal protocols were approved by the Harvard University Institutional Animal Care and Use Committee.
In situ hybridization of brain sections
Animals were anthestized and brains were removed and snap frozen in dry ice slurry. Sections were prepared as previously described (17) briefly, antisense and sense riboprobes corresponding to a 715 bp of ΔFosB was amplified and labeled with 35S-CTP (Amersham Biosciences, Piscataway, NJ). In situ hybridization of ΔFosB mRNA with cRNA probes were performed on brain sections anterior-posterior (−1.7 – −2.2 from Bregma) (17). Sections were developed with Emulsion NTB (Kodak, Rochester NY) for 1 week. (1 week in emulsion gives a signal intensity similar to 1 day exposed to film).
Immunostaining of brain sections
Animals were anesthetized and perfused transcardially with 4% paraformaldehyde in PBS. For immunostaining, brains were removed and incubated in 4% paraformaldehyde for 2 hrs, then placed in 20% glycerol in PBS for 6 hrs. 10 μm sections were prepared and labeled with antibodies to FosB, JunD or GFP at 1:1000 (Santa Cruz) and visualized with CY fluorophore-labeled secondary antibodies at 1:200 (Jackson Immunolabs).
RNA isolation and real-time PCR analysis
Total RNA was isolated from tissue samples using Trizol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. mRNA was converted to cDNA using Superscript II and an oligo-dT primer (Invitrogen, Carlsbad, CA), following manufacturer’s instructions. Transcripts were quantified by real-time PCR using an iCycler (Bio-Rad), with the iQ SYBR Green Super Mix (Bio-Rad) and primers to FosB and normalized to actin as previously described (15).
Stereotaxic injections into of the ventral hypothalamus
Adeno-associated viruses (AAV) encoding either ΔFosB-GFP, DNJunD-GFP or GFP alone (previously described in (18)), were injected bilaterally into the ventral hypothalamus of C57BL/6 mice. Stereotaxic coordinates of the injection site were anterior-posterior −2.1 mm, lateral ± 1.3 mm, and dorsoventral −5.7 mm at an angle of 10 degrees from Bregma. Animals were analyzed 6 weeks after injections.
CLAMS metabolic study
Mouse energy expenditure, food intake, and locomotor activity were measured using the Comprehensive Lab Animal Monitoring System (CLAMS) (Columbus Instruments, Columbus, OH). Food intake (Kcal/g/hr) was calculated from food consumption. Energy expenditure was calculated using indirect calorimetry as a measure of heat [(3.815 + 1.232 × RQ) × VO2] (Kcal/kg/hr). All data was normalized to the body weight during analysis.
GST pull-down
Glutathione-S-tranferase (GST) fusion proteins were produced and purified by binding to glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech.) as previously described (15). For interaction studies, DNJunD protein was produced by in vitro transcription-translation and incubated with GST fusion proteins bound to glutathione-Sepharose beads overnight at 4 °C. The samples were washed three times in mRIPA, the beads were sedimented and boiled in 10 μl of loading buffer, and the complexes were analyzed by Western blotting.
Electromobility Shift Assay
AP-1-binding double-stranded probes (forward oligonucleotide – GTC GAC GTG AGT CAG CGC GC; reverse oligonucleotide – GGG CGC GCT GAC TCA CGT) were labeled with 15 μCi [32P]-dCTP using the Amersham Random Prime kit and purified on NICK G50 Sephadex columns. Approximately equal amounts of in vitro-transcribed FosB, ΔFosB, Δ2ΔFosB, JunD and DNJunD proteins were incubated with labeled probe (25,000 cpm) in 340 mM KCl, 50 mM MgCl2, 1 mM DTT and 3 μg/ml poly-dIdC (Sigma) at room temperature for 30 minutes. Samples were separated on 6% non-denaturing polyacrylamide gels at 200 volts for 2 hrs. Gels were dried and autoradiographed.
Luciferase Reporter Assay
HEK-293 cells (ATCC) were plated at a density of 1×105 in 6-well plates and transfected using FuGENE 6 (Roche) with constructs encoding a 6X-TRE-luciferase reporter (6X-TRE-luc, Clontech) to measure AP-1 activity Cells were also transfected with either pcJunD or pcNDJunD and cDNAs encoding the FosB isoforms (pcFosB, pcΔFosB2i3i and pcΔ2ΔFosB) at the indicated concentrations. Transfection efficiency was assessed by co-transfecting an SV40 Renilla luciferase construct (Promega); the total amount of transfected DNA was maintained at 1.25 μg per well by adding empty vector (pcDNA3.1). Dual-Luciferase assay (Promega) was performed according to the manufacturer’s instructions.
Bone histomorphometry of the tibial metaphysic and calvarial segments
All mice were injected with calcein (Sigma, 40 mg/kg) and demeclocycline (Sigma, 20 mg/kg) at 10 and 3 days prior to sacrifice to label mineralizing fronts. Tibiae and calvaria were removed and fixed in 3.7% formaldehyde then infiltrated with a mixture of 90% methyl methacrylate (Sigma), 10% dibutyl phthalate (Sigma), and 0.15% benzoyl peroxide (Polyscience) at 4 °C and then embedded in a mixture composed of 85% methyl methacrylate (Sigma), 15% dibutyl phthalate (Sigma), and 0.05% benzoyl peroxide (Polyscience). Polymerization was performed at 37 °C. Standard undecalcified sections (4 μm) were prepared using a Reichert-Jung microtome (Cambridge Instrument), and were stained with toluidine blue or left unstained. A standard histomorphometric analysis of the tibial metaphysis and calvaria were performed using the Osteomeasure analyzing system (Osteometrics, Inc.) Tibial measurements were performed in a 1.28 mm2 area starting 0.3 mm from the proximal growth plate.
Statistical analysis
The data are presented as means ± standard error of the mean (SEM), statistical analysis was performed with Student’s t test for all in vitro experiments and 2-way ANOVAs for all in vivo experiments. P values of less than 0.05 were considered statistically significant.
RESULTS
Hypothalamic Expression of ΔFosB in the ENO2-ΔFosB mice
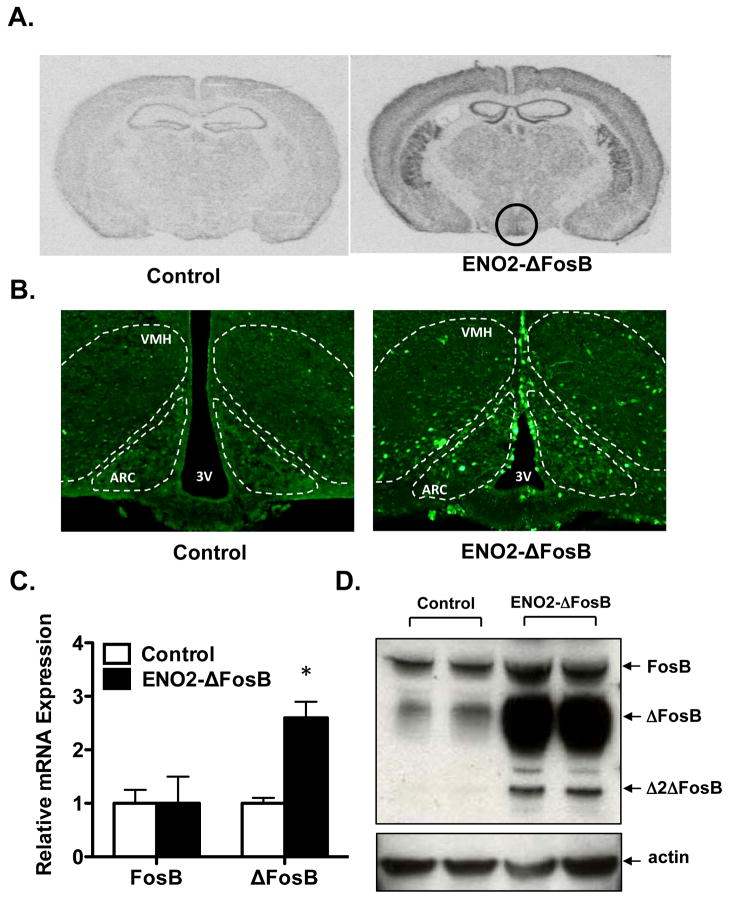
We have previously shown that targeted overexpression of ΔFosB to the osteoblastic or the adipocytic lineage had no effect on adipose mass and energy expenditure (15,16). We therefore sought to determine whether ΔFosB expression within the brain could be responsible for the increased energy expenditure observed in the ENO2-ΔFosB mice. Given the well-established role of the hypothalamus in maintaining energy homeostasis, we performed in situ hybridization and immunoflorescent staining of brain sections to determine whether ΔFosB was overexpressed in these neurons. Increased ΔFosB expression was observed in the ventral hypothalamus (black circle) (Figure 1A) and labeled neurons were identified in ventromedial hypothalamus (VMH) and to a greater extent in the arcuate nucleus (ARC) (Figure 1B). Given that our antibody detects both ΔFosB and full-length FosB, we confirmed the increased ΔFosB expression by both western blot analysis and real-time qPCR using primers which can discriminate between ΔFosB and FosB. We observed a significant increase in ΔFosB expression at both the protein (10 fold) and mRNA level in the hypothalamus of the ENO2-ΔFosB mice (Figure 1C and D). These data show that ΔFosB is expressed in the hypothalamus in wildtype mice and markedly overexpressed in the ENO2-ΔFosB mice, supporting the hypothesis that ΔFosB may be playing a role within this brain region.
Figure 1. Increased ΔFosB expression in the hypothalamus of ENO2-ΔFosB mice.
A) In situ hybridization of ΔFosB probes in coronal sections 2.1 mm posterior of Bregma in brains from ENO2-ΔFosB mice and control littermates showing high levels of ΔFosB overexpression within the striatum and ventral hypothalamus, B) Immunoflorescent staining of coronal sections 2.1 mm posterior of Bregma in brains from ENO2-ΔFosB mice and control littermates for FosB, C) Real-time expression of FosB and ΔFosB of hypothalamic punches from ENO2-ΔFosB mice and control littermates, and D) Western blot analysis of ΔFosB of hypothalamic punches from ENO2-ΔFosB mice and control littermates. Actin was used as loading control.
Central expression of ΔFosB reduces body weight and increases energy expenditure
Given the increased expression of ΔFosB within the hypothalamus of the ENO2-ΔFosB mice and the role of the hypothalamus in regulating energy expenditure, we sought to determine if increased expression of ΔFosB in the ventral hypothalamus was sufficient to alter the regulation of energy expenditure in wild-type mice. An adeno-associated virus (AAV) vector encoding either GFP alone or ΔFosB-GFP were stereotaxically injected bilaterally in the ventral hypothalamus of wildtype mice (Figure 2A). After 6 weeks, when the transgene effects become maximal (18), the mice were subjected to metabolic analysis; mice in which the injection had not been successful, as determined by post-mortem immunofluorescence analysis of brain sections (Figure 2A), were eliminated. Successfully injected AAV-ΔFosB animals exhibited a decrease in body weight, despite an apparent increase in food intake (Figure 2B and 2C). The AAV-ΔFosB injected animals also exhibited increased O2 consumption, CO2 production and energy expenditure, despite reduced locomotor activity (Figure 2D–G). These results demonstrated that specific overexpression of ΔFosB within the ventral hypothalamus is sufficient to decrease body weight and to increase energy expenditure in mice. Accordingly, histomorphometric analysis of adipocytes in visceral fat pads demonstrated a significant decrease (p<0.05) in the size of individual adipocytes and trend towards a reduction in adipose mass compared with wildtype animals (Figure S1A and S1B). Histological analysis of brown adipose tissue from these mice did not reveal any significant difference in BAT morphology, despite a trend towards smaller droplet size in the AAV-DNJunD injected mice (Figure S1D). Thus, targeted overexpression of ΔFosB in the ventral hypothalamus is sufficient to increase energy expenditure, reduce body mass and reduce fat mass adipocyte size, phenocopying the fat phenotype of the ENO2-ΔFosB mice.
Figure 2. AAV-ΔFosB expression to hypothalamus increases energy expenditure.
A) Target validation of AAV-GFP injection B) Body weight, C) Food intake, D) Locomotor activity, E) Oxygen consumption (VO2), F) Carbon dioxide production (VCO2), and G) Energy expenditure (kcal/h/kg) in AAV-GFP and AAV-ΔFosB hypothalamic injected mice. (n = 8–10; * - p < 0.05 relative to AAV-GFP).
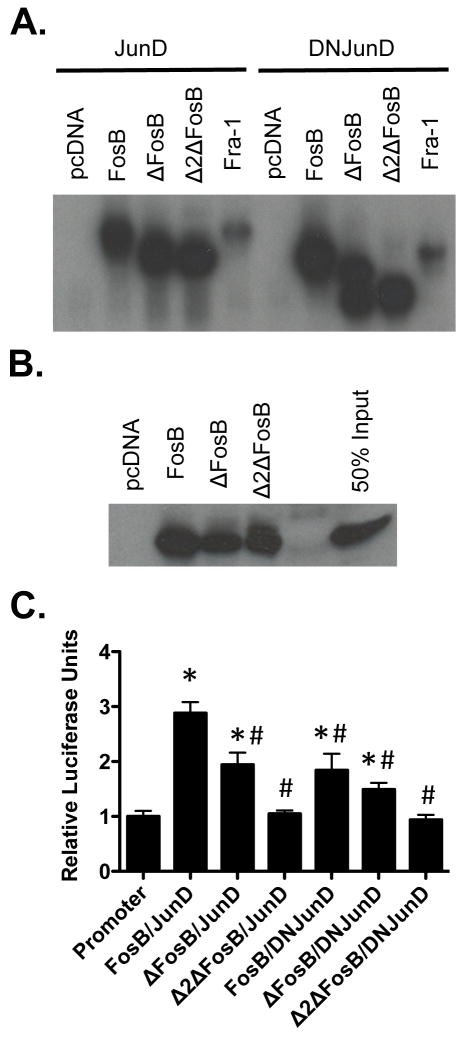
DNJunD binds Fos isoforms but has reduced transcriptional activity
We have previously shown that ENO2-driven overexpression of Δ2ΔFosB, a further truncated form of ΔFosB that has no AP1 transcriptional activity and behaves as a pure antagonist to AP1, was able to recapitulate the bone and fat phenotypes observed in the ENO2-ΔFosB mice (19). However, whether these effects could be attributed solely to repressed AP1 activity is unknown. To gain further insight into the possible mechanisms responsible for the phenotypes observed in the ENO2-ΔFosB mice and the hypothalamic expression of ΔFosB with AAV, we determined if the phenotype is the result of reduced AP1 activity within the hypothalamus. To test this possibility, we used DNJunD, which retains its DNA binding and dimerization ability similar to JunD (Figure 3A, 3B and 3C) but lacks the transactivation domain and therefore serves as an AP1 antagonist that reduces the transactivating potential of all complexes that incorporate a Jun protein as a binding partner (Figure 2C)(18,20). DNJunD therefore acts as a dominant-negative for all AP1 transcriptional activity.
Figure 3. DNJunD acts as an antagonist of AP-1 activity.
A) Gel shift of FosB isoforms with either JunD or DNJunD on AP1 DNA element, B) GST pull down of DNJunD by FosB and its truncated isoforms, C) AP1 reporter assay of FosB isoforms with either JunD or DNJunD on 6X TRE-luciferase (n = 3–4; * - p < 0.05 relative to promoter alone; # - p < 0.05 relative to FosB/JunD).
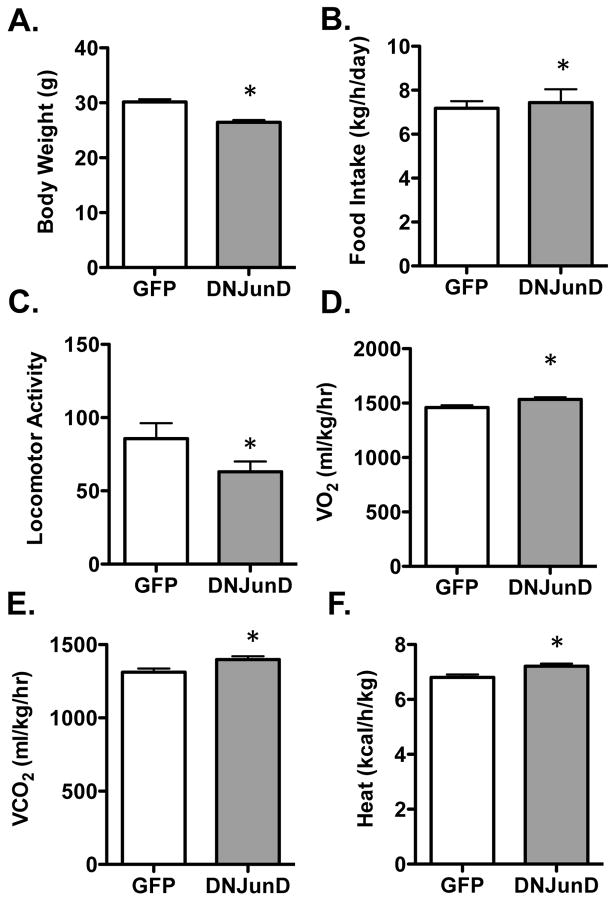
Hypothalamic expression of DNJunD reduces body weight and increases energy expenditure
Using DNJunD, we next tested the hypothesis that antagonizing AP1 activity within the hypothalamus would result in a decrease in adipose mass and an increase in energy expenditure. AAV vectors encoding DNJunD-GFP or GFP alone were stereotaxically injected within the ventral hypothalamus of wildtype mice similar to what was done for ΔFosB. Strikingly, targeted expression of DNJunD in the ventral hypothalamus was sufficient to induce a significant decrease in body weight and an increase in CO2 production and energy expenditure (Figure 4A–F), similar to what was observed in the ENO2-ΔFosB mice (16) and in the AAV- ΔFosB injected mice (Figure 2). The AAV-DNJunD injected mice also exhibited a decrease in ambulatory activity and an increased food intake (Figure 4C and 4B). Histological analysis of the adipose tissue revealed a reduction in the size of the adipocytes over that of control-injected mice (Figure S1A). Since what ΔFosB, Δ2ΔFosB and DNJunD have in common is their ability to antagonize AP1 transcriptional activity, these observations strongly suggest that AP1 activity within the hypothalamus down-regulates energy expenditure, such that expression of an AP1 antagonist increases energy expenditure and reduces fat mass in wild-type mice, recapitulating the adipose and energy phenotype observed in the ENO2-ΔFosB mice.
Figure 4. AAV-DNJunD expression in hypothalamus increases energy expenditure.
A) Body weight, B) Food intake, C) Locomotor activity, D) Oxygen consumption (VO2), E) Carbon dioxide production (VCO2), F) Energy expenditure (kcal/h/kg) in AAV-GFP and AAV-DNJunD hypothalamic injected mice. (n = 10–12; * - p < 0.05 relative to AAV-GFP).
Increased expression of ΔFosB or DNJunD in the ventral hypothalamus also increases bone formation
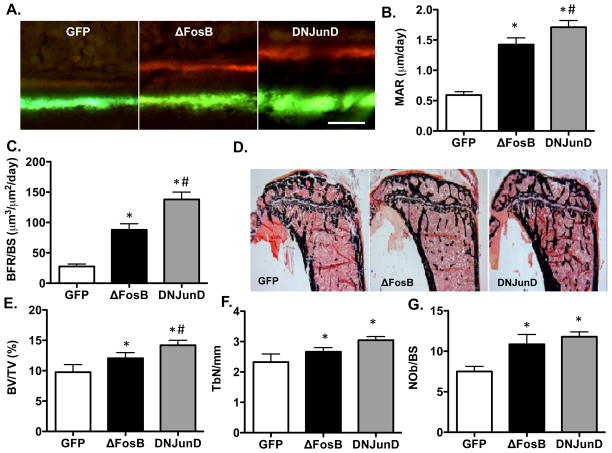
Having established that the energy expenditure and fat phenotype were the direct consequences of expression of ΔFosB and its antagonism of AP1 within the ventral region of the hypothalamus, we addressed the important question of whether this mechanism would also affect bone formation and bone mass, determining whether the bone phenotype observed in the ENO2-ΔFosB mice was also dependent on these properties. Strikingly, dynamic histomorphometric analysis of the bones from the ventral hypothalamus-targeted AAV-ΔFosB and AAV-DNJunD mice revealed a 3- and 5-fold increase in linear mineral apposition rates (MAR) resulting in a very pronounced increase in the bone formation rate (BFR) (3- and 7-fold, respectively). These observations demonstrate unequivocally an anabolic action on individual osteoblasts as well as marked increases in osteoblast numbers (Figure 5G). This massive increase in BFR was not associated with changes in bone resorption and resulted in a significant increase in bone volume (BV/TV) associated with significant changes in trabecular numbers (Figure 5A–F), despite the relatively short period of exposure to AAV-induced ΔFosB or DNJunD expression. Thus, restricted expression of ΔFosB or a pure AP1 antagonist in the ventral hypothalamus induces a massive increase in bone formation, and thereby recapitulates not only the energy and fat but also the bone phenotype observed in the ENO2-ΔFosB mice.
Figure 5. Antagonism of AP1 in the hypothalamus increases bone mass.
A) Fluorescent double labeling of mineralizing front with calcein and demeclocycline in AAV-injected mice. B) mineral apposition rate (MAR), C) Bone formation rate (BFR/BS), D) von Kossa staining of proximal tibias, E) bone volume (BV/TV), F) trabecular number (TbN/mm) and G) osteoblast number (N.Ob/BS) in AAV-injected mice. (n = 10–12; * - p < 0.05; ** - p < 0.005 relative to AAV-GFP; # - p < 0.05; ## - p < 0.005 relative to AAV-ΔFosB).
DISCUSSION
Our results demonstrate that ΔFosB, a member of the AP1 family of transcription factors, serves as a positive regulator of both energy expenditure and bone mass within the hypothalamus. This provides further support for the existence of a link between central regulation of energy expenditure and bone mass. Furthermore, our findings provide novel insight into the central transcriptional regulation of bone and fat. Independent of which neuronal circuitry is involved within, or downstream of, the ventral hypothalamus, these results provide the first direct evidence for a very potent and AP1-sensitive hypothalamic relay in the regulation of bone formation and skeletal homeostasis. Furthermore, these results demonstrate that both energy expenditure and fat and bone homeostasis share a common sensitivity to AP1 activity within the hypothalamus, which appears to play a potent role in down-regulating both energy expenditure and bone formation.
Notwithstanding our previous demonstration that the bone anabolic effects of ΔFosB is in part due to a cell-autonomous mechanism within the osteoblast lineage (15), the data reported here demonstrate that the increased bone formation observed in the ENO2-ΔFosB mice is predominantly due to ΔFosB’s activity in the hypothalamus. Although overexpression of Fra1 under the H2K promoter has recently been reported to reduce adipocytic differentiation and fat mass through cell-autonomous effects (21) we have not seen such effects in the ENO2-ΔFosB mice (16). Supporting this view, the results presented here demonstrate that the reduced adipose mass results exclusively from a centrally mediated increase in energy expenditure, without any detectable cell-autonomous effect of ΔFosB within the adipocyte lineage (16) and independent of the changes occurring in the osteoblasts and in bone formation (15). Indeed, this study demonstrates that expression of ΔFosB in the ventromedial region of the hypothalamus is sufficient to recapitulate the entire phenotype of the ENO2-ΔFosB mice, i.e. increased energy expenditure, decreased fat mass and increased bone formation and bone mass.
Thus, the expression levels of ΔFosB in the hypothalamus can simultaneously affect bone formation and energy expenditure in adult mice, leading to increases in bone formation and bone mass as well as a decrease in adipose mass. As the endogenous level of expression of ΔFosB within the hypothalamus has previously been shown to be regulated under several circumstances (22–24), our results raise the possibility that bone formation and energy expenditure may be physiologically co-regulated, at least in part, through this transcription factor.
Communication between the brain and adipose tissue has been well established (4). Adipose tissue secretes hormones such as leptin and adiponectin that are able to cross the blood-brain barrier and activate specific neurons in the arcuate nucleus of the hypothalamus, which in turn regulate food intake and lipid utilization (25–27). However, we do not observe any significant difference in adiponectin levels in our AAV-inject animals (Figure S1C). It is however only recently that studies have identified the potential importance of the central nervous system, and more specifically of the hypothalamus, in the regulation of bone mass. Signaling via hypothalamic-pituitary relays and the SNS are now recognized as contributors to the regulation of bone homeostasis (6,9,28–31). In addition, the hypothalamus controls the synthesis and secretion of pituitary hormones (FSH, TSH, GH and MSH), several of which affect bone homeostasis (29,30,32,33). We however did not find any significant alterations in these hormones in the ENO2-ΔFosB mice or AAV injected mice (Figure S2), indicating that the very pronounced increase in bone formation rates observed in both models cannot be explained by changes in pituitary hormones.
It is therefore possible that, independent of pituitary regulatory functions, one or more hypothalamic signaling pathways are regulated by ΔFosB to increase bone mass and energy expenditure, only secondarily affecting fat accumulation. As proposed by others, the relay to peripheral osteoblasts may operate via the SNS (6,34). If ΔFosB acts on the leptin/SNS pathway to induce the increase bone formation observed here, it would have to oppose the effects of leptin and/or NPY/PYY in the hypothalamus and/or the effects of beta-2-adrenergic receptor (β2AR) activation in osteoblasts. We have shown that restoring the normal levels of circulating leptin in the ENO2-ΔFosB mice, which are low due to the pronounced decrease in adipocyte size, failed to rescue the bone phenotype (35) and that leptin signaling in these mice is not altered (35). Thus, ΔFosB would have to act downstream of leptin, i.e. on NPY or β2AR signaling, to prevent their negative effects on bone formation. Analysis of NPY expression levels in the hypothalamus of the ENO2-ΔFosB mice revealed, however, that they were unchanged from control levels and β2AR sensitivity in the bone target cells, i.e. osteoblasts was also identical to controls (Figure S3). We therefore consider it unlikely that ΔFosB simply antagonizes the leptin/NPY/β2AR pathway.
In considering alternate possibilities, it is worth mentioning the fact that ΔFosB could be affecting bone and energy via two independent pathways that share only their hypothalamic localization and sensitivity to ΔFosB expression levels. Alternatively, although we have eliminated the possibility that changes in energy expenditure and fat are secondary to changes in bone (15), the reverse could be true, i.e. that the effects on bone formation could be secondary to the changes in energy and/or in fat. In this case, however, increased energy expenditure in muscle, as observed here, would affect bone formation directly or indirectly. Two findings contradict this hypothesis. First, we have found that increased UCP3 expression in muscle, which leads to massive increases in muscle energy expenditure, failed to alter bone formation or bone mass (Figure S4). Second, and although mechanical changes in the muscle could theoretically affect bone, the fact that we observe the same increase in bone formation in the calvaria, a non-weight bearing bone, in the ENO2-ΔFosB and AAV-infected mice makes it an unlikely explanation (Figure S5). Muscle could however exert its action on bone indirectly, via energy-dependent myokines which would induce an increase in bone formation, in addition to depleting fat from adipocytes. Alternatively, the fat depletion secondary to increased energy expenditure in the muscle could alter the levels of key adipokines, affecting positively bone formation. As leptin is not apparently a candidate in our model, the decrease in adiponectin that we observed in the ENO2-ΔFosB mice could be a good candidate since Cornish and colleagues have recently reported an increase in bone mass in adiponectin-null mice (36).
The detailed molecular mechanism by which ΔFosB affects transcriptional and signaling events in the hypothalamus to affect both energy and bone remains to be elucidated. The truncated ΔFosB splice variant lacks most of the C-terminal region of FosB, its major transcriptional domain, and therefore behaves as a relatively weak AP1 transcriptional activator that can also compete with normal FosB functions, leading to its activity as an AP1 antagonist as well (37). The weak AP-1 activity of ΔFosB in unlikely to play a role in the hypothalamic co-regulation described here, however. First, ubiquitous overexpression of FosB fails to induce changes in bone with no reported changes in fat (38 and unpublished results). Second, we have recently reported that expression of a further truncated isoform of ΔFosB (Δ2ΔFosB) under the control of the same ENO2 promoter induces the same bone and fat phenotypes as those of the ENO2-ΔFosB mice despite the fact that it has lost all intrinsic AP1 transcriptional activity (19). Third, the fact that DNJunD expression in the hypothalamus induced both a stronger bone formation phenotype and reduction in adipocyte size provides further support for the conclusion that it is not AP1 transactivation that is required for the co-regulation of energy and bone but rather that it is the AP1 antagonism common to all three proteins (ΔFosB, Δ2ΔFosB and DNJunD) that is essential in this co-regulatory activity. These observations suggest that the changes observed are most likely the result of either failure to induce or suppression of AP1 target genes within the hypothalamus. Further work will be required to test this hypothesis.
Importantly, changes in the expression of ΔFosB in the brain, which we show here affects both energy expenditure and bone formation in a coordinated manner, occur naturally as the result of fosB gene induction and alternative splicing as well as through stabilization of the FosB protein (13,39). Although the mechanisms that regulate the splicing event that generates the ΔFosB transcript are not elucidated, the relative stability of the FosB protein is a key mechanism regulating its accumulation in certain tissues (40). Moreover, both the stability and transactivation capabilities of ΔFosB are regulated by phosphorylation on specific serine residues (41,42). The regulation of ΔFosB levels under physiological conditions has been extensively studied in the nucleus accumbens and dorsal striatum (18,43,44). ΔFosB’s expression has been associated with changes in expression of particular neuropeptides, such as dynorphin, which is downregulated by ΔFosB in the nucleus accumbens (18). Interestingly, dynorphin-null mice have been recently reported as having a decrease in adipose mass and increased fatty acid oxidation, but no change in NPY expression within the hypothalamus (45). It is yet to be determined whether these mice also have an altered bone mass, given the recent reports on Nmu and signaling upstream of the Y2 receptors (7,8,10,46).
The expression of FosB and its shorter isoforms in specific regions of the brain is induced by many stimuli, including seizures, stress, drugs, and exposure to newborn pups (18,22,39,43,47,48). The temporal and spatial expression of FosB isoforms within the brain is markedly dependent on the stimulus (reviewed in (43)). Alteration in the relative levels of expression of the FosB isoforms results in changes in several specific behaviors in these animals, including locomotor activity and motivation for food (44,49). Expression of FosB/ΔFosB in the preoptic area of the hypothalamus of mice has been related to the nurturing of their pups by the fact that FosB/ΔFosB is induced in adult animals in this area upon exposure to pups, and FosB-null mice do not care for their pups appropriately. The adult mice had however no reported abnormality in eating behavior or body weight (22). Finally, recent studies have shown that FosB/ΔFosB expression is induced within the paraventricular nucleus of the hypothalamus upon both acute and repeated administration of cocaine (13,23). Apart from these observations, little is known about the consequences of altering the expression of ΔFosB within hypothalamic nuclei. In this context, our demonstration that variations in the level of ΔFosB in the hypothalamus co-regulate energy expenditure and bone formation is both novel and important.
In conclusion, we have shown that downregulation of AP1 activity, via ΔFosB or DNJunD expression, in hypothalamus simultaneously reduces adipocyte size by increasing energy expenditure and increases bone mass by increasing bone formation. This provides strong evidence for common central mechanisms in the regulation of both energy expenditure and adipose and bone mass. Furthermore our study shows that whether common or separate, the hypothalamic neuronal circuitry that regulates energy/fat and bone share a common sensitivity to AP1 transcriptional activity. These findings may open novel exploratory avenues for the treatment of osteoporosis and/or obesity.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH, NIAMS (AR 048218) and NIA (AG 040222) to R.B., NIMH (MH51399) to E.J.N. and a UNCF-Merck Dissertation Fellowship to G.C.R.
Footnotes
Author contributions: G.C.R., and R.B. designed research; G.C.R., V.V., K.S., H.S., Y.M., S.L., T.A.G., and M.K. performed research; G.C.R., W.C.H., E.J.N., and R.B. analyzed data, G.C.R., E.J.N. and R.B. wrote the paper.
References
- 1.Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. The Journal of clinical endocrinology and metabolism. 2010;95(11):4795–801. doi: 10.1210/jc.2010-1030. [DOI] [PubMed] [Google Scholar]
- 2.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. The American journal of medicine. 2009;122(5):409–14. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Mayer J, Thomas DW. Regulation of food intake and obesity. Science. 1967;156(773):328–37. doi: 10.1126/science.156.3773.328. [DOI] [PubMed] [Google Scholar]
- 4.Horvath TL. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci. 2005;8 (5):561–5. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- 5.He W, Lam TK, Obici S, Rossetti L. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci. 2006;9(2):227–33. doi: 10.1038/nn1626. [DOI] [PubMed] [Google Scholar]
- 6.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 7.Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109(7):915–21. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldock PA, Sainsbury A, Allison S, Lin EJ, Couzens M, Boey D, Enriquez R, During M, Herzog H, Gardiner EM. Hypothalamic control of bone formation: distinct actions of leptin and y2 receptor pathways. J Bone Miner Res. 2005;20(10):1851–7. doi: 10.1359/JBMR.050523. [DOI] [PubMed] [Google Scholar]
- 9.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 10.Sato S, Hanada R, Kimura A, Abe T, Matsumoto T, Iwasaki M, Inose H, Ida T, Mieda M, Takeuchi Y, Fukumoto S, Fujita T, Kato S, Kangawa K, Kojima M, Shinomiya K, Takeda S. Central control of bone remodeling by neuromedin U. Nat Med. 2007;13 (10):1234–40. doi: 10.1038/nm1640. [DOI] [PubMed] [Google Scholar]
- 11.Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, Bouali Y, Mukhopadhyay K, Ford K, Nestler EJ, Baron R. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6(9):985–90. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- 12.Sims NA, Sabatakos G, Chen JS, Kelz MB, Nestler EJ, Baron R. Regulating DeltaFosB expression in adult Tet-Off-DeltaFosB transgenic mice alters bone formation and bone mass. Bone. 2002;30(1):32–9. doi: 10.1016/s8756-3282(01)00622-6. [DOI] [PubMed] [Google Scholar]
- 13.McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6(11):1208–15. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 14.Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, Russo SJ, Vialou V, Chakravarty S, Kodadek TJ, Stack A, Kabbaj M, Nestler EJ. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62(3):335–48. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kveiborg M, Sabatakos G, Chiusaroli R, Wu M, Philbrick WM, Horne WC, Baron R. DeltaFosB induces osteosclerosis and decreases adipogenesis by two independent cell-autonomous mechanisms. Mol Cell Biol. 2004;24(7):2820–30. doi: 10.1128/MCB.24.7.2820-2830.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowe GC, Choi CS, Neff L, Horne WC, Shulman GI, Baron R. Increased energy expenditure and insulin sensitivity in the high bone mass DeltaFosB transgenic mice. Endocrinology. 2009;150(1):135–43. doi: 10.1210/en.2008-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang SX, Zhang JP, Fletcher DL, Zoeller RT, Sun GY. In situ hybridization of mRNA expression for IP3 receptor and IP3-3-kinase in rat brain after transient focal cerebral ischemia. Brain Res Mol Brain Res. 1995;32(2):252–60. doi: 10.1016/0169-328x(95)00085-7. [DOI] [PubMed] [Google Scholar]
- 18.Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9 (2):205–11. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- 19.Sabatakos G, Rowe GC, Kveiborg M, Wu M, Neff L, Chiusaroli R, Philbrick WM, Baron R. Doubly truncated FosB isoform (Delta2DeltaFosB) induces osteosclerosis in transgenic mice and modulates expression and phosphorylation of Smads in osteoblasts independent of intrinsic AP-1 activity. J Bone Miner Res. 2008;23(5):584–95. doi: 10.1359/JBMR.080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peakman MC, Colby C, Perrotti LI, Tekumalla P, Carle T, Ulery P, Chao J, Duman C, Steffen C, Monteggia L, Allen MR, Stock JL, Duman RS, McNeish JD, Barrot M, Self DW, Nestler EJ, Schaeffer E. Inducible, brain region-specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res. 2003;970(1–2):73–86. doi: 10.1016/s0006-8993(03)02230-3. [DOI] [PubMed] [Google Scholar]
- 21.Luther J, Driessler F, Megges M, Hess A, Herbort B, Mandic V, Zaiss MM, Reichardt A, Zech C, Tuckermann JP, Calkhoven CF, Wagner EF, Schett G, David JP. Elevated Fra-1 expression causes severe lipodystrophy. Journal of cell science. 2011;124(Pt 9):1465–76. doi: 10.1242/jcs.079855. [DOI] [PubMed] [Google Scholar]
- 22.Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86(2):297–309. doi: 10.1016/s0092-8674(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 23.Chocyk A, Czyrak A, Wedzony K. Acute and repeated cocaine induces alterations in FosB/DeltaFosB expression in the paraventricular nucleus of the hypothalamus. Brain Res. 2006;1090(1):58–68. doi: 10.1016/j.brainres.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32 (5):516–31. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyck DJ, Heigenhauser GJ, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) 2006;186(1):5–16. doi: 10.1111/j.1748-1716.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 26.Ahima RS, Qi Y, Singhal NS. Adipokines that link obesity and diabetes to the hypothalamus. Prog Brain Res. 2006;153:155–74. doi: 10.1016/S0079-6123(06)53009-2. [DOI] [PubMed] [Google Scholar]
- 27.Ahima RS, Qi Y, Singhal NS, Jackson MB, Scherer PE. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55(Suppl 2):S145–54. doi: 10.2337/db06-s018. [DOI] [PubMed] [Google Scholar]
- 28.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122(5):803–15. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Cornish J, Callon KE, Mountjoy KG, Bava U, Lin JM, Myers DE, Naot D, Reid IR. alpha -melanocyte-stimulating hormone is a novel regulator of bone. Am J Physiol Endocrinol Metab. 2003;284(6):E1181–90. doi: 10.1152/ajpendo.00412.2002. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, Davies TF, Blair HC, Abe E, Zaidi M. TSH and bone loss. Ann N Y Acad Sci. 2006;1068:309–18. doi: 10.1196/annals.1346.033. [DOI] [PubMed] [Google Scholar]
- 31.Daval M, Foufelle F, Ferre P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol. 2006;574(Pt 1):55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125(2):247–60. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 33.Olney RC. Regulation of bone mass by growth hormone. Med Pediatr Oncol. 2003;41 (3):228–34. doi: 10.1002/mpo.10342. [DOI] [PubMed] [Google Scholar]
- 34.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 35.Kveiborg M, Chiusaroli R, Sims NA, Wu M, Sabatakos G, Horne WC, Baron R. The increased bone mass in deltaFosB transgenic mice is independent of circulating leptin levels. Endocrinology. 2002;143(11):4304–9. doi: 10.1210/en.2002-220420. [DOI] [PubMed] [Google Scholar]
- 36.Cornish J, Williams GA, Callon KE, Watson M, Lin J, Naot D, Wang Y, Xu A, Reid IR. Adiponectin, another link between fat and bone mass? Bone. 2007;40(6):S117–S117. [Google Scholar]
- 37.Yen J, Wisdom RM, Tratner I, Verma IM. An alternative spliced form of FosB is a negative regulator of transcriptional activation and transformation by Fos proteins. Proc Natl Acad Sci U S A. 1991;88(12):5077–81. doi: 10.1073/pnas.88.12.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grigoriadis AE, Schellander K, Wang ZQ, Wagner EF. Osteoblasts are target cells for transformation in c-fos transgenic mice. J Cell Biol. 1993;122(3):685–701. doi: 10.1083/jcb.122.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci. 1997;17 (13):4933–41. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alibhai IN, Green TA, Potashkin JA, Nestler EJ. Regulation of fosB and DeltafosB mRNA expression: in vivo and in vitro studies. Brain Res. 2007;1143:22–33. doi: 10.1016/j.brainres.2007.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulery PG, Nestler EJ. Regulation of DeltaFosB transcriptional activity by Ser27 phosphorylation. Eur J Neurosci. 2007;25(1):224–30. doi: 10.1111/j.1460-9568.2006.05262.x. [DOI] [PubMed] [Google Scholar]
- 42.Carle TL, Ohnishi YN, Ohnishi YH, Alibhai IN, Wilkinson MB, Kumar A, Nestler EJ. Proteasome-dependent and -independent mechanisms for FosB destabilization: identification of FosB degron domains and implications for DeltaFosB stability. Eur J Neurosci. 2007;25(10):3009–19. doi: 10.1111/j.1460-9568.2007.05575.x. [DOI] [PubMed] [Google Scholar]
- 43.McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132(2):146–54. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26(36):9196–204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sainsbury A, Lin S, McNamara K, Slack K, Enriquez R, Lee NJ, Boey D, Smythe GA, Schwarzer C, Baldock P, Karl T, Lin EJ, Couzens M, Herzog H. Dynorphin knockout reduces fat mass and increases weight loss during fasting in mice. Mol Endocrinol. 2007;21(7):1722–35. doi: 10.1210/me.2006-0367. [DOI] [PubMed] [Google Scholar]
- 46.Wortley KE, Garcia K, Okamoto H, Thabet K, Anderson KD, Shen V, Herman JP, Valenzuela D, Yancopoulos GD, Tschop MH, Murphy A, Sleeman MW. Peptide YY regulates bone turnover in rodents. Gastroenterology. 2007;133(5):1534–43. doi: 10.1053/j.gastro.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Zhang Y, Kelz MB, Steffen C, Ang ES, Zeng L, Nestler EJ. Induction of cyclin-dependent kinase 5 in the hippocampus by chronic electroconvulsive seizures: role of [Delta]FosB. J Neurosci. 2000;20(24):8965–71. doi: 10.1523/JNEUROSCI.20-24-08965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24 (47):10594–602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401(6750):272–6. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.